Abstract
Today molecular imaging technologies play a central role in clinical oncology. The use of imaging techniques in early cancer detection, treatment response and new therapy development is steadily growing and has already significantly impacted clinical management of cancer. In this chapter we will overview three different molecular imaging technologies used for the understanding of disease biomarkers, drug development, or monitoring therapeutic outcome. They are (1) optical imaging (bioluminescence and fluorescence imaging) (2) magnetic resonance imaging (MRI), and (3) nuclear imaging (e.g, single photon emission computed tomography (SPECT) and positron emission tomography (PET)). We will review the use of molecular reporters of biological processes (e.g. apoptosis and protein kinase activity) for high throughput drug screening and new cancer therapies, diffusion MRI as a biomarker for early treatment response and PET and SPECT radioligands in oncology.
OPTICAL IMAGING
Introduction
The integration of genetically encoded imaging reporters into cells and animals provides a unique opportunity to monitor molecular, biochemical and cellular pathways in vivo as modifications of post- or co-translational events such as phosphorylation or glycosylation can be monitored in real-time. In this section we will discuss the use of such reporters for target validation and dose/schedule optimization and for identification of lead compounds from a library using cell-based, high-throughput screening. We will further review the use of transgenic animals expressing tissue specific fluorescent or bioluminescent reporters as a unique tool for studying cellular processes such as transcription or apoptosis in living cells and animals.
Clinical significance for imaging kinase activity
Dysregulation and mutation of kinases have been reported to play a causal role in many human diseases such as cancer, rheumatoid arthritis, cardiovascular and neurological disorders, asthma, and psoriasis [1–6]. Molecular profiling of pathological samples and corroborative development in bioinformatics in recent years have led to the identification of potential biomarkers [7–11]. Among the biomarkers identified, protein kinases have gained much attention as potential therapeutic targets. Thus it is not surprising that the first molecularly targeted drugs approved by the Food Drug Administration are tyrosine kinase inhibitors specific for abl (the Abelson protooncogene), PDGFR (platelet-derived growth factor receptor), Erlotinib, Avastin, Lapatinib, and Herceptin [12]. However, other than Imatinib, most kinase inhibitors such as Erlotinib, Lapatinib, Cetuximab, ABX-EGF, Bevacizumab have relatively modest activity as a single agent [13–14]. This suggests the use of multitargeted kinase inhibitors or the combination with radiation therapy to be more beneficial for cancer therapy than a single agent. There is an increasing need for the preclinical evaluation of drug efficacies and also the optimization of dosing and scheduling of such agents. For instance the combinatorial treatment of gemcitabine and gefitinb was determined efficacious in a preclinical study of head and neck cancer but only when gefitinib was administered prior to gemcitabine [15]. The ability to test drug interaction and agent combination in preclinical models will facilitate drug development and serve to provide for rational does/schedule development for clinical trials. We believe that the use of targeted agents in oncology will significantly benefit from molecular imaging techniques in preclinical studies with the goal of identifying most efficacious agents modulating signaling pathways in a targeted manner. The increased development of such preclinical model systems has already begun to provide us with the ability to screen large number of molecules and will ensure the translation of the most efficacious drug or drug combination into the clinic in the future.
Imaging of post and co-translational events
Screening and identification of new kinase inhibitors from a library of chemical compounds require robust methods. Western blotting with phospho-specific antibodies and in vitro kinase assays with radioisotopes are commonly used to assess kinase activity. However, these methods are invasive, require large numbers of animals, and only provide a snapshot of kinase activity at specific time points. Additionally, some of these assays are only suitable for in vitro studies and may not reflect the in vivo kinase activity or drug specificity. Recent discoveries in the field of optical imaging have overcome such limitations. The field of molecular imaging encompasses the noninvasive visual representation of biological processes at the cellular and molecular level in the whole organism and the modalities and instrumentation to support the visualization and measurement of these processes [16]. These imaging technologies are an attempt to bridge the gap between discovery of causal disease markers and identification of their inhibitor for potential therapeutic use.
We have recently developed a bioluminescent reporter to monitor activity of the serine/threonine kinase Akt/PKB, [17], whose expression profile has been linked to tumor initiation, progression, and also resistance to cancer therapy. This reporter was based on conformation-dependent complementation of firefly luciferase, wherein a monomeric reporter gene is split into two separate inactive components. The reporter activity is restored and can be measured when the two components are brought into close proximity. Fields and colleagues utilize this strategy first and developed the yeast two hybrid system [18], which is based on protein complementation of GAL4, a transcriptional activator. Since then the system has been used successfully to identify novel protein-protein interactions. Yet it has limited utility in the context of a living cell or animal. Subsequently a number of molecular reporters routinely used in mammalian biology were engineered for complementation studies. These include a plethora of fluorescent proteins and bioluminescent enzymes (firefly luciferase and renilla luciferase), β-galactosidase, dihydrofolate reductase (DHFR), and TME-1 β-lactamase [19–24].
The bioluminescence reporter approach has emerged as a useful technique for small animal imaging using complementation assays. In the presence of ATP and oxygen luciferase modifies its substrate luciferin by releasing photons. The ATP-dependent firefly (Photinus pyralis) is most commonly used for in vivo imaging due to the fact that 30% of its light generated emits at a spectra above 600 nm, a region where the signal attenuation by the absorption and scattering properties of mammalian tissue is minimal [25–26]. Hence compared with fluorescence imaging wherein the excitation light excites other fluorescent molecules in the body, thus creating background/autofluoresence, BLI is superior especially for deep tissue imaging.
Previously Luker and colleagues developed a luciferase complementation system, by which N-terminal and C-terminal luciferase fragments were fused with FRB of the mammalian target of rapamycin and FK506-binding protein12 (FKBP), respectively [27]. Interaction between FRB–N-Luc/C-Luc–FKBP upon single-site binding of rapamycin to FKBP, reconstituted luciferase activity in an FK506-competitive manner. Later the investigators used this strategy to demonstrate the phosphorylation-dependent interaction between human Cdc25C and 14-3-3ε in vivo [27].
To create a prototype kinase reporter for reporting changes in kinase activity, we adapted the previously mentioned complementation system further. In particular, we flanked an Akt consensus substrate peptide and the phosphorylated amino acid binding domain from FHA2 with an amino- (N-Luc) and carboxyl- (C-Luc) terminal domain of the firefly luciferase reporter molecule (Figure 1) [17] to develop a bioluminescent Akt reporter (BAR). In the presence of Akt kinase activity, phosphorylation of the Akt consensus substrate sequence results in its binding to the FHA2 domain, thus sterically preventing reconstitution of a functional luciferase protein. In the absence of Akt kinase activity, release of this steric constraint allows reconstitution of the luciferase enzyme resulting in detectable bioluminescence. The advantage of this novel bioluminescent reporter is that a) it can be adapted for monitoring other kinase activities and b) it allows imaging in living cells and animals in a quantitative, dynamic, and noninvasive manner.
Figure 1. Schematic representation of the Akt kinase reporter (BAR).
A, The chimeric Akt kinase reporter is a fusion of the N-Luc and C-Luc luciferase protein linked to an Akt peptide domain containing the Akt substrate sequence and a yeast FHA2 phospho-Ser/Thr binding domain. B, Phosphorylation of the Akt substrate peptide in the reporter results in interaction with the FHA2 phospho-peptide binding domain leading to steric constraints on the N-Luc and C-Luc. Inhibition of Akt kinase activity results in decreased binding of phospho-peptide and the binding domain which enables reconstitution of luciferase and restored bioluminescence.
Preclinical application of kinase reporters
Applications of a kinase reporter include studying signaling pathways in biological systems as well as testing drug efficacies for preclinical studies. We used the BAR reporter in cell culture to evaluate several inhibitors; an Akt inhibitor (API-2) along with a PI-3K inhibitor (Perifosine). Increased bioluminescence activity was observed in a time- and dose-dependent manner with both inhibitors indicating that BAR provides a surrogate for Akt activity in terms of quantity and dynamics. Further we confirmed Akt kinase inhibition with conventional western blotting using phosphor specific antibodies against Akt [17]. Bioluminescent kinase reporters may additionally aid in investigating signaling events that are upstream of the particular kinase and thus impinge on its activity. When BAR reporter–expressing cells were treated with EGF, changes in BAR bioluminescent activity were detected thus confirming its application for pathway analysis [17]. Our results indicated that activation of EGFR, which has previously been reported to feed into the Akt cascade, can be monitored by Akt bioluminescent imaging. To test the use of BAR as a surrogate marker for EGFR signaling different cell lines were treated with a known EGFR inhibitor, Erlotinib. Differential activation of the BAR reporter was observed in Erlotinib-sensitive and Erlotinib-resistant cell lines, thus confirming its use in sensing specific upstream signals [17].
As mentioned previously, a significant advantage of using bioluminescent kinase reporters lies in their potential to monitor signaling pathways in live animals, and therefore providing a unique understanding of pharmacokinetics and bioavailability of specific drugs. For example, when animals with glioma xenografts were treated with 20 mg/kg of the Akt inhibitor AP1-2, peak inhibition was observed 12 post-treatment, yet when treated with 40 mg/kg, BLI signals were detected up to 24 hours after treatment initiation (Figure 2). Interestingly when animals were treated with Perifosine, Akt kinase inhibition was detected by BLI at 2 hours post treatment and remained elevated for 7 days. This observation was confirmed by previously published data demonstrating high plasma concentration of the drug up to 7 days post treatment.
Figure 2. Imaging of Akt kinase inhibiton in live animals.

A, Mice transplanted with D54 cells expressing the Akt reporter were treated with vehicle control (20% DMSO in PBS), API-2 (20 mg/kg or 40 mg/kg), or Perifosine (30 mg/kg). Representative images are shown of mice prior to treatment or during maximal luciferase signal upon treatment (Max), and post-treatment. B, Tumor-specific bioluminescence activity of D54 cells stably expressing BAR in mice, treated with either the vehicle control (20% DMSO in PBS) or API-2 (20 mg/kg or 40 mg/kg), was monitored at various times. Fold induction of signal intensity over pretreatment values was plotted as mean ± SEM for each of the groups. C, Bioluminescence activity in tumor-bearing mice before and after treatment with 30 mg/kg perifosine was plotted as fold induction over pretreatment values (± SEM) for each of the groups. (From [17], with permission).
In summary such studies establish a preclinical application for BLI reporters in identifying drug–target interaction in cells and living animals. Furthermore can these reporter assays be adapted for their in vivo use of dose and schedule optimization and identification of efficacious combinatorial treatments.
Modification of bioluminescent kinase reporter for enhanced sensitivity
To increase reporter sensitivity we tested the hypothesis that subcellular localization may influence kinases specificity. Distinct subcellular localization harbor variable kinase concentration and thus may result in improved reporter activity. Akt for example is recruited to the plasma membrane by PI-3 kinase–generated D3-phosphorylated phosphoinositides which bind to the Akt PH domain and induce its translocation [28–29]. Kinase-1, which depends on phosphoinositides co-localizes at the same time and phosphorylates Akt within the activation loop [28–29]. We hypothesized that constructing a membrane-targeted bioluminescent Akt reporter would increase its sensitivity. By fusing 10 amino-terminal residues of Lyn kinase responsible for myristoylation and palmitoylation to BAR [30](Figure 3) sensitivity of the MyrPalm-BAR reporter was doubled compared with BAR (unpublished data). Thus, subcellular localization of the investigated kinase needs to be considered when designing the next generation reporters.
Figure 3. Membrane-targeted bioluminescent Akt reporter.
MyrPalm-BAR is generated by adding 10 amino acids from the N-terminus of Lyn kinase to BAR plasmid. The proposed basis of reporter activity for the MyrPalm-BAR reporter remains the same as that for BAR alone, which is described in Figure 1. This involves Akt-dependent phosphorylation of the Akt peptide domain that results in its interaction with the FHA2 domain. In this form (Akt-ON), the reporter has minimal bioluminescence activity (Light OFF). In the absence of Akt activity (Akt-OFF), association of the N-Luc and C-Luc domains restores bioluminescence activity (Light-ON).
Molecular imaging of N-linked glycosylation
N-linked glycosylation (NLG) is a complex biosynthetic process that regulates maturation of proteins through the secretory pathway. This co-translational modification is regulated by a series of enzymatic reactions, which results in the transfer of a core glycan from the lipid carrier to a protein substrate. Previous work has shown that inhibition of (NLG) in vitro [31] reduces protein levels of receptor tyrosine kinases (RTKs) (ie EGFR, ErbB2, Erbb3, and IGF-1R) commonly overexpressed in many cancers, resulting in decreased signaling through both dominant and redundant RTK pathways. Hence inhibiting NLG has become a feasible strategy for cancer therapy [31] and has also been shown to have radiosensitizing effects as demonstrated for other RTK targeting agents in glioblastoma [32–35]. Despite validation of therapeutic NLG inhibition in vitro its evaluation as a potential target for cancer therapy is required in vivo. We have recently developed a model system, which allows us to monitor NLG in vivo by using bioluminescent imaging techniques [36]. We utilized a modified luciferase reporter (ER-LucT) to monitor the co-translational transfer of glycan precursors from its lipid precursor to consensus NLG sites (NXS/T) within the Luciferase reporter (Figure 4A) [36]. Glycosylation of Luc in the ER disrupted the ability of this enzyme to use luciferin and ATP as substrates and therefore had low biolumiescent activity. On the contrary inhibition of NLG (and loss of the added glycan moiety) enhanced bioluminescent activity [36]. After in vitro validation, we tested this reporter in D54 glioma xenografts. With the use of this novel ER-LucT reporter we demonstrated by non-invasively imaging these tumors that inhibition of NLG correlated with a decrease in RTK protein levels and tumor regression [36]. Using this molecular imaging approach we further determined efficacious in vivo doses of the GlcNAc-1-phosphotransferase inhibitor, and tunicamycin, which blocks N-glycan precursor biosynthesis. In summary, this reporter allowed for the determination that NLG inhibition in D54 and U87MG glioma xenograft tumors is therapeutically beneficial since the combination of NLG inhibiton and radiation therapy led to a significant tumor regression compared to NLG inhibition and radiation therapy given as single therapies (Figure 4B,C) [36].
Figure 4. Schematic diagram illustrating the NLG reporter design.
A, the ER translation sequence of the epidermal growth factor receptor is fused to the N-terminus of the Luc enzyme. Constructs contain either three (ER-LucT) sites for N-linked glycosylation or a single (ER-LucS) site. B, Mice bearing wild type D54 or C, U87-MG xenograft tumors were randomized into four treatment groups: control, tunicamycin (Tn, 0.75mg/kg), radiation (IR, 5 daily fractions of 2Gy) and the combination of Tn with IR. Data points represent relative tumor growth compared to the tumor volume on Day 0, and error bars report the standard error. (From [36], with permission).
Clinical significance for imaging proteases
Biological processes such as cleavage of nascent polypeptide chains, posttranslational cleavage of inactive enzymes to yield functional enzymes and proteolytic degradation of enzyme are regulated through the actions of proteases. Proteolytic processing is modulated both temporally and positionally, and thus contributes to protein activation and subcellular localization. Enzymatic cleavage plays a critical role in several physiological processes ranging from embryogenesis, hormone maturation, immunity, blood clotting, pathogenesis of viral and bacterial diseases to programmed cell death. Proteases are very tightly controlled and in the case of caspases even determine the cells fate due to their critical role in coordinating proliferation and programmed cell death, which is essential for normal physiology. Dysregulation of signaling pathways culminating in caspase activation often result in diseases such as AIDS, neurodegenerative disorders, myelodysplastic syndromes, ischemia/reperfusion injury, autoimmune disease and cancer. Thus, quantitative noninvasive imaging of proteases is of importance for monitoring disease progression, and for screening and validation of experimental therapeutic agents.
Imaging of enzymatic activity
Programmed cell death culminates in the selective activation of caspases (cysteine-aspartic proteases) followed by the cleavage of specific target proteins and is either death receptor (extrinsic) initiated or intrinsically by inhibitors of cellular pathways, such as staurosporine [37–40]. The converging point of this complex protease cascade of both intrinsic and extrinsic apoptotic pathways is the activation of caspase-3 which cleaves key proteins leading to the concomitant appearance of apoptotic morphology [40–42].
To develop a molecular imaging tool, which would report on caspase-3 activity yet exhibit low levels of background bioluminescent activity when expressed in mammalian cells we utilized our previously described chimeric luciferase reporter [43]. The fusion of an estrogen receptor regulatory domain (ER) to both the N- and C-terminus of luciferase had successfully silenced any bioluminescent activity of this enzyme (Figure 5). The design of a novel apoptosis reporter included the protease cleavage site for caspase-3 (DEVD) at the junction of the luciferase and ER domains allowing protease-mediated activation of the reporter upon separation from the silencing domain (ie, ER). When tested in cells undergoing apoptosis, caspase-3–dependent cleavage of the reporter occurred and resulted in the restoration of luciferase activity [43]. Furthermore, the use of this bioluminescent reporter in a xenograft model enabled us to image caspase-3 activation noninvasively upon TRAIL treatment. It further provided insight into its potential application as a unique tool for evaluating therapeutic efficacy of experimental agents alone or in combination and for dose and schedule optimization. Using a combinatorial treatment regimen of 5-fluorouracil and TRAIL/Apo2L in a glioma xenograft model (D54 cells), the use of our caspase-3 reporter indeed allowed determination of increased efficacy of this drug combination versus each drug alone [44].
Figure 5. Bioluminescent imaging reporter for apoptosis.
Depicted is a recombinant reporter created by fusing residues 281 to 599 of the modified mouse estrogen receptor (ER) sequence to the N- and C-terminal ends of luciferase, respectively, yet separated by DEVD sequence on both ends. Caspase-3–dependent cleavage of the ER domains from the luciferase of the ER–DEVD–Luc–DEVD–ER reporter restores luciferase activity.
Development of caspase-3 Reporter variants
Three additional variations of the caspase-3 reporter were developed to improve signal-to-noise ratios:
we construction of a recombinant protein wherein Peptide A and B, a pair of peptides that had been reported to possess strong affinity for each other were fused to N-Luc (ANLuc) and C-Luc (BCLuc), respectively with an intervening caspase-3 cleavage site [27, 45–46]. This chimeric luciferase reporter had significantly reduced background luciferase activity as the N-Luc and C-Luc were unable to complement when expressed as a fusion protein (Figure 6). Recently published data with this optimized apoptosis reporter demonstrates that this is a highly sensitive, dynamic, and quantitative system for the detection of caspase-3 activity both in vitro and in vivo [47]. It further allowed in vivo optimization of dose, combination, and schedule of novel therapies in a dynamic, noninvasive manner [47].
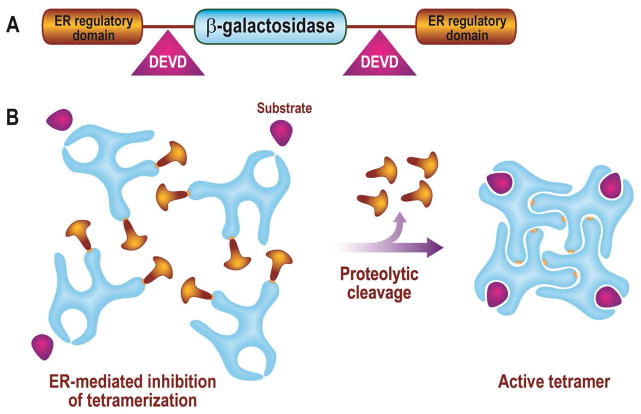
To minimize potential oligomerization of the reporter, two ER regulatory domains were added which inhibited the interaction of the substrate with the enzyme and replaced the monomeric luciferase with the tetrameric β-galactosidase which hindered the oligomerization [48–49] (Figure 7). Further advantages of using a β-galactosidase–based protease sensing reporter are the substrate variety available that are fluorogenic, para-magnetic, radioactive or chemiluminiscent and the increased stability of β-Galactosidase offering a robust and multimodality molecular imaging technology [50–53]. The use of this ER–LacZ–ER reporter for in vivo imaging in transgenic animals is described elsewhere in this chapter.
A non-bioluminescent reporter which contained a single chain antibody (harboring signal peptide, HA and myc tags, and a transmembrane domain), a Golgi retention signal and a caspase-3 recognition- and cleavage sequence (for details see reference [16]) who also constructed cells which undergo cell death actively translocate this chimeric single chain antibody, normally residing in the Golgi bodies, to the cell surface. Since this process is caspase-3 dependent, dying cells which contain the antibody on its cell surface were visualized (Figure 8). This strategy may not only enable a true three-dimensional imaging of apoptosis by may also allow the visualization of tumor cells undergoing apoptosis by other imaging modalities when coupled with nanoparticles embedded with contrast agents.
Figure 6. Noninvasive imaging of caspase-3 utilizing a split-luciferase reporter strategy.

A, Schematic representation of the bioluminescent caspase-3 reporter (AN-Luc-BC-Luc). Apoptosis imaging reporter constitutes the split luciferase (N-Luc and C-Luc) domains fused to interacting peptides, pepA and pepB, with an intervening caspase-3 cleavage motif. B, Upon induction of apoptosis, the reporter molecule is proteolytically cleaved by caspase-3 at the DEVD motif. This cleavage enables interaction between pepANLuc and pepBCLuc, thus reconstituting luciferase activity.
Figure 7. Tetrameric LacZ–based reporter for monitoring cell death.
A, Recombinant beta-galactosidase–based apoptosis reporter was created by fusing ER at both N- and C-terminus of LacZ separated by DEVD sequence on both ends. B, Addition of the ER to both end of LacZ had lead to inhibitory effect on the activity of ER–DEVD–LacZ–DEVD–ER: It inhibited the tetramerization of the enzyme, which is critical for its activity, leading to increased signal-to-noise ratio.
Figure 8. Strategy for imaging of apoptosis based on conditional expression of single chain antibody.
A, Constitutive imaging construct (CIC) was constructed from a single chain antibody, signal peptide, HA and myc tags, and a transmembrane domain. When expressed in cells, this fusion protein localizes to the cell surface. B, The inducible imaging construct (IIC) was CIC engineered to contain a Golgi retention signal, separated by caspase-3 recognition and cleavage sequence. The DEVD sequence was placed between the transmembrane domain and a Golgi retention signal to retain caspase-3 cleavage sequence in the cytoplasm (see inset). When expressed in cells, this chimeric protein localizes to Golgi bodies. Induction of apoptosis leads to caspase-3 activation and cleavage of the chimeric protein resulting in its translocation to cell surface.
Imaging of organelle-specific proteases
Versatile and sensitive assay systems to monitor the activity of proteases involved in the maturation of secretory proteins have been lacking. These proteases reside in the trans-Golgi network (TGN) where they proteolytically process newly formed proteins from the endoplasmic reticulum before packaging into secretory vesicles. Studying TGN protease biology noninvasively would allow the preservation of the unique intracellular TGN environment (low pH, high Ca2+) that is otherwise perturbed by commonly used biochemical methods. Such strategies are further advantageous for discovering novel pharmaceutical agents that can traverse plasma and Golgi membranes and retain inhibitory activity within the microenvironment of TGN. TGN-residing proteases include carboxypeptidases, prohormone convertase (PC) family members, and β-site amyloid precursor protein (APP)-cleaving enzyme (BACE) family members [54–57].
In an effort to develop a noninvasive reporter for TGN-residing proteases, we used three domains: a secreted alkaline phosphate (SEAP), a Golgi protease-specific recognition and cleavage site, and the cytoplasmic and transmembrane domains from BACE function to retain the reporter within the TGN (Figure 9). This GRAP reporter localizes to TGN until it is cleaved by a specific TGN protease after which SEAP is secreted into the extracellular medium. Thus, SEAP levels present in the media are indicative of intracellular TGN protease activity. Decreases in SEAP levels signify a loss of protease activity and allows positive identification of protease inhibitors.
Figure 9. TGN protease imaging reporter.
A, Fusion protein consisting of three functional domains, a secreted alkaline phosphatase (SEAP), a Golgi protease–specific recognition and cleavage site, and a Golgi retention signal from BACE that retains the reporter within the TGN, was created. This reporter localizes to TGN until it is cleaved by a specific TGN protease, after which SEAP is secreted into the extracellular media. The protease-specific recognition and cleavage sequence is located within the lumen of the Golgi to respond to specific TGN proteases residing in this compartment of the Golgi bodies (see inset).
The TGN-enriched protease Furin plays a critical role in processing a myriad of proteins such as serum proteins (proalbumin), coagulation factors (pro-von Willebrand factor), growth factors, hormones, cell surface receptors (insulin proreceptor), matrix metalloproteases (stromolysin-3 and MT1-MMP) and yet it also utilized by a number of pathogens such as HIV-1, ebola, and avian influenza and virulent bacterial pathogens such as anthrax, pseudomonas, and diphtheria.
Thus enhanced processing by Furin and other proteases has been linked to the development of several diseases, such as Alzheimer’s disease (AD), arthritis, and cancer. Remarkably, recent data suggest that the acquisition a glycoprotein effectively cleaved by Furin may extend the ability of the newly discovered H5N1 avian influenza virus thus contributing to its pathogenicity. The activation of the toxin anthrax through Furin cleavage is another example where one may envision benefits for inhibiting such protease activity.
To obtain the ability for noninvasive monitoring Furin activity we included a 10–amino acid recognition and cleavage site (GLSARNRQKR↓) of the furin substrate Stromolysin-3 (ST3) in our previously developed reporter and named it GRAPfurin. Overexpression of Furin in cells resulted in an observed increase in processing of GRAPfurin reporter as detected by western blotting and as an increase in SEAP activity in the media.
The specificity of this system was shown by construction of GRAPfurinmut in which the furin target recognition and cleavage sequence was mutated to GLSAANAQAA↓, rendering this reporter nonresponsive to Furin proteolytic activity. This led to a reduction in processed protein in both the lysate and extracellular medium and a concomitant decrease in SEAP activity in media [58–59]. Additionally, a notable decrease in SEAP activity was observed when CHO-GRAPfurin cells were treated with 25 μM of the Furin inhibitor, dec-RVKR-CMK, whereas similar treatment with dec-RVKR-CMK caused no decrease in SEAP activity in the control CHO cell line expressing SEAP constitutively [59]. In summary, using this technology, monitoring of Furin protease activity is now possible using molecular imaging [59].
Use of molecular reporters for high-throughput drug/target screening
The use of Furin inhibitors such as α1-PDX, D6R, D9R and dec-RVKR-CMK as pharmaceutical agents is unfortunately hampered by their large size, lack of stability, and/or toxicity. To date, the only non-protein/peptide inhibitor of Furin is a naturally occurring neoandrographolide and its succinoyl ester derivatives, with IC50 values ranging from high micromolar to low millimolar values. Thus there is an urgent need for identifying novel Furin inhibitors with desired characteristics in context to toxicity, solubility, and ability to interact with the protein target in its appropriate subcellular compartment. Such drug discovery can be achieved by modifying above described cell-based assays for high-throughput screening (HTS) of compound and siRNA libraries. Utilizing this approach, identification of Furin inhibitors utilizing our GRAPfurin reporter from a screen of 39,000 molecules was accomplished. CCG 8294, one of the major hits in the high-throughput assay, has shown promise as a Furin inhibitor with high efficacy in cells and has also shown inhibition of Furin-mediated processing of polypeptides within the secretory pathway [59].
This platform may also be used for imaging of kinase activity. These cell-based screens have unique advantages as only compounds interacting with the target in the correct cellular compartment and under normal cellular physiological conditions of that sub-cellular compartment (pH, concentrations of specific ions, etc) would be identified. In addition, because the assay involves live cells, the reporter enables monitoring of the kinase in question in context of other signaling pathways. Lastly, in contrast to other cell-based reporter screens, which are fraught with false positives, the kinase reporter such as BAR is a “gain-of-function assay” wherein the inhibition of kinase activity results in increase in bioluminescence. For example, compounds that are cytotoxic (and thus result in loss of signal) or those that inhibit the reporter directly (eg, luciferase inhibitors) may show up as false positives in traditional kinase assays but not with the BAR reporter platform. Such carefully designed screening methodologies would enable one to narrow down the number of hits to a smaller group of “true positives.”
In summary, we have developed imaging reporters for monitoring activities of kinases, proteases, and programmed cell death and thus provide imaging tools for high-throughput screening of targeted inhibitors, and pro- and antiapoptotic compounds and validation of such.
Design of transgenic reporter mice for optical imaging
Transgenic reporter mice contain transgenes, which typically consist of a promoter driving the expression of a fluorescent or bioluminescent reporter gene. The advantage of such reporter mice lies in the ability to follow the activation of specific promoters and transcription factors or even enzymatic activity in real time by utilizing optical imaging techniques. Biological processes such as transcription or apoptosis can be monitored both over time and in all organs of the animal. The combination of the Cre-loxP system with the use of transgenic reporter genes allows tissue specific Cre recombination and thereby activation of the fluorescent or bioluminescent reporter. The recent development of a reporter mouse (Luc-gal tg/reference), wherein global deletion of the loxP flanked EGFP by germ line Cre recombination lead to the expression of Luciferase and β-GAL exemplifies this concept [60]. This reporter mouse allows for noninvasive imaging of targeted Cre activation in vitro or in living animals and will be useful for future studies examining these events.
A wide range of human disorders involves the inappropriate regulation of NF-κB, including cancer, neurodegenerative diseases, asthma, and inflammatory diseases. With the development of transgenic mice reporting on NF-κB activity key processes regulating NF-κB dependent transcription can now be identified in vivo [61]. Another signaling molecule involved in the pathogenesis of many human diseases, including cancer, fibrotic disorders, and neurodegeneration is TGF-β. Transgenic mice containing a Smad-responsive luciferase reporter construct (SBE-luc) are useful instruments to assess Smad2/3 signaling activity. Smad 2/3 are anchor proteins important for downstream TGF-β signaling. The use of SBE-luc mice allows tissue specific detection of TGF-β pathway activation in response to systemic endotoxin challenges or brain injury [62].
Optical imaging plays also an important role in the preclinical assessment of drug target interaction and hence will continue to enhance our ability to fight cancer. The development of a transgenic mouse reporting on hydroxylase activity is an excellent example of such use [63]. The transcription factor hypoxia-inducible factor (HIF) consisting of a labile α subunit and stable β subunit is important for the cell’s adaptation to hypoxia. Under conditions of normal oxygen tension (normoxia), members of the EGLN family hydroxylate HIFα subunits on conserved prolyl residues. This signals the pVHL E3 containing complex to polyubiquitinate HIFα subunits resulting in its degradation. Under hypoxic conditions, or in the absence of pVHL, the alpha subunits accumulate in the cell and engage in transcriptional activation of genes involved in acute or chronic adaptation to hypoxia. With the fusion of a luciferase to the HIF1α region that binds pVHL in and oxygen-dependent manner Safran and colleagues [63] generated a transgenic mouse, which can report on EGLN hydroxylase activity. The ROSA26 ODD-Luc mouse expresses the oxygen-dependent domain of Hif1α subunit fused to luciferase protein (ODD-Luc) and responds to changes in oxygen tension. Moreover this mouse has been used to monitor the action of small molecule inhibitors of HIF prolyl hydroxylase activity [63].
Apoptosis is an essential process for the maintenance of normal physiology. Dysregulation of cell death has been defined as a hallmark of carcinogenesis. Thus monitoring apoptosis noninvasively in living animals will not only provide a unique insight into its function in normal and disease processes, but will also allow the assessment of preclinical drug efficacy. We have recently developed a novel reporter mouse, carrying a β-Galactosidase gene flanked by two regulatory domains of the estrogen receptor and intervening Asp-Glu-Val-Glu (DEVD) sequences which is controlled by the skin specific keratin 5 promoter (kRT5)[48]. The structure of the reporter is shown in Figure 10A. The activation of the excecutioner caspase (C-3) results in the cleavage of the reporter at the C-3 recognition site (DEVD), which was visualized by using a near-infrared fluorescent substrate of β-galactosidase (DDAOG (9H-{1,3-dichloro-9,9-dimethylacridin-2-one-7-yl} β-D-galactopyranoside) [48]. Immunohistochemical analysis of skin tissue from transgenic animals revealed the presence of β-galactosidase immunoreactivity in epidermal cells, which corresponded to cells that had KRT5-positive staining (Figure 10C). Control animals failed to show β-galactosidase-specific immunoreactivity (Figure 10B). To investigate whether the reporter in the transgenic animals was conditionally activated in response to an apoptotic stimulus, mice were UV-irradiated and fluorescence imaging was performed upon administration of the DDAOG substrate [48]. A significant increase in DDAO fluorescence was observed at 24 hours in UV-irradiated animals compared (Figures 11) control animals [48]. The analysis of skin samples from non-transgenic as well as unirradiated (transgenic CON) and UV-irradiated (transgenic UV) transgenic animals revealed the presence of the 190-kDa ER-LACZ-ER polypeptide in the transgenic animal. To validate the imaging studies, immunohistological studies using an antibody specific for active caspase-3 were accomplished to demonstrate the presence of apoptotic activity within UV-irradiated mouse skin samples. No significant staining was observed in unirradiated animals, whereas UV-treated animals had significant levels of active caspase 3 positivity within the epidermal cells. Finally, skin sections from an untreated control mouse and a UV-irradiated mouse were stained using 5-bromo-4-chloro-3-indolyl-b-D-galadctopyranoside to identify cells that possessed both active β-galactosidase and with an antibody specific for active caspase-3[48]. Untreated control cells had no significant staining for active caspase-3 and low β-galactosidase activity [48]. These results reveal co-localization of the β-galactosidase activity with activation of caspase-3, thus directly correlating detection of the fluorescent signal with apoptosis using this molecular imaging reporter system [48].
Figure 10. Schematic of the transgenic apoptosis reporter construct.
A, the transgene depicted contains the ER-LACZ-ER coding sequence (see Figure 7) under the transcriptional control of the keratin 5 (KRT5) promoter. B & C, Immunohistochemical analysis of formalin-fixed, paraffin-embedded dorsal skin sections from nontransgenic (control) (B) and transgenic (C) mice (ER-LACZ-ER transgenic). Presence of β-galactosidase protein and KRT5 was detected using the appropriate antisera. DAPI (4,6-diamidino-2-phenylindole) staining was used to identify nuclei. In control animals, KRT5 staining but not b-galactosidase staining was observed, whereas in transgenic animals, KRT5 staining and β-galactosidase staining was detected in similar populations within the section. Bar = 40 μm. (From [48], with permission).
Figure 11. Imaging of UV-induced apoptosis in transgenic animals.

Transgenic mice were shaved and UVB-irradiated (b) or mock irradiated (a). The irradiated area is outlined as a red square. After irradiation, mice were injected with the fluorescent substrate DDAOG (9H-{1,3-dichloro-9,9-dimethylacridin-2-one-7-yl} β-D-galactopyranoside) and imaged using a Xenogen IVIS system. As compared with the control animals, the UVB-treated animals showed a significant fluorescent signal 24 hours after radiation. (c) Control animals (open bar) showed an approximately 10% change in fluorescence, whereas UV-irradiated animals (solid bar) had an 80% mean increase in fluorescence activity. Error bars represent ± SD, n = 5 animals per group. (From [48], with permission).
In summary enzymatic activation of the reporter during apoptosis enabled us to monitor β-galactosidase activity noninvasively in living cells in a dose- and time dependent manner. Transgenic animals provide the ability to image apoptosis in the skin, and thus will enable unique insights into the role of apoptosis in skin biology, e.g. wound healing, UV-light induced DNA damage and melanoma to be obtained in vivo [48].
In conclusion, the development of transgenic reporter mice and their increasing use for preclinical drug/target identification, validation and dosing and schedule optimization will contribute in many ways to improving drug-development.
MAGNETIC RESONANCE IMAGING
Introduction
Magnetic resonance imaging (MRI) has proven to be one of the most important advances in the radiologic diagnosis of oncology patients. With high spatial resolution and soft tissue contrast, MRI allows precise non-invasive radiographic measurements of tumor location and size. Rapid advancements in functional MR technologies have facilitated rapid growth and widespread availability of clinical MR scanners. Routine MR acquisition sequences provide the capability of generating images of fundamental biophysical, physiologic, metabolic, or functional properties of tissues. This allows for characterization of tissue perfusion,[64] vascular permeability,[65–66] tissue oxygenation,[67] cellular status,[68] cellular density,[69] and microstructural organization,[70–71] all of which are used in research and clinical studies. Development and validation of MRI biomarkers, capable of monitoring the biology and behavior of tumors, are being investigated for their efficacy at predicting outcomes in the clinical management of individual cancer patients. This is of great interest since standard risk factors currently used cannot account for the variable and unpredictable treatment responses of patients with a similar risk profiles. The present chapter will highlight several key emerging functional and molecular imaging approaches as they are applied to translational imaging.
Diffusion magnetic resonance imaging
Molecular imaging is commonly refers to imaging techniques capable of measuring biologic processes on the cellular and molecular level. An example of an imaging readout serving as a biomarker for a cellular event, such as enzymatic expression from a targeted gene, qualifies as a molecular imaging modality. DW-MRI is sensitive to molecular water interactions, resulting from thermal motion, that occur at the cellular level. Water molecules are typically the signal source therefore, water mobility is probed in DW-MRI. In pure water, temperature is the only significant modulator of molecular mobility, and, in fact, diffusion MRI has been used to measure temperature noninvasively.[72] However, water mobility in cancer tissue is strongly impacted by biologic factors on the cellular level making DW-MRI a unique diagnostic tool.[73–74] Indeed, DWI is readily available and increasing in its use in clinical practice owing to its exquisite sensitivity to cellular status, cytotoxic edema, cellular density, and cellular organization of tissues.{Moseley, 1990 #275;Moseley, 1990 #275;Le Bihan, 1993 #273;Le Bihan, 1991 #272;Moseley, 1990 #276} The objective of this section will be to provide a broad overview of basic methods and applications of diffusion MRI as applied to cancer imaging.
Principles involved in diffusion imaging of cancer
First demonstrated in 1965, Stejskal and Tanner [78] measured water diffusivity by acquiring water signal using a spin echo sequence in the presence of bi-polar time-dependent field gradients. They showed that water signal attenuates at a rate proportional to the diffusivity of the molecule. Diffusion-weighted MRI was not applied to in vivo systems until the 1980s,[74, 79–80] Since then, DW-MRI has found wide applications as a research tool for studying biological systems resulting in reviews on the technical aspects and consensus biomarker recommendations using diffusion imaging.[77, 81–82] Molecular diffusion is the thermally driven random translational motion of molecules in media commonly referred to as Brownian motion. Key factors that influence the mobility of a diffusing molecule include media viscosity, temperature, and its molecular mass. Unlike, magnetization-related process such as T1 and T2 relaxation times which drive conventional MRI contrast diffusion measurements obtained by DW-MRI are unaffected by field strength. This allows for the noninvasive and consistent quantification (image) of water diffusion values spatially in vivo. Measurement of diffusion values are obtained in part through the use of magnetic field gradients which “encode” the initial locations of constituent water molecules in the tissue. Following a brief interval, the same gradients “decode” the molecular locations. The decoding of water molecules that undergo displacement during the time interval is incomplete resulting in the loss of signal through spin dephasing. The extent of dephasing increases proportionally to the distance translated between encode/decode diffusion gradient pulses. A larger loss of signal will be observed in highly mobile water molecules when compared to immobile water in more hindered/cellular tissue environments, which result in relatively strong signal on diffusion-weighted images. The extent of signal loss at various diffusion gradient settings provides to the means for calculating molecular mobility in complex systems, such as tumor tissue. However, water within tumor tissue maybe bound to macromolecules through hydrogen bonding [83] or compartmentalized and separated by semi-permeable membranes. Thus the concept of a single diffusion coefficient is not valid but rather a spectrum of diffusion values. As such diffusion measurements are typically reported as an “apparent diffusion coefficient” (ADC) when performing diffusion-sensitive sequences on tissues.[74, 77] ADC measurements can be used to assess a myriad of effects that impede molecular motions including cell membrane integrity, cell density, interactions with macromolecules, as well as processes that enhance mobility via active transport, convective motion, and perfusion.
Pure water at body temperature has a diffusion coefficient approximately 3 × 10–3 mm2/s, which results in a a displacement distance of 0.03 mm, or 30 μm, in 50 ms, which is on the order of the typical MR time interval used clinically. Tumor cells as well as other structures such as membranes, organelles, myelin layers, and macromolecules span smaller dimensions than those displaced by pure water. Thus, a water molecule will likely encounter many interactions with cellular or subcellular entities over this measurement interval. Transient association of water with obstacles residing within tumor tissue effectively reduce water mobility to an ADC lower than free water diffusion. The greater the bulk density of structures within a tumor tissue that hinders water mobility, the lower the ADC value for that tumor. For this reason, ADC is considered a noninvasive imaging biomarker of cellularity or cell density. However, if two tissues have different ADC values, the lower ADC tissue may not necessarily have the greater number of cells per unit volume. Other factors such as cell size, relative sizes compartment volumes, and membrane permeability also affect water mobility and ADC. For a given tissue, ADC is useful as an indicator of the relative cellularity, such as in the evolution of tumor over time following therapy. Alterations in the cellular makeup of the tumor due to disease or intervention, as well as changes in cellular organization or integrity of cellular elements, are available for study by DW-MRI.
Accurate diffusion measurement is attainable in spite of the presence of physiologic motions. A single-shot echo-planar imaging (EPI) approach[84] has become the standard MR sequence in clinical studies as its rapid acquisition speed allows the entire set of echoes for an image to be collected within one single scan period; essentially eliminating bulk tissue motion. However, images generated by EPI are sensitive to other artifacts such as image distortion and signal loss as a result of magnetic susceptibility. These limitations aside, EPI combined with diffusion-sensitization gradient pulses is the most commonly used clinical sequence for DW-MRI.
Diffusion Imaging in Tissue Characterization
Tumor ADC maps generated from DW-MRI data aid in defining solid enhancing tumor, non–contrast enhancing lesions, peritumoral edema, and necrotic or cystic regions from normal surrounding tissue. Progressively increasing ADC values have been widely observed in dense cellular tumors to necrotic cysts which is consistent with known histological properties of tumors. The ADCs for highly cellular dense tumors are 0.6 to 0.8 × 10–3 mm2/s,[85] whereas the ADCs for solid enhancing high-grade glioma span a range from 0.8 to 1.3 × 10–3 mm2/s.[85] ADC values of edematous brain are in the range of 1.3 to 1.4 × 10–3 mm2/s, and a necrotic tumor core typically has an ADC of 1.8 to 2.4 × 10–3 mm2/s. ADC measurements obtained by diffusion MRI has also been reliably shown in abdominal organs and tumors within organ sites such as renal, liver and pancreas.[86] ADC values have also been evaluated in colorectal hepatic metastases.[87–88] This ability to reliably obtain ADC measurements in internal organs has allowed the investigation of pretreatment ADC as a predictive biomarker of chemotherapeutic response of hepatic metastatic lesions from colorectal cancer.[88] It was observed that significantly high mean pretreatment ADC values were found in metastatic lesions that responded to chemotherapy, which may have implications for future development of individualized therapy.
Diffusion measurements have also been investigated for differentiating benign and malignant lesions in liver, breast, and prostate, where increased cellularity of malignant lesions hinders water motion in a reduced extracellular space.[89] Whole-body diffusion MRI has recently been reported for screening malignancies throughout the body.[90] This approach of acquiring whole-body diffusion images has been demonstrated on freely breathing patients. Figure 12 shows whole-body MRI, including DW-MRI of a 60-year-old male with stage III diffuse large B-cell lymphoma. Coronal T1-weighted and slab maximum intensity projection DW-MRI images reveal lymph node involvement on both sides of the diaphragm (see arrows). The highest lymph node-to-background contrast was observed in the whole-body DW-MRI. Whole-body diffusion MRI for applications in tumor detection and monitoring of treatment response will continue to be an active area of investigation.
Figure 12.
Whole-body anatomical and DW-MR images of a 60-year-old male with stage III diffuse large B-cell lymphoma. (A) Coronal T1-weighted and (B) maximum intensity projection DW-MRI obtained with a b-value of 1000 s/mm2 show lymph node involvement on both sides of the diaphragm (arrows). Note that whole-body DW-MRI provides superior lymph node-to-background contrast. (Figure kindly provided by Dr. Thomas Kwee, Department of Radiology, University Medical Centre Utrecht, Utrecht.)
Diffusion Imaging in Tumor Grading
Investigation into differentiating tumor type and grade has also been explored using DW-MRI and diffusion tensor MRI (DT-MRI) in adult as well as pediatric populations. Preliminary results using diffusion MRI for detecting pancreatic adenocarcinoma have been reported with high sensitivity and specificity.[91] Diffusion MRI has also provided useful diagnostic information for discriminating poorly differentiated from undifferentiated carcinomas[92] and benign from malignant salivary gland tumors.[93] Several studies have also observed high ADC values in low-grade astrocytoma, whereas low ADC values were reported in high-grade malignant glioma. These findings reflect a more hindered diffusion with increasing tumor cellularity.[69, 94] Although tumor anisotropy has been investigated, it remains uncertain whether tumor type and grade can be differentiated by anisotropy indices derived from DT-MRI. Tumor cytoarchitecture is predominantly random; therefore, negligible anisotropy is observed in tumor. In addition, normal tissue anisotropy depends heavily on its location in the brain,[95] which implies that the contrast of tumor to normal background, as depicted by anisotropy, will depend on lesion location. There is justifiable optimism that anisotropy will be valuable in assessing the effect of tumor on normally unidirectional white matter structures. Displace and compress white matter tracks by tumor mass effects as well as destruction of track organization by tumor infiltration have been documented by anisotropy-based DT-MRI, which suggest that this technology may have a role in pre-surgical planning.[96–98]
Diffusion Imaging to Assess Tumor Cellularity and Treatment Response
It is traditionally viewed that as cellular density increases, the added tortuosity reduces water mobility. The inverse relationship between ADC and cellular density has been noted by several groups.[69, 97, 99–100] A recently proposed biphasic model relating ADC values to cellularity assumes water resides in two pools within tissue, a fast diffusion and a slow diffusion pool.[83] The slow diffusion pool is proposed to consist of a water layer trapped by electrostatic forces, i.e. hydrogen bonding, of the protein membranes and associated cytoskeleton. The fast diffusion pool is thought to belong to water compartmentalized in intra- and extracellular spaces, which are slower than free water. Based on either the traditional, water resides in intra- and extracellular compartments, and biphasic diffusion models water diffusion is expected to decrease during cell swelling or cell proliferation and increase during treatment-induced loss of cellular viability or density. Whatever the specific underlying mechanism governing water diffusion in tissue, the fact remains that tumor diffusion values increase as tumor tissue progresses from a solid, cellular lesion to an acellular, necrotic tumor during successful cytotoxic therapy. This characteristic of tumor water diffusion values provides a key opportunity to use this quantifiable ADC parameter as a sensitive biomarker for detecting the underlying changes of tumor cytoarchitecture associated with treatment.[101]
Because treatment-induced cellular changes precede gross volumetric changes in tumor size, diffusion MRI can provide early detection of changes in tumor structure. Preclinical and clinical cancer studies could thus utilize this imaging biomarker as an early response indicator. Sixteen years of research in preclinical studies have supported this notion that diffusion MR can be used to noninvasively detect cellular changes associated with treatment-induced cell killing in animal models.{Ross, 2003 #296;Lyng, 2000 #295;Lee, 2007 #52;Lee, 2007 #291;Lee, 2006 #288;Lee, 2007 #292;Lee, 2006 #288;Hamstra, 2004 #286;Guo, 2002 #266;Chenevert, 1997 #299;Lee, 2006 #289} The key findings in these studies were that changes in ADC values precede changes in tumor volume regression and were treatment-independent and dose-dependent, all supporting the claim that this imaging biomarker may indeed be used as an early surrogate for treatment outcome.
Tumor burden is typically assessed between pre-treatment scans and those obtained weeks to months after the conclusion of a therapeutic protocol.[107–108] Clinical studies have found that early changes in tumor ADC correlate with a delayed clinical response to therapy.[100, 109–144] In general, a significant difference in the mean ADC between responders and non-responders to therapy have been reported, as well as a linear correlation between the relative change in ADC and the normalized change in tumor volume.[122] The correlation of increasing ADC with a positive clinical outcome was also observed in head and neck squamous cell carcinomas[138]. Presented in Figure 13 are the corresponding pre-treatment and 3 week post treatment initiation T1-weighted and ADC maps with corresponding histograms of a patient treated by nonsurgical organ preservation therapy. The diffusion histogram reflects a broad ADC distribution with some areas exhibiting a very low ADC consistent with high cellularity (mean ADC of 120 × 10−5 mm2/sec). Following 3 weeks of treatment, the nodal tumor showed a negligible increase in tumor volume, whereas the ADC had increased by 28% (153 × 10−5 mm2/sec). Given the large nodal disease and minimal volumetric response, this patient underwent a clinically necessitated cervical lymph node dissection which revealed no evidence of residual disease (complete responder) and was alive and free of disease 35 months from the completion of treatment. DW-MRI of a breast cancer patient who has undergone two cycles of neoadjuvant therapy revealed an increase in the tumor diffusion values [117]. This indicates a reduction in the tumor cell density as a result of treatment but, no significant reduction in tumor size was observed. A significant decrease in tumor volume was noted prior to the second treatment cycle. Although initial increases in tumor ADC values during treatment are typically associated with cell death, a drop in ADC values has been observed later within the tumor, even to pretreatment levels. This trend in ADC may indicate tumor regrowth or fibrosis. This is supported by findings of lower ADC values in contrast-enhancing portions of recurrent high-grade gliomas when compared with those obtained in patients with radiation injury and necrosis where higher diffusion values are observed in necrotic regions of osteosarcomas.[111, 145]
Figure 13.
Representative functional diffusion map (fDM) analysis at 1, 3, and 10 weeks of clinically (left panels) responding and (right panels) non-responding patients to fractionated radiation therapy. Presented images are single slices of the T1-weighted contrast-enhanced scans at each time point with a pseudocolor overlay of the fDM. Red voxels indicate regions with a significant rise in apparent diffusion coefficient (ADC) at each time point compared with pretreatment, green regions had unchanged ADC, and blue voxels indicate areas of significant decline in ADC. The scatter plots display data for the entire tumor volume and not just for the depicted slice at each time point, with the pretreatment ADC on the x-axis and post-treatment ADC on the y-axis. The central red line represents unity, and the flanking blue lines represent the 95% CIs. (From [276], with permission.)
A major confounding factor in assigning a single indicator for patient tumor response is tumor heterogeneity. A given lesion often contains wide gradations of viable cellularity and necrosis resulting in a nonuniform response of tumor sub-regions to treatment. Whole-tumor histogram-based analysiss of ADC values is one approach to address spatial heterogeneity to response. Nevertheless, the extent of regional changes may be underestimated by whole-tumor averages. To deal with intrinsic heterogeneity of diffusion values within a tumor, an alternative approach for image analysis has been proposed, referred to as functional diffusion mapping (fDM).[119] A key component of fDM is spatial alignment, i.e. registration, of all three-dimensional image sets into a common geometrical framework. Once aligned, changes in diffusion values are determined on a voxel-by-voxel basis from pretreatment, mid-treatment, and post-treatment ADC maps. To visualize changes in ADC within the tumor, tumor volumes are segmented into three categories representing voxels for which ADC (1) increased by a specified threshold (red voxels), (2) decreased (blue voxels), and (3) did not change outside this threshold range (green voxels). Illustrated in Fig. 14 are a series of fDM color overlays and corresponding voxel-wise scatter plots of ADC pretreatment versus at 1, 3 and 10 weeks post-treatment initiation for a therapeutically responding and non-responding patient. The relative volume of tumor that exhibited a significant increase in ADC, shown as red voxels in the color overlay and scatter plots, is used as a biomarker to predict treatment outcome.
Figure 14.
Pre- (top row) and 3 week post-treatment initiation (bottom row) A) T2-weighted, B) apparent diffusion coefficient (ADC) maps with corresponding C) ADC histograms from the whole-tumor of a patient treated for HNSCC of the left base of the tongue. The tumor is outlined by the yellow contour line. Mean ADC values increased from 120×10−5 mm2/s to 156×10−5 mm2/s following treatment initiation of a nonsurgical organ preservation therapy. This patient was subsequently determined to be a complete responder to therapy.(From [169], with permission.)
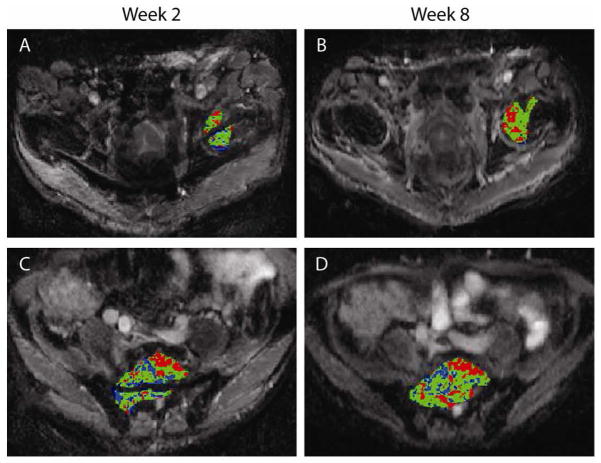
It was found in previous work that the relative volume of tumor that exhibiting a significant increase in ADC values as determined by fDM measured at 3 weeks into treatment was predictive of radiographic response measured at 10 weeks.[119, 137] Moreover, tumor assessment by fDM at 3 weeks into treatment provided an early indicator of the eventual clinical responses of disease time to progression and overall survival in patients with malignant glioma.[119, 137] Preliminary work was performed to determine the feasibility of clinically translating the functional diffusion map (fDM) imaging biomarker for for use in quantifying bone tumor response in a patient treated for metastatic prostate cancer to the bone [146]. DW-MRI was performed prior to treatment and again at 2 and 8 weeks post-treatment initiation to quantify changes in tumor diffusion values. Three metastatic lesions were identified for fDM analysis, of which two are presented in Fig 15. All tumors demonstrated early changes in diffusion values at 2 weeks and continued to increase further by week 8 post-treatment initiation. When compared to the percent change in tumor mean ADC, the fDM analysis offered improved sensitivity over the histogram-based approach when tumors have heterogeneous changes over time. A strong correlation in fDM was observed with patient’s prostate-specific antigen (PSA) levels, which is suggestive of patient response. In contrast, CT, bone scans, and anatomic MRI images obtained post-treatment were not useful for the assessment of treatment efficacy. In general, quantification of tumor response by fDM may provide a standardized approach for treatment response assessment using diffusion MRI. Extension of this image post-processing approach to other tumor types is possible. While further validation of DW-MRI measurements as a biomarker for early treatment response is needed, recent studies have shown promising results.
Figure 15.
Presented is the fDM analysis in a patient with metastatic prostate cancer to the bone treated with hormone therapy. Areas with increased ADC (red voxels), decreased ADC (blue voxels), and areas where ADC did not change significantly (green voxels) are visually apparent. fDM analysis of the femoral head and sacral lesions at (A, C) 2 and (B, D) 8 weeks after treatment initiation revealed distinct regions of red voxels signifying areas with significant increases in ADC (>26 ×105 mm2/s). (Reprint from Ref. [146] with permission from Neoplasia).
Summary
MRI methods such as DW-MRI and DT-MRI, which are based on tissue biophysical properties, are rapidly being incorporated into routine imaging protocols for improving diagnosis, characterization, and management of cancer patients. In the future, these imaging techniques when combined with other physiology-based methods, such as MR permeability and magnetic resonance spectroscopy (MRS) metabolite mapping, as well as excellent anatomic images are anticipated to improve tumor diagnosis, biopsy guidance, pre-treatment and pre-surgical planning, and the assessment of early therapeutic efficacy in individuals. Research continues in determining how best to use diffusion information to positively impact patient management.
Permeability magnetic resonance imaging
An active area for treating tumors is the development of drugs that target the vascular support network of the tumor. In response to this growing field, imaging biomarkers for tumor angiogenesis are under investigation for detection and quantification of pharmacodynamic drug activity. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is presently being applied for assessment of tumor pathophysiology and treatment response against radiotherapy as well as antiangiogenic and vascular disruption agents. This MRI technique is rapidly progressing as a noninvasive imaging-based biomarker for drug efficacy studies in clinical trials.[147–163]
Principles of DCE-MRI
DCE-MRI is an imaging technique used to investigate microvascular structure and function by recording the pharmacokinetics of injected low-molecular-weight contrast agents as they pass through the tumor vasculature. Following an intravenous bolus injection of a paramagnetic contrast agent, the contrast agent enters the tumor arterioles, passes through capillary beds, and finally drains via the veins within the tumor. Commonly used is a gadolinium-based contrast agent which shortens the T1 relaxation time of blood resulting in a concentration-dependent spatially varying enhancement of signal (contrast) on T1-weighted images. Collection of rapidly acquired serial T1-weighted images prior and early postinjection of the contrast agent provides the initial area under the contrast-agent concentration time curve, which can be analyzed for kinetic information. Signal enhancement within the tumor depends on a variety of physiological factors such as tissue perfusion, arterial input function, capillary surface area, capillary permeability, and the volume of the extracellular extravascular space (EES). Imaging data are typically analyzed from a defined region of interest that may encompass all or part of the tumor. The data is fit to a compartmental model of tumor microvasculature that generates parameters that describe the behavior of the contrast agent time-dependent concentration curve, which represents a combination of flow, blood volume, vessel permeability, and EES. Standardized terms for the kinetic variables within the model are commonly used in these studies.[164] The two-compartment model regards the EES and plasma as the two compartments that are well mixed with contrast agent and which have a constant permeability. Transport between these compartments is determined by the parameter Ktrans (volume transfer constant between the blood plasma and the EES) and kep (rate constant between the EES and the blood plasma). The EES fractional volume (ve) is related to Ktrans and kep via the equation, ve = (Ktrans/κep). Although a primary end point used in clinical trials, changes in Ktrans may represent different physiological processes in different individuals within a patient population (e.g., a reduction of Ktrans could represent reduced permeability, reduced blood flow, or a combination of the two). There are a variety of analytical approaches used for post-processing and analysis of DCE-MRI data. To date, there remains no consensus on a recommended model for deriving the volume transfer coefficient of contrast between the blood plasma and the EES (Ktrans) and the size of the EES (ve), as well as should descriptive parameters such as the initial area under the contrast-agent concentration time curve be used for in assessing antiangiogenic and vascular disrupting agents in clinical trials.[165]
Dynamic contrast-enhanced magnetic resonance imaging for detection of residual disease
DCE-MRI following therapy has been proposed to aid in detecting residual disease or early recurrence, which may be difficult to detect in tissue regions exposed to radiotherapy. DCE-MRI measurements in tumor treated with radiotherapy have been investigated in the cervix, lung, head and neck, and bladder tumors wherein high enhancement was associated with an increase in local recurrence and poor survival.[166–169] Following treatment completion, a contrast agent enhancement pattern that persists or has returned early has been attributed to viable tumor cells. It has been shown in cervical cancer that DCE-MRI enhancement obtained early in therapy was associated with early recurrence and poor survival.[169]
DCE-MRI also shows promise as a non-invasive imaging technique for determining the malignancy of a tumor. As presented in Figure 16, a large tumor is observed in a series of T1-weighted contrast-enhanced MR images, which show strong signal enhancement over time post-injection of contrast. Using the serial images, tumor hemodynamics can be quantified. These values, such as permeability constant, Ktrans, and extracellular/extravascular fraction, ve, have been shown to suggest the extent of tumor malignancy. In a study involving head and neck tumor patients, the presence of lesion enhancement was found to have a positive correlation with viable tumor cells in post-radiation surgical specimens.[166] Finally, in meningioma patients examined using DCE-MRI following radiotherapy, pharmacokinetic analysis revealed a decrease in the exchange rate constant in patients who responded relative to non-responders. Overall, although there is emerging evidence to the potential use of DCE-MRI for detecting post-treatment residual disease, further studies are required in order to adequately validate this approach for routine clinical use.
Figure 16.
Demonstration of Dynamic Contrast Enhance (DCE)-MRI shown as maximum intensity projections (MIPs) and mid-tumor axial images of the modeled permeability constant Ktrans and extracellular/extravascular fraction ve. Note marked intratumoral heterogeneity of vasculature denoting high malignancy.
Dynamic contrast-enhanced magnetic resonance imaging as predictor of treatment response
DCE-MRI is capable of provide anatomic and physiological information using conventional clinical MRI sequences. These sequences are incorporated into standard imaging protocols that can be used for treatment assessment. Many studies have evaluated the prognostic value of DCE-MRI in assessing treatment response to radiotherapy, antivascular, and antiangiogenic therapies.[147–148] Most studies consisted of small cohort single-center phase I trials, although a few phase II trials have also incorporated DCE-MRI.[170] In addition, the efficacy of antiangiogenic drugs has been demonstrated using DCE-MRI in clinical trials. For example, a colorectal cancer treated with a vascular disrupting agent, combretastatin, resulted in a rapid tumor vascular shutdown within 4 hours following the first dose.[171] Quantitative maps of area under gadolinium contrast medium curve at 60 seconds revealed significant decreases in permeability. The permeability constant (Ktrans) and extracellular/extravascular space (ve) had also revealed that the vascular shutdown was induced by this drug with negligible morphologic change in tumor.
DCE-MRI biomarkers have been used as early indicators of efficacy, dose, and outcome as well as assist in defining the biologically active and maximum tolerated doses. However, few trials have demonstrated that DCE-MRI measurements correlate with clinical outcome measures. Thus, observed changes in DCE-MRI biomarkers should not be considered a surrogate of a successful outcome measure in randomized phase III trials. Advancement of DCE-MRI as a diagnostic/predictive indicator of treatment response will require added attention to the design of a clinical protocol where the acquisition of the imaging data must be at well-conceived scanning interval after administration of the agent. For example, an agent that causes vascular disruption in the tumor may require image time points pretreatment, 4- to 6-hours, and 24-hour post-treatment initiation. In comparison to a trial involving an antiangiogenic agent (or radiotherapy), DCE-MRI may be more sensitive to treatment-induced effects at longer interscan intervals of days to weeks or months in order to reach maximal change in the DCE-MRI biomarkers.
Another area for potential improvement of the employment of DCE-MRI as a predictive biomarker of tumor response to treatment may be in the choice of the specific imaging biomarker(s) or parameters selected for a given therapeutic intervention. Moreover, the criteria for selecting regions of interest within the tumor along with the specific pharmacokinetic model used to analyze the imaging data, which both provide for the quantification of the DCE-MRI biomarkers, may impact the final results. Alternative methods for image data analysis may have a significant role in DCE-MRI, especially in tumors with significant regions of heterogeneity.
Summary
As with any biomarker, the routine application of DCE-MRI as a noninvasive biomarker of tumor angiogenesis and response to therapy requires validation through statistical correlation with traditional clinical outcome measures (i.e., radiologic response, overall survival). Presently, there is a lack of clinical data correlating changes in quantified DCE-MRI biomarkers with outcome measures. Thus, this measurements adoption as a surrogate end point in drug efficacy studies is limited. New contrast agents, higher magnetic fields (7 and 9.4 T), and image acquisition and analysis tools are currently in development, which may help to improve the prognostic value of DCE-MRI in cancer trials.
NUCLEAR IMAGING
Introduction
Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) are nuclear imaging techniques used to map physiological and biological processes in humans and animals following the administration of radiolabeled tracers. A unique advantage of PET and SPECT imaging techniques is their potential for detecting disease-related biochemical and physiologic abnormalities prior to the appearance of anatomical changes which are visualized by conventional imaging modalities such as CT and MRI. PET uses radioisotopes that decay via emission of positrons, whereas, SPECT radioisotopes decay by electron capture and/or gamma emission.
Table 1 lists some of the most commonly used PET and SPECT radioisotopes and their physical data. The short half lives of the positron-emitters carbon-11, nitrogen-13 and oxygen-15 dictates that radioligand synthesis with these isotopes can only be accomplished in close proximity to a cyclotron. On the other hand, radioisotopes such as fluorine-18, copper-64, indium-111, iodine-123 and iodine-124 are sufficiently long-lived to allow transportation from regional commercial sites. Additionally, the radioisotopes gallium-68, copper-62 and technetium-99m can be conveniently obtained from an in-house generator. At the present time, clinical SPECT imaging is more frequently conducted than PET imaging due to its cost effectiveness and the greater availability of SPECT scanners at most clinical centers.
Table 1. Commonly used PET and SPECT Radioisotopes.
| Isotope | Imaging Mode | Production Method | Half-Life | Decay Mode(s) |
|---|---|---|---|---|
| 11C | PET | Cyclotron | 20.4 min | β+ (99+%) |
| 13N | PET | Cyclotron | 10 min | β+ (100%) |
| 15O | PET | Cyclotron | 2.03 min | β+ (99.9%) |
| 18F | PET | Cyclotron | 110 min | β+ (97%) EC (3%) |
| 124I | PET | Accelerator | 4.2 days | EC (74.4%) β+ (25.6%) |
| 68Ga | PET | Generator | 68.3 min | β+ (90%) EC (10%) |
| 62Cu | PET | Generator | 9.73 min | β+ (98%) EC (2%) |
| 64Cu | PET | Reactor | 12.7 hours | β+ EC |
| 99mTc | SPECT | Generator | 6.02 hours | IT |
| 111In | Gamma Scintigraphy | Accelerator | 2.8 days | EC x-ray |
| 123I | SPECT | Accelerator | 13.3 hours | EC |
β+ = positron emission; EC = electron capture; IT = Isomeric transition.
In PET, positrons (positively charged electrons, β+) ejected from the nucleus during radioactive decay travel a few millimeters in tissue, after which, they undergo annihilation by collision with electrons. Each annihilation event releases two γ-ray photons of equal energy (511 keV) in opposite trajectories (1800 apart). PET scanners utilize the simultaneous detection of these two photons (coincidence detection) to precisely locate the source of the annihilation event. Subsequently, the event data is processed by computers to reconstruct the spatial distribution of the annihilation events. SPECT scanners on the other hand, use collimators (lead shields containing narrow parallel holes) to acquire only those photons that have a parallel trajectory. Thus, the original path of the detected photon can be linearly extrapolated from knowledge of the collimators orientation. Coincidence detection is significantly more efficient than collimation at recording annihilation events as the latter approach results in discarding a high percentage of useful emitted photons. Thus, PET provides a much better sensitivity (2 – 3 orders of magnitude), quantitation capability and spatial resolution than SPECT.
The process of developing a useful nuclear imaging radiotracer for biological imaging has several requirements that can pose special challenges. Incorporation of the radionuclide (including its chelating functionality in some cases) in a target ligand should have a negligible effect on its binding affinity. Radioligand binding sites (receptor, enzyme, etc.,) usually exist in low concentration (micromolar to nanomolar). Thus, the specific activity of the radioligand should be sufficiently high to represent a high radiative emission from a very small quantity (mass) of radiodiagnostic to avoid producing a pharmacologic effect. Due to the constraints of working with a short radioisotope half-life, the overall synthetic strategy for radioligand preparation should be short, the individual reaction steps rapid and high yielding, and the entire process should be adaptable to microscale manipulation. From an in vivo standpoint, the radioligand should display low or negligible non-specific binding so as to provide a high target-to-background signal and the in vivo kinetics of radioligand target uptake and washout should be compatible with the half-life of the radioisotope. Additionally, the radioligand should not be extensively metabolized and metabolites, if present, should not compete with the radioligand at its intended binding site. Despite these rigorous requirements, many radioligands have been developed which display demonstrated clinical utility for biological imaging (e.g. [18F]fluoro-deoxyglucose ([18F]FDG), [18F]FLT, radiolabeled somatostatin analogs, etc.,
Currently, [18F]FDG (Fig. (17); a radioligand marker for tumor glucose metabolism), is the workhorse of PET, reportedly used in at least 90% of human PET studies. The majority of these studies have been in oncology where [18F]FDG PET is the primary method used for detection and staging of many cancers [172]. However, [18F]FDG is not tumor specific and is known to accumulate in many benign inflammatory processes leading to false-positive interpretation [173]. The past decade has also seen the investigation and validation of several alternative radioligands to [18F]FDG that target specific aspects of tumor biology. These targets include molecular biomarkers such as growth factor receptors, protein kinases, specific receptor over-expression or biological events such as angiogenesis, apoptosis, hypoxia and tumor proliferation. This review will highlight recent developments in PET and SPECT radioligand development in the area of oncology and CNS imaging with a major focus on its application in oncology.
Figure 17.

Structure of D-glucose and [18F]FDG.
Radioligands for imaging angiogenesis
Angiogenesis, the formation of new blood vessels through capillary sprouting from pre-existing vasculature, plays a key role in the growth and metastatic potential of solid tumors [174–175]. Tumor growth beyond a 1 – 2 mm3 volume requires an independent vasculature for the cellular supply of oxygen and nutrients and removal of waste products [176]. Consequently, tumors that outgrow their existing blood supply frequently display oxygen deficiency (hypoxia) which can trigger the secretion of various pro-angiogenic growth factors, such as, vascular endothelial growth factors (VEGF’s) for initiating new blood vessel growth [174] [177]. Binding of VEGF’s to the VEGF family of receptors (VEGFR) initiates a signaling cascade that promotes the proliferation, migration and survival of endothelial cells, ultimately leading to angiogenesis [178], [179]. The angiogenic effects of the VEGF family are believed to be primarily mediated through VEGF-A. To date, VEGF-A (also referred to as VEGF) and its receptors are the most characterized signaling pathways in developmental and tumor angiogenesis.
Alternative splicing of RNA has revealed the existence of at least seven different molecular isoforms for VEGF-A, comprising, 121, 145, 148, 165, 189 or 206 amino acids [180]. The angiogenic actions of VEGF-A are mediated primarily via two closely related endothelium-specific receptor tyrosine kinases (VEGFR-1 and VEGFR-2) [181], [182]. All of the VEGF-A isoforms bind to both VEGFR-1 and VEGFR-2, of which, VEGFR-2 is the major mediator of the mitogenic, angiogenic and permeability enhancing effects of VEGF-A [182]. VEGFR’s are over-expressed in a variety of solid tumors with over-expression of VEGFR-2 or VEGF-A in particular, serving as poor prognostic markers [178], [183].
PET imaging of VEGFR expression in vivo was first demonstrated using VEGF121 (a molecular isoform of VEGF) radiolabeled with 64Cu [184]. Radiolabeling was achieved via 64Cu chelation to a DOTA-VEGF121 conjugate (DOTA is an abbreviation for 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid). In vivo evaluation of 64Cu-DOTA-VEGF121 using microPET imaging of athymic nude mice bearing U87MG human glioblastoma xenografts showed rapid and high specific accumulation of the radioligand in small U87MG tumors (16% injected dose per gram [ID/g]) at 4 h postinjection. Larger tumors showed significantly lower uptake (1 – 3% ID/g). Differences in tumor localization between large and small tumors showed a good correlation with tumor VEGF receptor expression (VEGR2). In vivo VEGFR2 specificity of the radioligand was also confirmed by pharmacological blocking experiments and ex vivo studies (immunofluorescence staining, western blot analysis). This study also demonstrated the dynamic nature of VEGFR expression during tumor expression within the same animal model. Subsequently, these authors also reported on the development of a 64Cu-labeled vasculature-targeting fusion toxin (VEGF121/rGel) composed of a VEGF121 linked recombinant plant toxin gelonin construct (rGel) for multimodality imaging and therapy of glioblastoma [185]. High sustained tumor accumulation and tumor-to-target ratios were demonstrated by this radioligand in mice bearing glioblastoma xenografts up to 48 h postinjection. Based on the pharmacokinetic information obtained from the imaging studies, therapeutic administration of VEGF121/rGel to mice bearing orthotopic U87MG glioblastomas revealed specific tumor neovasculature damage by histological analysis after a 4 dose treatment regimen [185].
Myocardial infarction (MI) is known to activate many biological pathways including VEGF/VEGFR signaling. PET imaging studies conducted in a rat model of MI with 64Cu-DOTA-VEGF121 revealed a 3- 4 higher myocardial uptake of radioactivity for up to 2 weeks following infarction as compared to controls [186]. In addition, PET imaging of VEGFR expression with 64Cu-DOTA-VEGF121 in a rat stroke model showed peak tracer uptake in the stroke border zone at approximately 10 days post-surgery indicating neovascularization as confirmed by histopathology studies [187].
Chan and coworkers have reported on the synthesis and evaluation in tumor-bearing mice of an 111In-labeled recombinant VEGF isoform VEGF165 (111In-hn-Tf-VEGF) as a tumor angiogenesis marker [188]. VEGF165 was fused through a flexible polypeptide linker to the n-lobe of human transferrin. The latter construct permitted labeling of the radioligand with 111In at a site remote from the VEGF receptor-binding domain. In radioligand stability studies, 111In-hn-Tf-VEGF demonstrated a moderate loss of 111In to transferrin in human plasma in vitro over a 72 h period (21.3% ± 3.4% per day). Radioligand biodistribution studies and whole-body gamma camera imaging were conducted in athymic mice bearing subcutaneous U87MG human glioblastoma xenografts. 111In-hn-Tf-VEGF displayed tumor and blood radioactivity accumulations of 6.7 ± 1.1 %ID/g and 1.6 ± 0.4 %ID/g, respectively, at 72 h post-injection. Co-administration of a 100-fold excess of VEGF led to a 15-fold decrease in tumor uptake of radioactivity. High uptake of radioactivity was also observed in liver (45.5 ± 7.5 %ID/g), kidneys (39.4 ± 7.0 %ID/g) and spleen (35.6 ± 4.4 %ID/g) at this time interval. The authors present evidence to indicate that uptake of radioactivity in these organs is due to 111In-hn-Tf-VEGF and not due to 111In-transferrin via transchelation of 111In from the radioligand to transferrin.
An indirect approach to angiogenesis imaging has focused on radioligands targeting the αvβ3 class of cell adhesion molecule integrins [189]. Integrin αvβ3 receptors are significantly up-regulated in endothelial cells during angiogenesis but not in mature vessels or non-neoplastic epithelium [190], [191], [192]. Integrin αvβ3 is also expressed in a variety of tumor cells, including melanoma, late-stage glioblastoma, ovarian, breast and prostate cancer [193]. The ability to visualize and quantify integrin αvβ3 expression in vivo would allow for appropriate selection of patients for anti-integrin treatment and also monitor treatment efficacy in such patients.
Radioligand development for αvβ3 imaging has focused primarily on small RGD peptide antagonists [194]. The tripeptide sequence motif, arginine-glycine-aspartate (RGD), is found in proteins of the extracellular matrix. Many integrins, including αvβ3, link the intracellular cytoskeleton of cells with the extracellular matrix via recognition and binding to this RGD motif. Wu and coworkers have reported on the enhanced αvβ3 receptor binding characteristics of dimeric and multimeric RGD peptides over monomeric peptides which has been attributed to an increased local concentration of RGD domains at the receptor vicinity (polyvalency effect) [195]. Accordingly, several [18F]- and [64Cu]-labeled, dimeric and tetrameric RGD peptide analogs have been recently synthesized and evaluated by this group for integrin-targeted imaging in lung, brain and breast cancer [195–197]. As an example, microPET imaging studies with a dimeric RGD peptide coupled to 4-[18F]Fluorobenzoate {[18F]-FB-E[c(RGDyK)]2} showed predominantly renal excretion and twice as much tumor uptake in the same animal model as the monomeric analog [18F]-FB-c(RGDyK) [198]. Binding potentials derived from tracer kinetic modeling studies showed good correlation with tumor integrin expression levels as measured by SDS-PAGE/autoradiography in the six tumor models tested [198].
Matrix metalloproteinases (MMP’s), a family of zinc-and calcium-dependent endopeptidases, facilitate endothelial cell migration during angiogenesis via the enzymatic degradation of connective tissue [199]. Consequently, several MMP-specific peptides as well as small-molecule inhibitors (MMPI) have been radiolabeled with 125I, 99mTc, 111In, 64Cu, 11C or 18F for PET or SPECT imaging of angiogenesis [200–201]. However, their utility for angiogenesis imaging has yet to be demonstrated in most cases due to their low tumor targeting and in vivo instability.
Radioligands for imaging apoptosis
Apoptosis (programmed cell death) plays a critical role in the homeostasis of multicellular organisms. Initiation of apoptotic cell death leads to activation of a family of cysteine proteases (caspases) which act as central executioners of the apoptotic process [39]. Radiation as well as anticancer drug treatment can induce apoptosis in tumor cells. Consequently, imaging methods that provide information on the rate and extent of apoptosis are of interest in monitoring the efficacy of anticancer treatment. A vast majority of the work on apoptosis-targeted radioligands has focused on Annexin V and its derivatives [202]. Annexin V is a member of the calcium and phospholipid binding superfamily of Annexin proteins that displays selective, nanomolar affinity (Kd ~ 0.5 – 7 nM) toward phosphatidylserine (PS) residues. Induction of apoptosis results in a rapid externalization of PS from the inner leaflet of the plasma membrane to its outer surface [203]. Accordingly, Annexin-mediated imaging of PS has been extensively investigated for identifying cells at the early stages of apoptosis. Annexin V and its derivatives have been radiolabeled with a wide variety of radioisotopes including radioiodine (123I, 124I, 125I), 18F, 99mTc, 111In, 11C, and 64Cu [204]. Tait and coworkers have compared the apoptosis-specific liver uptake of several [99mTc]-labeled Annexin V derivatives prepared by amine-directed modification with that labeled site-specifically at the N-terminus [205]. A clear improvement was seen for site-specific labeling as compared to amine-directed modification. Use of [99mTc]-labeled hydrazinenicotinamide-annexin imaging for assessment of response to chemotherapy has also been reported [206]. The reported imaging protocol was able to distinguish responders from non-responders with 94% accuracy (16/17 patients) and a sensitivity and specificity of 86% and 100%, respectively.
Radiolabeled caspase substrates, inhibitors and monoclonal antibodies targeted to human Annexin V have also been reported as alternative approaches for apoptosis imaging [207] [208]. An excellent review on these developments has been recently published by Lahorte and coworkers [204].
More recent approaches towards apoptosis imaging radioligand development have focused on small-molecule probes that target the early stages of the apoptotic cascade as exemplified by the 4-[18F]Fluorobenzyltriphenylphosphonium cation ([18F]-FBnTP) and the [18F]-labeled malonic acid analog ([18F]-ML-10) (Figure 18) [209–210]. The latter radioligand which is capable of distinguishing between apoptotic and necrotic cells has shown promising initial results in several small-scale clinical trials [211].
Figure 18.

Structure of Radioligands for Imaging Apoptosis.
Radioligands for imaging hypoxia
The growth of solid malignant tumors is frequently accompanied by tissue hypoxia due to outgrowth of its blood supply. Hypoxia in tumor cells leads to resistance to both radiation and anticancer treatment [212]. Noninvasive imaging methods for identification and quantitation of tumor hypoxia status could play a central role in predicting and monitoring treatment response. Initial approaches to hypoxia imaging focused on radiolabeled 2-nitroimidazole derivatives, a class of hypoxia-activated prodrugs [213]. These bioreductive prodrugs undergo reductive metabolism in the hypoxic environment of tumors thereby releasing toxic metabolites that can lead to cell damage [214]. Among the 2-nitroimidazole class of radioligands, [18F]fluoromisonidazole ([18F]FMISO) is currently the most widely used clinical PET hypoxia tracer (Fig. (19)) [215].
Figure 19.
Structure of Misonidazole (MISO), [18F]FMISO and [18F]FAZA.
More recently, Rischin et al. have shown that [18F]FMISO PET imaging is a useful method for identifying head and neck cancer patients most likely to benefit from treatment with the hypoxic cell cytotoxin, tirapazamine in a chemoradiotherapy regimen [216]. In an extensive study involving 73 patients with head and neck cancer, Rajendran et al. have shown that [18F]FMISO PET imaging is a predictor of survival prior to radiation therapy [217]. A 18F-labeled 2-nitroimidazole derivative, fluoroazomycin arabinoside ([18F]FAZA; Fig. (19)) that displays enhanced in vivo stability to enzymatic cleavage has been described by Piert and colleagues [218]. Studies indicate that [18F]FAZA may be superior to [18F]FMISO for hypoxia imaging due to its superior biokinetic profile [218]. These authors have also shown that [18F]FAZA imaging has predictive value for the determination of radiotherapy success when used in combination with tirapazamine [219]. Copper-64 labeled Cu-Diacetyl-bis(N4-methylthiosemicarbazone) (64Cu-ATSM) has also been proposed as a PET hypoxia imaging agent. Differences in tumor type selectivity for this radiotracer, however, raises questions regarding its use as a universal PET hypoxia marker [220].
Radioligands for imaging EGF receptors
The search for radioligands that target the ErbB family of receptor tyrosine kinases is an active area of research. This receptor family includes four members: epidermal growth factor receptor (EGFR/ErbB1/HER1), HER2 (ErbB2/neu), HER3 (ErbB3) and HER4 (ErbB4) [221] [222]. Over-expression of these receptors, particularly HER1 and HER2, has been documented in many epithelial cancers including breast, non-small cell lung (NSCLC), ovarian and bladder cancer [223] [224]. It has also been shown that such over-expression is frequently associated with a poor prognosis [225], [226], [227].
Preclinical evaluation of a 68Ga-labeled, recombinant human epidermal growth factor DOTA conjugate (68Ga-DOTA-hEGF) has been reported for HER1 imaging [228]. In vitro studies with 68Ga-DOTA-hEGF conducted on EGFR-expressing cell lines, U343 glioma and A431 cervical carcinoma, demonstrated high affinity binding (2 nM), rapid internalization of radioligand and good retention of radioactivity. Radioligand biodistribution in mice bearing A431 tumor xenografts showed a tumor-to-blood ratio of 4.5 at 30 min postinjection (2.7% ID/g in tumor). Interestingly, tumor uptake was dependent on the specific activity of the radioligand: a two-fold increase in tumor uptake was observed with a 10-fold lower specific activity material. Tumors were clearly visualized by microPET imaging in a tumor-bearing mouse although the kinetics of tumor uptake of radioactivity was slow compared to that of liver and kidney.
An 111In-labeled human EGF-diethylenetriaminepentaacetic acid (DTPA) conjugate (111In-DTPA-hEGF) is under investigation for future Phase 1 clinical trials as a radiotherapeutic for breast cancer [229]. Preclinical pharmacokinetic, toxicology and dosimetry studies conducted in mice and rabbits with 111In-DTPA-hEGF showed no acute toxicity in female BALB/c mice at 42 times the maximum planned human dose. Highest uptake of radioactivity was seen in liver (41.3% ID/g) and kidney (18.6% ID/g) at 1 h postinjection, although these values had decreased to 4.5 – 4.9% at 72 h. The radiotracer showed fast blood clearance following intravenous injection and no morphologic changes were seen by light microscopy in 19 sampled tissues.
Cetuximab (Erbitux; ImClone Systems, Inc.), a chimeric anti-EGFR monoclonal antibody, is an FDA-approved drug for the treatment of advanced metastatic colorectal cancer. Cai and coworkers describe microPET imaging with a 64Cu-labeled DOTA-cetuximab conjugate (64Cu-DOTA-cetuximab) in seven xenograft tumor mouse models [230]. Uptake of radioactivity for 64Cu-labeled DOTA-cetuximab was similar in major organs and tissues in all seven of the tested tumor models (U87MG human glioblastoma, PC-3 human prostate carcinoma, CT-26 murine colorectal carcinoma, HCT-8, HCT-116, SW620 human colorectal carcinoma and MDA-MB-435 human breast cancer). High uptake of radioactivity was seen for the high EGFR-expression U87MG and PC-3 tumors (13.2%ID/g and 12.8% ID/g, respectively) at 48 h postinjection. Tumor radioactivity uptake determined by microPET imaging showed a good correlation with EGFR expression levels as measured by western blot analysis.
An Affibody dimer, His6-(ZHER2:4)2, has been recently described as a high affinity HER2/neu ligand [231]. Orlova and coworkers have prepared 99mTc- and 125I-labeled His6-(ZHER2:4)2 and compared their biodistribution in tumor-bearing BALB/c nu/nu mice [232]. Significantly, higher levels of radioactivity were observed in tumor as compared to liver for the 125I-labeled ligand. These studies indicate that the radioiodinated ligand may be more suitable than the corresponding [99mTc]-labeled ligand for imaging tumor HER2 expression levels, particularly in liver. A 99mTc-labeled Affibody compound MAG3-(ZHER2:342) labeled via a MAG3 chelator has also been recently reported by this group [233]. Smith-Jones and colleagues have used mouse microPET imaging with a 68Ga-labeled F(ab′)2 fragment of herceptin (68Ga-DOTA-F(ab′)2-herceptin) to image HER2 downregulation after heat shock protein (Hsp90) inhibition [234]. The Ansamycin antibiotic, geldanamycin is known to cause HER2 degradation via inhibition of the Hsp90 chaperone protein. PET imaging was conducted on mice bearing BT474 breast tumor xenografts with 68Ga-DOTA-F(ab′)2-herceptin and [18F]-fluorodeoxyglucose demethoxygeldanamycin (17-AAG). A significant decrease of HER2 expression was seen within 24 h of 17-AAG treatment with 68Ga-DOTA-F(ab′)2-herceptin imaging. In contrast, tumor uptake of [18F]FDG (a marker of glycolysis) was unchanged. The authors conclude that PET imaging with the HER2 radioligand, 68Ga-DOTA-F(ab′)2-herceptin is superior to [18F]FDG imaging for evaluating tumor response to 17-AAG therapy.
Blakenberg and colleagues have recently described a new generation of radiotracers based on a recombinant human EGF expressing a cysteine-containing fusion tag. Incorporation of the cysteine-fusion tag allowed for site-specific conjugation with either 99mTc or 64Cu for imaging EGFR expression with SPECT or PET, respectively [235]. Tumor accumulation of the radiotracers were demonstrated in orthotopic MDA231luc breast carcinoma by PET and SPECT imaging and also in the spontaneous mouse lung carcinoma using SPECT in a bitransgenic mouse model [235].
Radioligands for imaging somatostatin receptors
The utilization of radiolabeled peptide analogs of the hormone somatostatin for the diagnostic imaging and therapy of neuroendocrine tumors (NET) has had notable success [236] [237]. Somatostatin exists in two isoforms: a short peptide having 14 amino acids, and a second peptide with 28 amino acids, both of which bind with high affinity to five receptor subtypes (sst1 – sst5) [238]. A majority of malingnant tumors (e.g., NET, small cell lung cancer, malignant lymphoma and breast tumors) over-express multiple sst receptor subtypes relative to non-tumor tissues, of which the sst2 subtype is frequently more predominantly expressed [239]. Since these G-protein coupled receptors undergo internalization on ligand binding they are uniquely suited for radionuclide imaging. Internalization of the receptor-radioligand complex allows for extended tumor retention times which could enhance diagnostic sensitivity due to improved tumor-to-background ratios. Receptor-radioligand complex internalization could also be an important advantage in targeted radiotherapy applications [240]. A recent elegant study by Cescato and colleagues describes the use of new immunocytochemical methods to quantitatively measure sst2, sst3 and sst5 receptor subtype internalization induced by a variety of somatostatin analogs in human embryonic kidney 293 (HEK293) cells expressing these subtypes [241].
Somatostatin has a short plasma half-life (approximately 2 min) making it unsuitable for radioligand development [242]. The discovery of the short octapeptide somatostatin analog, octreotide, which displays a superior pharmacological profile, played a major role in the development of radioligands for somatostatin receptor imaging [243]. Octreotide which displays high affinity for sst2 (IC50 = 2 nM) has a plasma half-life of 1.7 hours and higher metabolic stability than somatostatin [244]. Subsequently, [111In]-labeled DTPA conjugated octreotide (Octreoscan; Mallinkrodt Medical) was developed and introduced for scintigraphic imaging of sst-expressing NET. Octreoscan is currently the gold standard for the localization, staging and management of NET [245]. However, for imaging purposes, use of the 111In radionuclide has certain disadvantages, including high cost, limited availability, less than optimum image quality and elevated patient radiation dose [245]. Consequently, several newer somatostatin analogs labeled with single photon emitters (99mTc, 123I) or positron-emitters (68Ga, 18F, 64Cu) for SPECT or PET application, respectively, have been described [246], [247], [248], [249], [250], [251]. A recent review describes the progress of functional imaging of NET using PET [252].
Current work in sst radioligand development has been directed primarily towards modulation of radioligand subtype specificity or evaluation of different radionuclides for improved tumor-targeted radiotherapy [244]. Ginj et al. have reported on novel 111In-labeled DOTA-conjugated octreotide analogs that display high affinity binding (1.4 – 13 nM) to several sst receptor subtypes (sst2, sst3 and sst5) [240]. Animal biodistribution studies showed high, specific uptake of radioactivity in sst receptor-expressing tumors (AR4-2J) in a rat model. Both radiopeptides were more efficiently internalized than [111In-DOTA,Tyr3]-octreotide. The authors propose that the high-affinity, broad sst specificity of these radioligands will be advantageous for the diagnosis and targeted radiotherapy of a broader range of sst-expressing tumors.
In an extensive study involving 84 patients, Gabriel and coworkers have compared the utility of a new PET somatostatin analog, 68Ga-D-Phe1-Tyr3-octreotide DOTA conjugate (68Ga-DOTA-TOC), for NET imaging with that of SPECT and CT [247]. 68Ga-DOTA-TOC was designed by replacement of the Phe3 residue in the corresponding octapeptide by Tyr which leads to increased hydrophillicity and improved kidney clearance [253], [254]. This modification also provides enhanced affinity for human sst2 [255], [253]. Gabriel et al. compared PET imaging with 68Ga-DOTA-TOC to SPECT imaging with the following radioligands: (111In-DOTA-TOC) and 99mTc-labeled hydrazinonicotinyl-Tyr3-octreotide (99mTc-HYNIC-TOC) [247]. CT imaging was also performed on each patient. Comparison of the three imaging modalities revealed an accuracy for PET imaging of 96% which was significantly higher than that of CT (63%) and SPECT (58%). In addition, 68Ga-DOTA-TOC imaging results were true-positive in 32 patients whose SPECT results were false-negative and it was able to detect more lesions than either SPECT or CT. Moreover, PET detected more metastatic tumors (lymph node, bone and liver) than SPECT thus permitting more accurate disease staging. The authors conclude that PET imaging with 68Ga-DOTA-TOC in conjunction with CT is superior to SPECT in the clinical diagnosis of NET.
Radiolabeled somatostatin analogs that incorporate beta-emitting (90Y, 177Lu) or alpha-emitting (213Bi ) radioisotopes for targeted tumor radiotherapy are under active investigation [256], [257]. Preliminary, preclinical data suggest that the radionuclide 90Y (high energy, pure beta-emitter; Emax = 2.25 MeV) may be more effective for treating larger tumors while the use of 177Lu (low energy, beta-emitter; Emax = 0.497 Mev) leads to fewer relapses when treating smaller lesions [258–259]. These developments have been recently reviewed [244].
Radioligands for imaging tumor cell proliferation
Tritium-labeled thymidine ([3H]thymidine) has been shown to be rapidly incorporated into newly synthesized DNA. Hence [3H]thymidine has been the gold standard for many years for assessing cell proliferation in cell culture and animal studies [260]. [11C]Thymidine was subsequently developed as a PET radioligand for monitoring cell proliferation in vivo. However, general use of this radioligand is hampered by its rapid catabolism and short half-life [261]. Two PET radiolgands that were subsequently developed to address these limitations include the [18F]-labeled analogs, 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT) and 1-(2′-deoxy-2′-[18F]fluoro-beta-D-arabinofuranosyl)thymine ([18F]FMAU) (Fig. (20)) [1]. Of these, [18F]FLT has been the most studied to date [172]. [18F]FLT is taken up by cells and phosphorylated by thymidine kinase 1 (TK-1) with subsequent trapping within the cell [260]. Intracellular [18F]FLT retention can thus be used as a measure of cellular TK-1 activity. Since TK-1 enzyme activity closely parallels the DNA synthesis pathway, cellular retention of [18F]FLT is also a measure of cellular proliferation.
Figure 20.
Structure of [18F]FLT and [18F]FMAU.
Leyton and coworkers have published several reports describing the use of PET imaging of [18F]FLT uptake as a surrogate marker for early in vivo quantitative imaging of drug-induced changes in cell proliferation. In one example, PET imaging with [18F]FLT was used for noninvasive measurement of the biological activity of a novel histone deacetylase inhibitor (LAQ824) [262]. Histone deacetylase inhibitors have been shown to cause growth inhibition of cancer cells in vitro and in animal models [263]. Treatment of mice bearing HCT116 colon-carcinoma xenografts with LAQ824 resulted in a dose-dependent decrease in [18F]FLT tumor uptake. Dose-dependent decreases of tumor TK1 and protein levels were also observed with LAQ824 pretreatment.
In a second study, [18F]FLT PET imaging was found to be superior to [18F]FDG PET imaging for quantitative measurement of tumor cell proliferation following cisplatin treatment [264]. Cisplatin is a widely used chemotherapeutic drug for cervical, lung, bladder and prostate cancer [265]. Decreased [18F]FLT tumor uptake was seen in mice bearing radiation-induced fibrosarcoma 1 (RIF-1) tumors subject to cisplatin treatment, despite a lack of change in tumor size. The decrease in [18F]FLT uptake was associated with a decrease in cell proliferation determined by immunohistochemical analysis. [18F]FLT PET imaging was also successful in quantification of the activity of an administered replicating oncolytic viral vector (dl922-947) in mice bearing IGROVI ovarian carcinoma xenografts [266].
Waldherr and colleagues have used [18F]FLT PET imaging to measure the effect of the ErbB-selective kinase inhibitor PK1-166 on tumor cell proliferation in SCID mice having ErbB1-over-expressing A431 xenograft tumors [267]. Treatment with PK1-166 markedly lowered tumor [18F]FLT uptake within 48 h of drug exposure and led to a 79% decrease of [18F]FLT uptake within a week of treatment. [18F]FLT PET imaging has also been used to monitor decreased tumor proliferation in a murine squamous cell carcinoma following radiation therapy [268].
Taken together, these studies provide good initial evidence that [18F]FLT PET imaging may be useful for monitoring therapeutic response early in the course of treatment. However, present studies suggest that [18F]FDG may be more useful for tumor detection and staging [269].
Radioligands for imaging β-amyloid
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by irreversible impairment of memory, cognitive function and behavior changes. The pathological features of the disease are the presence of intracellular neurofibrillary tangles (NFT) and extracellular amyloid plagues consisting of dense aggregates of β-amyloid (Aβ) [270]. Aβ is a 39 – 43 amino acid metalloprotein resulting from the proteolytic cleavage of amyloid precursor protein by β- and γ-secretases. Although the precise mechanism of neuronal death in AD remains unknown, it is widely accepted that progressive accumulation of Aβ in brain due an imbalance between its production and removal is central to AD pathogenesis. Currently, postmortem analysis of brain tissue remains the only definitive method of AD diagnosis. For these reasons, intense efforts have been directed towards the development of PET and SPECT radioligands for the diagnostic imaging of amyloid deposits in AD.
Prominent among these is [11C]PIB (Figure 21), a carbon-11 labeled derivative of the fluorescent dye Thioflavin T routinely used to quantify beta-sheet fibrilization in vitro. [11C]PIB displays high in vitro binding affinity for Aβ plagues (Ki = 4.3 nM) and a high initial brain uptake (7.0% ID/g at 2 min) followed by a rapid clearance from normal mouse brain (0.6% ID/g at 30 min) following i.v. administration. Initial clinical studies conducted with [11C]PIB in patients with mild AD showed marked retention in areas of association cortex, which are known to contain large amounts of amyloid deposits in AD, with respect to control subjects (Figure 22) [271]. An F-18 labeled stilbene derivative ([18F]AV45) developed at the University of Pennsylvania is also under clinical evaluation for imaging of AD with PET [272]. [18F]AV45 displays rapid reversible binding characteristics which permits subject scanning at 45 min postinjection and several studies have confirmed its ability to discriminate between AD and age-matched controls [273]. Several [123I]-labeled SPECT Aβ radioligands as exemplified by [123I]IMPY and [123I]CQ are also under evaluation due to the advantage of more widespread application [274]. [123I]IMPY displayed selective labeling of Aβ plaques in ex vivo autoradiography studies using a double transgenic (PSAPP) mouse model of AD [275].
Figure 21.

Structure of Radioligands for Imaging beta-Amyloid.
Figure 22. Comparative PET images of [11C]PIB binding in an AD and Control Subject.
(Image courtesy of http://www.thinkgene.com).
Summary and conclusion
The ability of PET and SPECT imaging to non-invasively image physiological and biochemical processes has opened up numerous applications in clinical as well as basic scientific research. Additionally, PET and SPECT imaging could play an important role in many aspects of the drug development process. At the preclinical stage, noninvasive imaging studies with radiolabeled investigational drugs can provide vital proof-of-concept information to aid proper candidate selection. These studies would allow noninvasive determination of a drug’s pharmacokinetic behavior and target versus non-target accumulation. Importantly, such studies could provide information early in the development process if a drug is, in fact, reaching its target and also identify potential toxicity issues. Drug analogs under investigation could also be screened in vivo in animal models against a validated radioligand selective for the same biological target (e.g., receptor, enzyme binding site, etc.) to provide direct measures of receptor occupancy. Such data could be used to design optimal dosing and timing schedules in clinical trials thereby improving their efficiency and cost-effectiveness.
At the clinical level, microdosing studies with suitable radioligands could provide useful metabolic and toxicology data prior to the conducting of classical Phase I trials. The FDA Critical Path Initiative has emphasized the development of validated imaging-based biomarkers as an important tool for streamlining the drug development process. For example in oncological drug development, clinically-validated radioligand probes that image downstream biological effects of cancer treatment such as, apoptosis, angiogenesis and decreased tumor cell proliferation could be useful surrogate markers for monitoring the therapeutic response of new oncology drugs in development.
[18F]FDG PET imaging currently plays an increasingly important role particularly in cancer treatment by virtue of its ability to diagnose, stage and assess tumor response to chemotherapy and chemo-radiotherapy. It is highly likely that the future availability of validated molecularly-targeted radioligands will likewise play a major role in therapeutic drug development. Such achievements will greatly advance the ultimate goal of personalized medical treatment in providing the right drug to the right patient.
Acknowledgments
We thank Steven Kronenberg for designing the figures and the members of the Center for Molecular Imaging (CMI) for critical reading of the manuscript. The authors acknowledge support from the following National Institutes of Health grants (PO1 CA85878, P50 CA93990, and R24 CA83099) during the preparation of this review.
References
- 1.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 2.Westra J, Limburg PC. p38 mitogen-activated protein kinase (MAPK) in rheumatoid arthritis. Mini Rev Med Chem. 2006;6(8):867–74. doi: 10.2174/138955706777934982. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Bassat H. Biological activity of tyrosine kinase inhibitors: novel agents for psoriasis therapy. Curr Opin Investig Drugs. 2001;2(11):1539–45. [PubMed] [Google Scholar]
- 4.Blease K. Targeting kinases in asthma. Expert Opin Investig Drugs. 2005;14(10):1213–20. doi: 10.1517/13543784.14.10.1213. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Singh VP, Baker KM. Kinase inhibitors for cardiovascular disease. J Mol Cell Cardiol. 2007;42(1):1–11. doi: 10.1016/j.yjmcc.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4(5):387–98. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- 7.Alizadeh A, et al. The lymphochip: a specialized cDNA microarray for the genomic-scale analysis of gene expression in normal and malignant lymphocytes. Cold Spring Harb Symp Quant Biol. 1999;64:71–8. doi: 10.1101/sqb.1999.64.71. [DOI] [PubMed] [Google Scholar]
- 8.Margalit O, et al. Microarray-based gene expression profiling of hematologic malignancies: basic concepts and clinical applications. Blood Rev. 2005;19(4):223–34. doi: 10.1016/j.blre.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Morris DS, et al. Integrating biomedical knowledge to model pathways of prostate cancer progression. Cell Cycle. 2007;6(10):1177–87. doi: 10.4161/cc.6.10.4247. [DOI] [PubMed] [Google Scholar]
- 10.Nishiu M, et al. Microarray analysis of gene-expression profiles in diffuse large B-cell lymphoma: identification of genes related to disease progression. Jpn J Cancer Res. 2002;93(8):894–901. doi: 10.1111/j.1349-7006.2002.tb01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tschoep K, et al. Gene expression profiling in sarcomas. Crit Rev Oncol Hematol. 2007;63(2):111–24. doi: 10.1016/j.critrevonc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Radford IR. Imatinib. Novartis. Curr Opin Investig Drugs. 2002;3(3):492–9. [PubMed] [Google Scholar]
- 13.Melnikova I, Golden J. Targeting protein kinases. Nat Rev Drug Discov. 2004;3(12):993–4. doi: 10.1038/nrd1600. [DOI] [PubMed] [Google Scholar]
- 14.Melnikova I, Golden J. Apoptosis-targeting therapies. Nat Rev Drug Discov. 2004;3(11):905–6. doi: 10.1038/nrd1554. [DOI] [PubMed] [Google Scholar]
- 15.Chun PY, et al. Synergistic effects of gemcitabine and gefitinib in the treatment of head and neck carcinoma. Cancer Res. 2006;66(2):981–8. doi: 10.1158/0008-5472.CAN-05-2665. [DOI] [PubMed] [Google Scholar]
- 16.Bhojani MS, et al. Imaging of proteolytic activity using a conditional cell surface receptor. Mol Imaging. 2006;5(2):129–37. [PubMed] [Google Scholar]
- 17.Zhang L, et al. Molecular imaging of Akt kinase activity. Nat Med. 2007;13(9):1114–9. doi: 10.1038/nm1608. [DOI] [PubMed] [Google Scholar]
- 18.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340(6230):245–6. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 19.Luker KE, Piwnica-Worms D. Optimizing luciferase protein fragment complementation for bioluminescent imaging of protein-protein interactions in live cells and animals. Methods Enzymol. 2004;385:349–60. doi: 10.1016/S0076-6879(04)85019-5. [DOI] [PubMed] [Google Scholar]
- 20.Michnick SW, et al. Universal strategies in research and drug discovery based on protein-fragment complementation assays. Nat Rev Drug Discov. 2007;6(7):569–82. doi: 10.1038/nrd2311. [DOI] [PubMed] [Google Scholar]
- 21.Remy I, Ghaddar G, Michnick SW. Using the beta-lactamase protein-fragment complementation assay to probe dynamic protein-protein interactions. Nat Protoc. 2007;2(9):2302–6. doi: 10.1038/nprot.2007.356. [DOI] [PubMed] [Google Scholar]
- 22.Remy I, Michnick SW. A highly sensitive protein-protein interaction assay based on Gaussia luciferase. Nat Methods. 2006;3(12):977–9. doi: 10.1038/nmeth979. [DOI] [PubMed] [Google Scholar]
- 23.Remy I, Michnick SW. Application of protein-fragment complementation assays in cell biology. Biotechniques. 2007;42(2):137, 139, 141. doi: 10.2144/000112396. passim. [DOI] [PubMed] [Google Scholar]
- 24.Stefan E, et al. Quantification of dynamic protein complexes using Renilla luciferase fragment complementation applied to protein kinase A activities in vivo. Proc Natl Acad Sci U S A. 2007;104(43):16916–21. doi: 10.1073/pnas.0704257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Contag CH, Ross BD. It’s not just about anatomy: in vivo bioluminescence imaging as an eyepiece into biology. J Magn Reson Imaging. 2002;16(4):378–87. doi: 10.1002/jmri.10178. [DOI] [PubMed] [Google Scholar]
- 26.Greer LF, 3rd, Szalay AA. Imaging of light emission from the expression of luciferases in living cells and organisms: a review. Luminescence. 2002;17(1):43–74. doi: 10.1002/bio.676. [DOI] [PubMed] [Google Scholar]
- 27.Luker KE, et al. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci U S A. 2004;101(33):12288–93. doi: 10.1073/pnas.0404041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 29.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 30.Kovarova M, et al. Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fcepsilon receptor I aggregation. Mol Cell Biol. 2001;21(24):8318–28. doi: 10.1128/MCB.21.24.8318-8328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Contessa JN, et al. Inhibition of N-linked glycosylation disrupts receptor tyrosine kinase signaling in tumor cells. Cancer Res. 2008;68(10):3803–9. doi: 10.1158/0008-5472.CAN-07-6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002;62(1):200–7. [PubMed] [Google Scholar]
- 33.Damiano V, et al. Cooperative antitumor effect of multitargeted kinase inhibitor ZD6474 and ionizing radiation in glioblastoma. Clin Cancer Res. 2005;11(15):5639–44. doi: 10.1158/1078-0432.CCR-05-0174. [DOI] [PubMed] [Google Scholar]
- 34.Dote H, et al. ErbB3 expression predicts tumor cell radiosensitization induced by Hsp90 inhibition. Cancer Res. 2005;65(15):6967–75. doi: 10.1158/0008-5472.CAN-05-1304. [DOI] [PubMed] [Google Scholar]
- 35.Lammering G, et al. Radiosensitization of malignant glioma cells through overexpression of dominant-negative epidermal growth factor receptor. Clin Cancer Res. 2001;7(3):682–90. [PubMed] [Google Scholar]
- 36.Contessa JN, et al. Molecular Imaging of N-linked Glycosylation Suggests Glycan Biosynthesis Is a Novel Target for Cancer Therapy. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-09-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ameisen JC. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Differ. 2002;9(4):367–93. doi: 10.1038/sj.cdd.4400950. [DOI] [PubMed] [Google Scholar]
- 38.Bhojani MS, Rossu BD, Rehemtulla A. TRAIL and anti-tumor responses. Cancer Biol Ther. 2003;2(4 Suppl 1):S71–8. [PubMed] [Google Scholar]
- 39.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–6. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 40.Nicholson DW, Thornberry NA. Apoptosis. Life and death decisions. Science. 2003;299(5604):214–5. doi: 10.1126/science.1081274. [DOI] [PubMed] [Google Scholar]
- 41.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22(8):299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y. Apoptosome: the cellular engine for the activation of caspase-9. Structure. 2002;10(3):285–8. doi: 10.1016/s0969-2126(02)00732-3. [DOI] [PubMed] [Google Scholar]
- 43.Laxman B, et al. Noninvasive real-time imaging of apoptosis. Proc Natl Acad Sci U S A. 2002;99(26):16551–5. doi: 10.1073/pnas.252644499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee KC, et al. Noninvasive molecular imaging sheds light on the synergy between 5-fluorouracil and TRAIL/Apo2L for cancer therapy. Clin Cancer Res. 2007;13(6):1839–46. doi: 10.1158/1078-0432.CCR-06-1657. [DOI] [PubMed] [Google Scholar]
- 45.Thormeyer D, et al. Characterization of lacZ complementation deletions using membrane receptor dimerization. Biotechniques. 2003;34(2):346–50. 352–5. doi: 10.2144/03342rr05. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, Zhu W, Kodadek T. Selection and application of peptide-binding peptides. Nat Biotechnol. 2000;18(1):71–4. doi: 10.1038/71951. [DOI] [PubMed] [Google Scholar]
- 47.Coppola JM, Ross BD, Rehemtulla A. Noninvasive imaging of apoptosis and its application in cancer therapeutics. Clin Cancer Res. 2008;14(8):2492–501. doi: 10.1158/1078-0432.CCR-07-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khanna D, et al. A Transgenic Mouse for Imaging Caspase-Dependent Apoptosis within the Skin. J Invest Dermatol. 2010;130(7):1797–806. doi: 10.1038/jid.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matthews BW. The structure of E. coli beta-galactosidase. C R Biol. 2005;328(6):549–56. doi: 10.1016/j.crvi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Celen S, et al. Synthesis and evaluation of 18F- and 11C-labeled phenyl-galactopyranosides as potential probes for in vivo visualization of LacZ gene expression using positron emission tomography. Bioconjug Chem. 2008;19(2):441–9. doi: 10.1021/bc700216d. [DOI] [PubMed] [Google Scholar]
- 51.Olesen CE, et al. Novel methods for chemiluminescent detection of reporter enzymes. Methods Enzymol. 2000;326:175–202. doi: 10.1016/s0076-6879(00)26055-2. [DOI] [PubMed] [Google Scholar]
- 52.Smith AD, Trempe JP. Luminometric quantitation of photinus pyralis firefly luciferase and Escherichia coli beta-galactosidase in blood-contaminated organ lysates. Anal Biochem. 2000;286(1):164–72. doi: 10.1006/abio.2000.4797. [DOI] [PubMed] [Google Scholar]
- 53.Van Poucke SO, Nelis HJ. Rapid detection of fluorescent and chemiluminescent total coliforms and Escherichia coli on membrane filters. J Microbiol Methods. 2000;42(3):233–44. doi: 10.1016/s0167-7012(00)00193-7. [DOI] [PubMed] [Google Scholar]
- 54.Molloy SS, et al. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9(1):28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- 55.Reznik SE, Fricker LD. Carboxypeptidases from A to z: implications in embryonic development and Wnt binding. Cell Mol Life Sci. 2001;58(12–13):1790–804. doi: 10.1007/PL00000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vassar R. Beta-secretase (BACE) as a drug target for Alzheimer’s disease. Adv Drug Deliv Rev. 2002;54(12):1589–602. doi: 10.1016/s0169-409x(02)00157-6. [DOI] [PubMed] [Google Scholar]
- 57.Vassar R, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 58.Coppola JM, et al. A small-molecule furin inhibitor inhibits cancer cell motility and invasiveness. Neoplasia. 2008;10(4):363–70. doi: 10.1593/neo.08166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coppola JM, et al. Identification of inhibitors using a cell-based assay for monitoring Golgi-resident protease activity. Anal Biochem. 2007;364(1):19–29. doi: 10.1016/j.ab.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishikawa TO, Herschman HR. Conditional Bicistronic Cre Reporter Line Expressing Both Firefly Luciferase and beta-galactosidase. Mol Imaging Biol. 2010 doi: 10.1007/s11307-010-0333-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlsen H, et al. In vivo imaging of NF-kappa B activity. J Immunol. 2002;168(3):1441–6. doi: 10.4049/jimmunol.168.3.1441. [DOI] [PubMed] [Google Scholar]
- 62.Lin AH, et al. Global analysis of Smad2/3-dependent TGF-beta signaling in living mice reveals prominent tissue-specific responses to injury. J Immunol. 2005;175(1):547–54. doi: 10.4049/jimmunol.175.1.547. [DOI] [PubMed] [Google Scholar]
- 63.Safran M, et al. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci U S A. 2006;103(1):105–10. doi: 10.1073/pnas.0509459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosen BR, et al. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990;14(2):249–65. doi: 10.1002/mrm.1910140211. [DOI] [PubMed] [Google Scholar]
- 65.Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991;17(2):357–67. doi: 10.1002/mrm.1910170208. [DOI] [PubMed] [Google Scholar]
- 66.Schwickert HC, et al. Contrast-enhanced MR imaging assessment of tumor capillary permeability: effect of irradiation on delivery of chemotherapy. Radiology. 1996;198(3):893–8. doi: 10.1148/radiology.198.3.8628889. [DOI] [PubMed] [Google Scholar]
- 67.Su FC, et al. Temporal resolving power of perfusion- and BOLD-based event-related functional MRI. Med Phys. 2004;31(1):154–60. doi: 10.1118/1.1634480. [DOI] [PubMed] [Google Scholar]
- 68.Warach S, et al. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol. 1995;37(2):231–41. doi: 10.1002/ana.410370214. [DOI] [PubMed] [Google Scholar]
- 69.Guo AC, et al. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002;224(1):177–83. doi: 10.1148/radiol.2241010637. [DOI] [PubMed] [Google Scholar]
- 70.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 71.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 72.Gellermann J, et al. Methods and potentials of magnetic resonance imaging for monitoring radiofrequency hyperthermia in a hybrid system. Int J Hyperthermia. 2005;21(6):497–513. doi: 10.1080/02656730500070102. [DOI] [PubMed] [Google Scholar]
- 73.Sorensen AG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology. 1996;199(2):391–401. doi: 10.1148/radiology.199.2.8668784. [DOI] [PubMed] [Google Scholar]
- 74.Le Bihan D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 75.Moseley ME, et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176(2):439–45. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- 76.Le Bihan D, et al. Diffusion and perfusion magnetic resonance imaging in brain tumors. Top Magn Reson Imaging. 1993;5(1):25–31. [PubMed] [Google Scholar]
- 77.Le Bihan D. Molecular diffusion nuclear magnetic resonance imaging. Magn Reson Q. 1991;7(1):1–30. [PubMed] [Google Scholar]
- 78.Stejskal E, Tanner J. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J Chem Phys. 1965;42(1):288–292. [Google Scholar]
- 79.Thomsen C, Henriksen O, Ring P. In vivo measurement of water self diffusion in the human brain by magnetic resonance imaging. Acta Radiol. 1987;28(3):353–61. [PubMed] [Google Scholar]
- 80.Merboldt KD, et al. MRI of “diffusion” in the human brain: new results using a modified CE-FAST sequence. Magn Reson Med. 1989;9(3):423–9. doi: 10.1002/mrm.1910090316. [DOI] [PubMed] [Google Scholar]
- 81.Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol. 2003;45(3):169–84. doi: 10.1016/s0720-048x(02)00303-0. [DOI] [PubMed] [Google Scholar]
- 82.Padhani AR, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11(2):102–25. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Le Bihan D. The ‘wet mind’: water and functional neuroimaging. Phys Med Biol. 2007;52(7):R57–90. doi: 10.1088/0031-9155/52/7/R02. [DOI] [PubMed] [Google Scholar]
- 84.Edelman RR, Wielopolski P, Schmitt F. Echo-planar MR imaging. Radiology. 1994;192(3):600–12. doi: 10.1148/radiology.192.3.8058920. [DOI] [PubMed] [Google Scholar]
- 85.Provenzale JM, et al. Peritumoral brain regions in gliomas and meningiomas: investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology. 2004;232(2):451–60. doi: 10.1148/radiol.2322030959. [DOI] [PubMed] [Google Scholar]
- 86.Yoshikawa T, et al. ADC measurement of abdominal organs and lesions using parallel imaging technique. AJR Am J Roentgenol. 2006;187(6):1521–30. doi: 10.2214/AJR.05.0778. [DOI] [PubMed] [Google Scholar]
- 87.Koh DM, et al. Colorectal hepatic metastases: quantitative measurements using single-shot echo-planar diffusion-weighted MR imaging. Eur Radiol. 2006;16(9):1898–905. doi: 10.1007/s00330-006-0201-x. [DOI] [PubMed] [Google Scholar]
- 88.Koh DM, et al. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007;188(4):1001–8. doi: 10.2214/AJR.06.0601. [DOI] [PubMed] [Google Scholar]
- 89.Charles-Edwards EM, deSouza NM. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging. 2006;6:135–43. doi: 10.1102/1470-7330.2006.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takahara T, et al. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22(4):275–82. [PubMed] [Google Scholar]
- 91.Ichikawa T, et al. High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: preliminary results. AJR Am J Roentgenol. 2007;188(2):409–14. doi: 10.2214/AJR.05.1918. [DOI] [PubMed] [Google Scholar]
- 92.Sumi M, Ichikawa Y, Nakamura T. Diagnostic ability of apparent diffusion coefficients for lymphomas and carcinomas in the pharynx. Eur Radiol. 2007;17(10):2631–7. doi: 10.1007/s00330-007-0588-z. [DOI] [PubMed] [Google Scholar]
- 93.Eida S, et al. Apparent diffusion coefficient mapping of salivary gland tumors: prediction of the benignancy and malignancy. AJNR Am J Neuroradiol. 2007;28(1):116–21. [PMC free article] [PubMed] [Google Scholar]
- 94.Bulakbasi N, et al. The added value of the apparent diffusion coefficient calculation to magnetic resonance imaging in the differentiation and grading of malignant brain tumors. J Comput Assist Tomogr. 2004;28(6):735–46. doi: 10.1097/00004728-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 95.Shimony JS, et al. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology. 1999;212(3):770–84. doi: 10.1148/radiology.212.3.r99au51770. [DOI] [PubMed] [Google Scholar]
- 96.Mori S, et al. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Ann Neurol. 2002;51(3):377–80. doi: 10.1002/ana.10137. [DOI] [PubMed] [Google Scholar]
- 97.Chenevert TL, McKeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res. 1997;3(9):1457–66. [PubMed] [Google Scholar]
- 98.Brunberg JA, et al. In vivo MR determination of water diffusion coefficients and diffusion anisotropy: correlation with structural alteration in gliomas of the cerebral hemispheres. AJNR Am J Neuroradiol. 1995;16(2):361–71. [PMC free article] [PubMed] [Google Scholar]
- 99.Lyng H, Haraldseth O, Rofstad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med. 2000;43(6):828–36. doi: 10.1002/1522-2594(200006)43:6<828::aid-mrm8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 100.Chenevert TL, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92(24):2029–36. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 101.Chenevert TL, Sundgren PC, Ross BD. Diffusion imaging: insight to cell status and cytoarchitecture. Neuroimaging Clin N Am. 2006;16(4):619–32. viii–ix. doi: 10.1016/j.nic.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 102.Ross BD, et al. Evaluation of cancer therapy using diffusion magnetic resonance imaging. Mol Cancer Ther. 2003;2(6):581–7. [PubMed] [Google Scholar]
- 103.Lee KC, et al. Prospective early response imaging biomarker for neoadjuvant breast cancer chemotherapy. Clin Cancer Res. 2007;13(2 Pt 1):443–50. doi: 10.1158/1078-0432.CCR-06-1888. [DOI] [PubMed] [Google Scholar]
- 104.Lee KC, et al. Dynamic imaging of emerging resistance during cancer therapy. Cancer Res. 2006;66(9):4687–92. doi: 10.1158/0008-5472.CAN-05-3205. [DOI] [PubMed] [Google Scholar]
- 105.Lee KC, et al. An imaging biomarker of early treatment response in prostate cancer that has metastasized to the bone. Cancer Res. 2007;67(8):3524–8. doi: 10.1158/0008-5472.CAN-06-4236. [DOI] [PubMed] [Google Scholar]
- 106.Hamstra DA, et al. The use of 19F spectroscopy and diffusion-weighted MRI to evaluate differences in gene-dependent enzyme prodrug therapies. Mol Ther. 2004;10(5):916–28. doi: 10.1016/j.ymthe.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 107.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 108.Sorensen AG, et al. Comparison of diameter and perimeter methods for tumor volume calculation. J Clin Oncol. 2001;19(2):551–7. doi: 10.1200/JCO.2001.19.2.551. [DOI] [PubMed] [Google Scholar]
- 109.Yoshida S, et al. Initial experience of diffusion-weighted magnetic resonance imaging to assess therapeutic response to induction chemoradiotherapy against muscle-invasive bladder cancer. Urology. 75(2):387–91. doi: 10.1016/j.urology.2009.06.111. [DOI] [PubMed] [Google Scholar]
- 110.Yankeelov TE, et al. Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Magn Reson Imaging. 2007;25(1):1–13. doi: 10.1016/j.mri.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Uhl M, et al. Osteosarcoma: preliminary results of in vivo assessment of tumor necrosis after chemotherapy with diffusion- and perfusion-weighted magnetic resonance imaging. Invest Radiol. 2006;41(8):618–23. doi: 10.1097/01.rli.0000225398.17315.68. [DOI] [PubMed] [Google Scholar]
- 112.Tomura N, et al. Diffusion changes in a tumor and peritumoral tissue after stereotactic irradiation for brain tumors: possible prediction of treatment response. J Comput Assist Tomogr. 2006;30(3):496–500. doi: 10.1097/00004728-200605000-00024. [DOI] [PubMed] [Google Scholar]
- 113.Theilmann RJ, et al. Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia. 2004;6(6):831–7. doi: 10.1593/neo.03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun YS, et al. Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology. 254(1):170–8. doi: 10.1148/radiol.2541082230. [DOI] [PubMed] [Google Scholar]
- 115.Schubert MI, et al. Diffusion-weighted magnetic resonance imaging of treatment-associated changes in recurrent and residual medulloblastoma: preliminary observations in three children. Acta Radiol. 2006;47(10):1100–4. doi: 10.1080/02841850600990300. [DOI] [PubMed] [Google Scholar]
- 116.Rhee TK, et al. Tumor response after yttrium-90 radioembolization for hepatocellular carcinoma: comparison of diffusion-weighted functional MR imaging with anatomic MR imaging. J Vasc Interv Radiol. 2008;19(8):1180–6. doi: 10.1016/j.jvir.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 117.Pickles MD, et al. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging. 2006;24(7):843–7. doi: 10.1016/j.mri.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 118.Okuma T, et al. Assessment of early treatment response after CT-guided radiofrequency ablation of unresectable lung tumours by diffusion-weighted MRI: a pilot study. Br J Radiol. 2009;82(984):989–94. doi: 10.1259/bjr/13217618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moffat BA, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci U S A. 2005;102(15):5524–9. doi: 10.1073/pnas.0501532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marugami N, et al. Early detection of therapeutic response to hepatic arterial infusion chemotherapy of liver metastases from colorectal cancer using diffusion-weighted MR imaging. Cardiovasc Intervent Radiol. 2009;32(4):638–46. doi: 10.1007/s00270-009-9532-8. [DOI] [PubMed] [Google Scholar]
- 121.Mardor Y, et al. Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res. 2001;61(13):4971–3. [PubMed] [Google Scholar]
- 122.Mardor Y, et al. Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J Clin Oncol. 2003;21(6):1094–100. doi: 10.1200/JCO.2003.05.069. [DOI] [PubMed] [Google Scholar]
- 123.Liu Y, et al. Diffusion-weighted imaging in predicting and monitoring the response of uterine cervical cancer to combined chemoradiation. Clin Radiol. 2009;64(11):1067–74. doi: 10.1016/j.crad.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 124.Lin YC, et al. Significant temporal evolution of diffusion anisotropy for evaluating early response to radiosurgery in patients with vestibular schwannoma: findings from functional diffusion maps. AJNR Am J Neuroradiol. 31(2):269–74. doi: 10.3174/ajnr.A1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liapi E, et al. Assessment of response of uterine fibroids and myometrium to embolization using diffusion-weighted echoplanar MR imaging. J Comput Assist Tomogr. 2005;29(1):83–6. doi: 10.1097/01.rct.0000146111.48570.64. [DOI] [PubMed] [Google Scholar]
- 126.Kremser C, et al. Preliminary results on the influence of chemoradiation on apparent diffusion coefficients of primary rectal carcinoma measured by magnetic resonance imaging. Strahlenther Onkol. 2003;179(9):641–9. doi: 10.1007/s00066-003-1045-9. [DOI] [PubMed] [Google Scholar]
- 127.Koh DM, et al. Reproducibility and changes in the apparent diffusion coefficients of solid tumours treated with combretastatin A4 phosphate and bevacizumab in a two-centre phase I clinical trial. Eur Radiol. 2009;19(11):2728–38. doi: 10.1007/s00330-009-1469-4. [DOI] [PubMed] [Google Scholar]
- 128.Kim S, et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15(3):986–94. doi: 10.1158/1078-0432.CCR-08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim SH, et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009;253(1):116–25. doi: 10.1148/radiol.2532090027. [DOI] [PubMed] [Google Scholar]
- 130.Kato H, et al. Head and neck squamous cell carcinoma: usefulness of diffusion-weighted MR imaging in the prediction of a neoadjuvant therapeutic effect. Eur Radiol. 2009;19(1):103–9. doi: 10.1007/s00330-008-1108-5. [DOI] [PubMed] [Google Scholar]
- 131.Kamel IR, et al. Functional MR imaging assessment of tumor response after 90Y microsphere treatment in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2007;18(1 Pt 1):49–56. doi: 10.1016/j.jvir.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 132.Kamel IR, et al. The role of functional MR imaging in the assessment of tumor response after chemoembolization in patients with hepatocellular carcinoma. J Vasc Interv Radiol. 2006;17(3):505–12. doi: 10.1097/01.RVI.0000200052.02183.92. [DOI] [PubMed] [Google Scholar]
- 133.Jain R, et al. Imaging response criteria for recurrent gliomas treated with bevacizumab: role of diffusion weighted imaging as an imaging biomarker. J Neurooncol. 96(3):423–31. doi: 10.1007/s11060-009-9981-6. [DOI] [PubMed] [Google Scholar]
- 134.Jacobs MA, Herskovits EH, Kim HS. Uterine fibroids: diffusion-weighted MR imaging for monitoring therapy with focused ultrasound surgery--preliminary study. Radiology. 2005;236(1):196–203. doi: 10.1148/radiol.2361040312. [DOI] [PubMed] [Google Scholar]
- 135.Hayashida Y, et al. Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: Initial results. Eur Radiol. 2006;16(12):2637–43. doi: 10.1007/s00330-006-0342-y. [DOI] [PubMed] [Google Scholar]
- 136.Harry VN, et al. Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol. 2008;111(2):213–20. doi: 10.1016/j.ygyno.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 137.Hamstra DA, et al. Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proc Natl Acad Sci U S A. 2005;102(46):16759–64. doi: 10.1073/pnas.0508347102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Galban CJ, et al. A feasibility study of parametric response map analysis of diffusion-weighted magnetic resonance imaging scans of head and neck cancer patients for providing early detection of therapeutic efficacy. Transl Oncol. 2009;2(3):184–90. doi: 10.1593/tlo.09175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dzik-Jurasz A, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360(9329):307–8. doi: 10.1016/S0140-6736(02)09520-X. [DOI] [PubMed] [Google Scholar]
- 140.Dirix P, et al. Dose painting in radiotherapy for head and neck squamous cell carcinoma: value of repeated functional imaging with (18)F-FDG PET, (18)F-fluoromisonidazole PET, diffusion-weighted MRI, and dynamic contrast-enhanced MRI. J Nucl Med. 2009;50(7):1020–7. doi: 10.2967/jnumed.109.062638. [DOI] [PubMed] [Google Scholar]
- 141.Deng J, et al. Diffusion-weighted MR imaging for determination of hepatocellular carcinoma response to yttrium-90 radioembolization. J Vasc Interv Radiol. 2006;17(7):1195–200. doi: 10.1097/01.RVI.0000227234.81718.EB. [DOI] [PubMed] [Google Scholar]
- 142.Cui Y, et al. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008;248(3):894–900. doi: 10.1148/radiol.2483071407. [DOI] [PubMed] [Google Scholar]
- 143.Byun WM, et al. Diffusion-weighted MR imaging of metastatic disease of the spine: assessment of response to therapy. AJNR Am J Neuroradiol. 2002;23(6):906–12. [PMC free article] [PubMed] [Google Scholar]
- 144.Eccles CL, et al. Change in diffusion weighted MRI during liver cancer radiotherapy: preliminary observations. Acta Oncol. 2009;48(7):1034–43. doi: 10.1080/02841860903099972. [DOI] [PubMed] [Google Scholar]
- 145.Hein PA, et al. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol. 2004;25(2):201–9. [PMC free article] [PubMed] [Google Scholar]
- 146.Lee KC, et al. A feasibility study evaluating the functional diffusion map as a predictive imaging biomarker for detection of treatment response in a patient with metastatic prostate cancer to the bone. Neoplasia. 2007;9(12):1003–11. doi: 10.1593/neo.07954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zahra MA, et al. Dynamic contrast-enhanced MRI as a predictor of tumour response to radiotherapy. Lancet Oncol. 2007;8(1):63–74. doi: 10.1016/S1470-2045(06)71012-9. [DOI] [PubMed] [Google Scholar]
- 148.O’Connor JP, et al. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer. 2007;96(2):189–95. doi: 10.1038/sj.bjc.6603515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ah-See ML, et al. Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2008;14(20):6580–9. doi: 10.1158/1078-0432.CCR-07-4310. [DOI] [PubMed] [Google Scholar]
- 150.Baar J, et al. A vasculature-targeting regimen of preoperative docetaxel with or without bevacizumab for locally advanced breast cancer: impact on angiogenic biomarkers. Clin Cancer Res. 2009;15(10):3583–90. doi: 10.1158/1078-0432.CCR-08-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bauerle T, et al. Imaging anti-angiogenic treatment response with DCE-VCT, DCE-MRI and DWI in an animal model of breast cancer bone metastasis. Eur J Radiol. 2010;73(2):280–7. doi: 10.1016/j.ejrad.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 153.Jain RK, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6(6):327–38. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jarnagin WR, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20(9):1589–95. doi: 10.1093/annonc/mdp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kamoun WS, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27(15):2542–52. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim S, et al. Prediction of response to chemoradiation therapy in squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2010;31(2):262–8. doi: 10.3174/ajnr.A1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lockhart AC, et al. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010;28(2):207–14. doi: 10.1200/JCO.2009.22.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meyer T, et al. A phase I trial of radioimmunotherapy with 131I-A5B7 anti-CEA antibody in combination with combretastatin-A4-phosphate in advanced gastrointestinal carcinomas. Clin Cancer Res. 2009;15(13):4484–92. doi: 10.1158/1078-0432.CCR-09-0035. [DOI] [PubMed] [Google Scholar]
- 159.Sorensen AG, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69(13):5296–300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.van Laarhoven HW, et al. Phase I clinical and magnetic resonance imaging study of the vascular agent NGR-hTNF in patients with advanced cancers (European Organization for Research and Treatment of Cancer Study 16041) Clin Cancer Res. 2010;16(4):1315–23. doi: 10.1158/1078-0432.CCR-09-1621. [DOI] [PubMed] [Google Scholar]
- 161.Vriens D, et al. Chemotherapy response monitoring of colorectal liver metastases by dynamic Gd-DTPA-enhanced MRI perfusion parameters and 18F-FDG PET metabolic rate. J Nucl Med. 2009;50(11):1777–84. doi: 10.2967/jnumed.109.064790. [DOI] [PubMed] [Google Scholar]
- 162.Wong CI, et al. Phase I and biomarker study of ABT-869, a multiple receptor tyrosine kinase inhibitor, in patients with refractory solid malignancies. J Clin Oncol. 2009;27(28):4718–26. doi: 10.1200/JCO.2008.21.7125. [DOI] [PubMed] [Google Scholar]
- 163.Zahra MA, et al. Semiquantitative and quantitative dynamic contrast-enhanced magnetic resonance imaging measurements predict radiation response in cervix cancer. Int J Radiat Oncol Biol Phys. 2009;74(3):766–73. doi: 10.1016/j.ijrobp.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 164.Tofts PS, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10(3):223–32. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 165.Leach MO, et al. The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: issues and recommendations. Br J Cancer. 2005;92(9):1599–610. doi: 10.1038/sj.bjc.6602550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Semiz Oysu A, et al. Dynamic contrast-enhanced MRI in the differentiation of posttreatment fibrosis from recurrent carcinoma of the head and neck. Clin Imaging. 2005;29(5):307–12. doi: 10.1016/j.clinimag.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 167.Ohno Y, et al. Prognostic value of dynamic MR imaging for non-small-cell lung cancer patients after chemoradiotherapy. J Magn Reson Imaging. 2005;21(6):775–83. doi: 10.1002/jmri.20297. [DOI] [PubMed] [Google Scholar]
- 168.Dobson MJ, et al. The assessment of irradiated bladder carcinoma using dynamic contrast-enhanced MR imaging. Clin Radiol. 2001;56(2):94–8. doi: 10.1053/crad.2000.0560. [DOI] [PubMed] [Google Scholar]
- 169.Boss EA, et al. Post-radiotherapy contrast enhancement changes in fast dynamic MRI of cervical carcinoma. J Magn Reson Imaging. 2001;13(4):600–6. doi: 10.1002/jmri.1084. [DOI] [PubMed] [Google Scholar]
- 170.Wedam SB, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24(5):769–77. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 171.Goh V, Padhani AR, Rasheed S. Functional imaging of colorectal cancer angiogenesis. Lancet Oncol. 2007;8(3):245–55. doi: 10.1016/S1470-2045(07)70075-X. [DOI] [PubMed] [Google Scholar]
- 172.Shields AF. Positron emission tomography measurement of tumor metabolism and growth: its expanding role in oncology. Mol Imaging Biol. 2006;8(3):141–50. doi: 10.1007/s11307-006-0039-2. [DOI] [PubMed] [Google Scholar]
- 173.Metser U, Even-Sapir E. Increased (18)F-fluorodeoxyglucose uptake in benign, nonphysiologic lesions found on whole-body positron emission tomography/computed tomography (PET/CT): accumulated data from four years of experience with PET/CT. Semin Nucl Med. 2007;37(3):206–22. doi: 10.1053/j.semnuclmed.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 174.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 175.Sturk, editor. Basic Science of Oncology. 2005. [Google Scholar]
- 176.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 177.Safran M, Kaelin WG., Jr HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111(6):779–83. doi: 10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 179.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 180.Renner W, Pilger E. Simultaneous in vivo quantitation of vascular endothelial growth factor mRNA splice variants. J Vasc Res. 1999;36(2):133–8. doi: 10.1159/000025636. [DOI] [PubMed] [Google Scholar]
- 181.Sato Y, et al. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann N Y Acad Sci. 2000;902:201–5. doi: 10.1111/j.1749-6632.2000.tb06314.x. discussion 205–7. [DOI] [PubMed] [Google Scholar]
- 182.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. Exs. 2005;(94):209–31. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- 183.Rudlowski C, et al. Prognostic significance of vascular endothelial growth factor expression in ovarian cancer patients: a long-term follow-up. Int J Gynecol Cancer. 2006;16(Suppl 1):183–9. doi: 10.1111/j.1525-1438.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- 184.Cai W, et al. PET of vascular endothelial growth factor receptor expression. J Nucl Med. 2006;47(12):2048–56. [PubMed] [Google Scholar]
- 185.Hsu AR, et al. Multimodality molecular imaging of glioblastoma growth inhibition with vasculature-targeting fusion toxin VEGF121/rGel. J Nucl Med. 2007;48(3):445–54. [PubMed] [Google Scholar]
- 186.Rodriguez-Porcel M, et al. Imaging of VEGF receptor in a rat myocardial infarction model using PET. J Nucl Med. 2008;49(4):667–73. doi: 10.2967/jnumed.107.040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cai W, et al. Positron emission tomography imaging of poststroke angiogenesis. Stroke. 2009;40(1):270–7. doi: 10.1161/STROKEAHA.108.517474. [DOI] [PubMed] [Google Scholar]
- 188.Chan C, et al. A human transferrin-vascular endothelial growth factor (hnTf-VEGF) fusion protein containing an integrated binding site for (111)In for imaging tumor angiogenesis. J Nucl Med. 2005;46(10):1745–52. [PubMed] [Google Scholar]
- 189.Danen EH. Integrins: regulators of tissue function and cancer progression. Curr Pharm Des. 2005;11(7):881–91. doi: 10.2174/1381612053381756. [DOI] [PubMed] [Google Scholar]
- 190.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2(2):91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 191.Kumar CC. Integrin alpha v beta 3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr Drug Targets. 2003;4(2):123–31. doi: 10.2174/1389450033346830. [DOI] [PubMed] [Google Scholar]
- 192.Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer. 2002;2(2):83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 193.Cai W, et al. In vitro and In vivo Characterization of 64Cu-Labeled AbegrinTM, a Humanized Monoclonal Antibody against Integrin {alpha}v{beta}3. Cancer Res. 2006;66(19):9673–81. doi: 10.1158/0008-5472.CAN-06-1480. [DOI] [PubMed] [Google Scholar]
- 194.Cai W, Chen X. Anti-angiogenic cancer therapy based on integrin alphavbeta3 antagonism. Anticancer Agents Med Chem. 2006;6(5):407–28. doi: 10.2174/187152006778226530. [DOI] [PubMed] [Google Scholar]
- 195.Wu Y, et al. microPET imaging of glioma integrin {alpha}v{beta}3 expression using (64)Cu-labeled tetrameric RGD peptide. J Nucl Med. 2005;46(10):1707–18. [PubMed] [Google Scholar]
- 196.Chen X, et al. Integrin alpha v beta 3-targeted imaging of lung cancer. Neoplasia. 2005;7(3):271–9. doi: 10.1593/neo.04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cai W, et al. A thiol-reactive 18F-labeling agent, N-[2-(4-18F-fluorobenzamido)ethyl]maleimide, and synthesis of RGD peptide-based tracer for PET imaging of alpha v beta 3 integrin expression. J Nucl Med. 2006;47(7):1172–80. [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang X, et al. Quantitative PET imaging of tumor integrin alphavbeta3 expression with 18F-FRGD2. J Nucl Med. 2006;47(1):113–21. [PMC free article] [PubMed] [Google Scholar]
- 199.Pendas AM, et al. Diet-induced obesity and reduced skin cancer susceptibility in matrix metalloproteinase 19-deficient mice. Mol Cell Biol. 2004;24(12):5304–13. doi: 10.1128/MCB.24.12.5304-5313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wagner S, et al. Molecular imaging of matrix metalloproteinases in vivo using small molecule inhibitors for SPECT and PET. Curr Med Chem. 2006;13(23):2819–38. doi: 10.2174/092986706778522002. [DOI] [PubMed] [Google Scholar]
- 201.Medina OP, et al. Radionuclide imaging of tumor xenografts in mice using a gelatinase-targeting peptide. Anticancer Res. 2005;25(1A):33–42. [PubMed] [Google Scholar]
- 202.Blankenberg FG. Recent advances in the imaging of programmed cell death. Curr Pharm Des. 2004;10(13):1457–67. doi: 10.2174/1381612043384790. [DOI] [PubMed] [Google Scholar]
- 203.Corsten MF, et al. Counting heads in the war against cancer: defining the role of annexin A5 imaging in cancer treatment and surveillance. Cancer Res. 2006;66(3):1255–60. doi: 10.1158/0008-5472.CAN-05-3000. [DOI] [PubMed] [Google Scholar]
- 204.Lahorte CM, et al. Apoptosis-detecting radioligands: current state of the art and future perspectives. Eur J Nucl Med Mol Imaging. 2004;31(6):887–919. doi: 10.1007/s00259-004-1555-4. [DOI] [PubMed] [Google Scholar]
- 205.Tait JF, et al. Improved detection of cell death in vivo with annexin V radiolabeled by site-specific methods. J Nucl Med. 2006;47(9):1546–53. [PubMed] [Google Scholar]
- 206.Rottey S, et al. Sequential 99mTc-hydrazinonicotinamide-annexin V imaging for predicting response to chemotherapy. J Nucl Med. 2006;47(11):1813–8. [PubMed] [Google Scholar]
- 207.Haberkorn U, et al. Investigation of a potential scintigraphic marker of apoptosis: radioiodinated Z-Val-Ala-DL-Asp(O-methyl)-fluoromethyl ketone. Nucl Med Biol. 2001;28(7):793–8. doi: 10.1016/s0969-8051(01)00247-5. [DOI] [PubMed] [Google Scholar]
- 208.Bauer C, et al. 131I-labeled peptides as caspase substrates for apoptosis imaging. J Nucl Med. 2005;46(6):1066–74. [PubMed] [Google Scholar]
- 209.Reshef A, et al. Small-molecule biomarkers for clinical PET imaging of apoptosis. J Nucl Med. 51(6):837–40. doi: 10.2967/jnumed.109.063917. [DOI] [PubMed] [Google Scholar]
- 210.Madar I, et al. Detection and quantification of the evolution dynamics of apoptosis using the PET voltage sensor 18F-fluorobenzyl triphenyl phosphonium. J Nucl Med. 2009;50(5):774–80. doi: 10.2967/jnumed.108.061283. [DOI] [PubMed] [Google Scholar]
- 211.Cohen A, et al. From the Gla domain to a novel small-molecule detector of apoptosis. Cell Res. 2009;19(5):625–37. doi: 10.1038/cr.2009.17. [DOI] [PubMed] [Google Scholar]
- 212.Harrison LB, et al. Impact of tumor hypoxia and anemia on radiation therapy outcomes. Oncologist. 2002;7(6):492–508. doi: 10.1634/theoncologist.7-6-492. [DOI] [PubMed] [Google Scholar]
- 213.Chapman JD. Hypoxic sensitizers--implications for radiation therapy. N Engl J Med. 1979;301(26):1429–32. doi: 10.1056/NEJM197912273012606. [DOI] [PubMed] [Google Scholar]
- 214.Denny WA. Prodrug strategies in cancer therapy. Eur J Med Chem. 2001;36(7–8):577–95. doi: 10.1016/s0223-5234(01)01253-3. [DOI] [PubMed] [Google Scholar]
- 215.Rajendran JG, Mankoff DA. Beyond Detection: Novel Applications for PET Imaging to Guide Cancer Therapy. J Nucl Med. 2007;48(6):855–6. doi: 10.2967/jnumed.107.039768. [DOI] [PubMed] [Google Scholar]
- 216.Rischin D, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006;24(13):2098–104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]
- 217.Rajendran JG, et al. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res. 2006;12(18):5435–41. doi: 10.1158/1078-0432.CCR-05-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Piert M, et al. Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J Nucl Med. 2005;46(1):106–13. [PubMed] [Google Scholar]
- 219.Beck R, et al. Pretreatment 18F-FAZA PET Predicts Success of Hypoxia-Directed Radiochemotherapy Using Tirapazamine. J Nucl Med. 2007;48(6):973–980. doi: 10.2967/jnumed.106.038570. [DOI] [PubMed] [Google Scholar]
- 220.Yuan H, et al. Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) J Nucl Med. 2006;47(6):989–98. [PubMed] [Google Scholar]
- 221.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 222.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 223.Baselga J, Hammond LA. HER-targeted tyrosine-kinase inhibitors. Oncology. 2002;63(Suppl 1):6–16. doi: 10.1159/000066198. [DOI] [PubMed] [Google Scholar]
- 224.Nahta R, Hortobagyi GN, Esteva FJ. Growth factor receptors in breast cancer: potential for therapeutic intervention. Oncologist. 2003;8(1):5–17. doi: 10.1634/theoncologist.8-1-5. [DOI] [PubMed] [Google Scholar]
- 225.Selvaggi G, et al. Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer. Ann Oncol. 2004;15(1):28–32. doi: 10.1093/annonc/mdh011. [DOI] [PubMed] [Google Scholar]
- 226.Witton CJ, et al. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200(3):290–7. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 227.Memon AA, et al. The relation between survival and expression of HER1 and HER2 depends on the expression of HER3 and HER4: a study in bladder cancer patients. Br J Cancer. 2006;94(11):1703–9. doi: 10.1038/sj.bjc.6603154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Velikyan I, et al. Preparation and evaluation of (68)Ga-DOTA-hEGF for visualization of EGFR expression in malignant tumors. J Nucl Med. 2005;46(11):1881–8. [PubMed] [Google Scholar]
- 229.Reilly RM, et al. Preclinical pharmacokinetic, biodistribution, toxicology, and dosimetry studies of 111In-DTPA-human epidermal growth factor: an auger electron-emitting radiotherapeutic agent for epidermal growth factor receptor-positive breast cancer. J Nucl Med. 2006;47(6):1023–31. [PubMed] [Google Scholar]
- 230.Cai W, et al. Quantitative PET of EGFR expression in xenograft-bearing mice using (64)Cu-labeled cetuximab, a chimeric anti-EGFR monoclonal antibody. Eur J Nucl Med Mol Imaging. 2007;34(6):850–8. doi: 10.1007/s00259-006-0361-6. [DOI] [PubMed] [Google Scholar]
- 231.Steffen AC, et al. In vitro characterization of a bivalent anti-HER-2 affibody with potential for radionuclide-based diagnostics. Cancer Biother Radiopharm. 2005;20(3):239–48. doi: 10.1089/cbr.2005.20.239. [DOI] [PubMed] [Google Scholar]
- 232.Orlova A, et al. Comparative in vivo evaluation of technetium and iodine labels on an anti-HER2 affibody for single-photon imaging of HER2 expression in tumors. J Nucl Med. 2006;47(3):512–9. [PubMed] [Google Scholar]
- 233.Engfeldt T, et al. Imaging of HER2-expressing tumours using a synthetic Affibody molecule containing the (99m)Tc-chelating mercaptoacetyl-glycyl-glycyl-glycyl (MAG3) sequence. Eur J Nucl Med Mol Imaging. 2007;34(5):722–33. doi: 10.1007/s00259-006-0266-4. [DOI] [PubMed] [Google Scholar]
- 234.Smith-Jones PM, et al. Early tumor response to Hsp90 therapy using HER2 PET: comparison with 18F-FDG PET. J Nucl Med. 2006;47(5):793–6. [PMC free article] [PubMed] [Google Scholar]
- 235.Levashova Z, et al. SPECT and PET imaging of EGF receptors with site-specifically labeled EGF and dimeric EGF. Bioconjug Chem. 2009;20(4):742–9. doi: 10.1021/bc800443w. [DOI] [PubMed] [Google Scholar]
- 236.Mariani G, Erba PA, Signore A. Receptor-mediated tumor targeting with radiolabeled peptides: there is more to it than somatostatin analogs. J Nucl Med. 2006;47(12):1904–7. [PubMed] [Google Scholar]
- 237.Schillaci O. Somatostatin receptor imaging in patients with neuroendocrine tumors: not only SPECT? J Nucl Med. 2007;48(4):498–500. doi: 10.2967/jnumed.106.038653. [DOI] [PubMed] [Google Scholar]
- 238.Bodei L, Paganelli G, Mariani G. Receptor radionuclide therapy of tumors: a road from basic research to clinical applications. J Nucl Med. 2006;47(3):375–7. [PubMed] [Google Scholar]
- 239.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30(5):781–93. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 240.Ginj M, et al. Preclinical evaluation of new and highly potent analogues of octreotide for predictive imaging and targeted radiotherapy. Clin Cancer Res. 2005;11(3):1136–45. [PubMed] [Google Scholar]
- 241.Cescato R, et al. Internalization of sst2, sst3, and sst5 receptors: effects of somatostatin agonists and antagonists. J Nucl Med. 2006;47(3):502–11. [PubMed] [Google Scholar]
- 242.Scarpignato C, Pelosini I. Somatostatin analogs for cancer treatment and diagnosis: an overview. Chemotherapy. 2001;47(Suppl 2):1–29. doi: 10.1159/000049157. [DOI] [PubMed] [Google Scholar]
- 243.Lamberts SW, et al. Somatostatin-receptor imaging in the localization of endocrine tumors. N Engl J Med. 1990;323(18):1246–9. doi: 10.1056/NEJM199011013231805. [DOI] [PubMed] [Google Scholar]
- 244.Forrer F, et al. Neuroendocrine tumors. Peptide receptor radionuclide therapy. Best Pract Res Clin Endocrinol Metab. 2007;21(1):111–29. doi: 10.1016/j.beem.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 245.Storch D, et al. Evaluation of [99mTc/EDDA/HYNIC0]octreotide derivatives compared with [111In-DOTA0,Tyr3, Thr8]octreotide and [111In-DTPA0]octreotide: does tumor or pancreas uptake correlate with the rate of internalization? J Nucl Med. 2005;46(9):1561–9. [PubMed] [Google Scholar]
- 246.Guggenberg EV, et al. Radiopharmaceutical development of a freeze-dried kit formulation for the preparation of [99mTc-EDDA-HYNIC-D-Phe1, Tyr3]-octreotide, a somatostatin analog for tumor diagnosis. J Pharm Sci. 2004;93(10):2497–506. doi: 10.1002/jps.20148. [DOI] [PubMed] [Google Scholar]
- 247.Gabriel M, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48(4):508–18. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 248.Krenning EP, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20(8):716–31. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 249.Henze M, et al. Characterization of 68Ga-DOTA-D-Phe1-Tyr3-octreotide kinetics in patients with meningiomas. J Nucl Med. 2005;46(5):763–9. [PubMed] [Google Scholar]
- 250.Meisetschlager G, et al. Gluc-Lys([18F]FP)-TOCA PET in patients with SSTR-positive tumors: biodistribution and diagnostic evaluation compared with [111In]DTPA-octreotide. J Nucl Med. 2006;47(4):566–73. [PubMed] [Google Scholar]
- 251.Anderson CJ, et al. 64Cu-TETA-octreotide as a PET imaging agent for patients with neuroendocrine tumors. J Nucl Med. 2001;42(2):213–21. [PubMed] [Google Scholar]
- 252.Mottaghy FM, Reske SN. Functional imaging of neuroendocrine tumours with PET. Pituitary. 2006;9(3):237–42. doi: 10.1007/s11102-006-0269-y. [DOI] [PubMed] [Google Scholar]
- 253.Reubi JC, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27(3):273–82. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 254.Heppeler A, et al. Receptor targeting for tumor localisation and therapy with radiopeptides. Curr Med Chem. 2000;7(9):971–94. doi: 10.2174/0929867003374516. [DOI] [PubMed] [Google Scholar]
- 255.Rufini V, Calcagni ML, Baum RP. Imaging of neuroendocrine tumors. Semin Nucl Med. 2006;36(3):228–47. doi: 10.1053/j.semnuclmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 256.Forrer F, et al. Treatment with 177Lu-DOTATOC of patients with relapse of neuroendocrine tumors after treatment with 90Y-DOTATOC. J Nucl Med. 2005;46(8):1310–6. [PubMed] [Google Scholar]
- 257.van Essen M, et al. Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J Nucl Med. 2006;47(10):1599–606. [PubMed] [Google Scholar]
- 258.de Jong M, et al. Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. J Nucl Med. 2005;46(Suppl 1):13S–7S. [PubMed] [Google Scholar]
- 259.De Jong M, et al. Somatostatin receptor-targeted radionuclide therapy of tumors: preclinical and clinical findings. Semin Nucl Med. 2002;32(2):133–40. doi: 10.1053/snuc.2002.31027. [DOI] [PubMed] [Google Scholar]
- 260.Shields AF, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4(11):1334–6. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 261.Mankoff DA, Shields AF, Krohn KA. PET imaging of cellular proliferation. Radiol Clin North Am. 2005;43(1):153–67. doi: 10.1016/j.rcl.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 262.Leyton J, et al. In vivo biological activity of the histone deacetylase inhibitor LAQ824 is detectable with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Cancer Res. 2006;66(15):7621–9. doi: 10.1158/0008-5472.CAN-05-3962. [DOI] [PubMed] [Google Scholar]
- 263.McLaughlin F, La Thangue NB. Histone deacetylase inhibitors open new doors in cancer therapy. Biochem Pharmacol. 2004;68(6):1139–44. doi: 10.1016/j.bcp.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 264.Leyton J, et al. Early detection of tumor response to chemotherapy by 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography: the effect of cisplatin on a fibrosarcoma tumor model in vivo. Cancer Res. 2005;65(10):4202–10. doi: 10.1158/0008-5472.CAN-04-4008. [DOI] [PubMed] [Google Scholar]
- 265.Boulikas T, Vougiouka M. Recent clinical trials using cisplatin, carboplatin and their combination chemotherapy drugs (review) Oncol Rep. 2004;11(3):559–95. [PubMed] [Google Scholar]
- 266.Leyton J, et al. Quantifying the activity of adenoviral E1A CR2 deletion mutants using renilla luciferase bioluminescence and 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography imaging. Cancer Res. 2006;66(18):9178–85. doi: 10.1158/0008-5472.CAN-06-1539. [DOI] [PubMed] [Google Scholar]
- 267.Waldherr C, et al. Monitoring antiproliferative responses to kinase inhibitor therapy in mice with 3′-deoxy-3′-18F-fluorothymidine PET. J Nucl Med. 2005;46(1):114–20. [PubMed] [Google Scholar]
- 268.Yang YJ, et al. Use of 3′-deoxy-3′-[18F]fluorothymidine PET to monitor early responses to radiation therapy in murine SCCVII tumors. Eur J Nucl Med Mol Imaging. 2006;33(4):412–9. doi: 10.1007/s00259-005-0011-4. [DOI] [PubMed] [Google Scholar]
- 269.Yap CS, et al. Evaluation of thoracic tumors with 18F-fluorothymidine and 18F-fluorodeoxyglucose-positron emission tomography. Chest. 2006;129(2):393–401. doi: 10.1378/chest.129.2.393. [DOI] [PubMed] [Google Scholar]
- 270.Masters C. Neuropathology of Alzheimer’s Disease. In: Burns A, O’Brien J, Ames D, editors. Dememtia. London: Hodder Arnold; 2005. pp. 393–407. [Google Scholar]
- 271.Klunk WE, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 272.Lin K-J, et al. Whole-body biodistribution and brain PET imaging with [18F]AV-45, a novel amyloid imaging agent- a pilot study. Nuclear Medicine and Biology. 2010;37:497–508. doi: 10.1016/j.nucmedbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 273.Choi SR, et al. Preclinical Properties of 18F-AV-45: a PET imaging agent for mapping beta-amyloid plagues in the brain. Journal of Nuclear Medicine. 2009;50:1887–1894. doi: 10.2967/jnumed.109.065284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ono M. Development of positron-emission tomography/single-photon emission computed tomography imaging probes for in vivo detection of beta-amyloid plaques in Alzheimer’s brains. Chem Pharm Bull (Tokyo) 2009;57(10):1029–39. doi: 10.1248/cpb.57.1029. [DOI] [PubMed] [Google Scholar]
- 275.Kung MP, et al. Characterization of IMPY as a potential imaging agent for beta-amyloid plaques in double transgenic PSAPP mice. Eur J Nucl Med Mol Imaging. 2004;31(8):1136–45. doi: 10.1007/s00259-004-1487-z. [DOI] [PubMed] [Google Scholar]
- 276.Hamstra DA, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol. 2008;26(20):3387–94. doi: 10.1200/JCO.2007.15.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]