Abstract
Pediatric kidney transplant recipients experience a high-risk age window of increased graft loss during late adolescence and early adulthood that has been attributed primarily to sociobehavioral mechanisms such as non-adherence. An examination of how this age window affects recipients of other organs may inform the extent to which sociobehavioral mechanisms are to blame or whether kidney-specific biologic mechanisms may also exist. Graft loss risk across current recipient age was compared between pediatric kidney (n=17, 446), liver (n=12, 161), and simultaneous liver-kidney (n=224) transplants using piecewise-constant hazard rate models. Kidney graft loss during late adolescence and early adulthood (ages 17–24 years) was significantly greater than during ages <17 (aHR=1.79, 95%CI=1.69–1.90, p<0.001) and ages >24 (aHR=1.11, 95%CI=1.03–1.20, p=0.005). In contrast, liver graft loss during ages 17–24 was no different than during ages <17 (aHR=1.03, 95%CI=0.92–1.16, p=0.6) or ages >24 (aHR=1.18, 95%CI=0.98–1.42, p=0.1). In simultaneous liver-kidney recipients, a trend toward increased kidney compared to liver graft loss was observed during ages 17–24 years. Late adolescence and early adulthood are less detrimental to pediatric liver grafts compared to kidney grafts, suggesting that sociobehavioral mechanisms alone may be insufficient to create the high-risk age window and that additional biologic mechanisms may also be required.
INTRODUCTION
Graft survival in pediatric kidney transplant (KT) recipients is strongly associated with recipient age. Recipients of a KT during their adolescent years have poorer long-term graft survival compared to recipients in other age groups (1–7). This disparity in graft survival is likely due to a significantly increased rate of graft loss during ages 17–24 (8, 9), a high-risk age window through which all pediatric KT recipients must eventually pass regardless of the age at which transplantation is performed.
The exact etiology of increased graft loss during the high-risk age window is unknown. Potential sociobehavioral explanations include poor adherence to immunosuppression (10–16), loss of insurance coverage (17–19), and transitions from pediatric to adult care (14, 20–25) during the patients’ teens to early twenties. Possible biologic mechanisms that may be specific to kidney grafts, such as hyperfiltration injury (26) during this period of increased growth or enhanced susceptibility to immunosuppression withdrawal, may also be involved or interact with the proposed sociobehavioral mechanisms. An examination of the extent to which the high-risk age window is equally detrimental to pediatric recipients of other transplants may shed light on which mechanisms are primarily at work in creating the high-risk age window in pediatric KT recipients.
If the mechanisms behind the high-risk age window of KT recipients are primarily non-biologic and not specific to the kidney, one would expect a similar high-risk age window for LT recipients given the likely similar difficulties with adherence, insurance, and care transitions. In addition, one would expect liver and kidney grafts in pediatric simultaneous liver-kidney (SLK) recipients to be similarly subject to graft loss during the high-risk age window. On the other hand, however, differential kidney and liver graft loss (especially within the same patient) would instead suggest that biologic in addition to sociobehavioral mechanisms must be involved. To better understand the mechanisms behind the high-risk age window after pediatric kidney transplantation, the objective of this study was to compare graft loss of pediatric KT, liver transplant (LT), and SLK transplant recipients during the high-risk age window of late adolescence and early adulthood.
MATERIALS AND METHODS
Study Population
This study utilized data from the Scientific Registry of Transplant Recipients (SRTR), a national registry of all solid organ transplants. The SRTR includes data on all donors, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere (27). The Health Resources and Services Administration, U.S. Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors.
All pediatric (recipient age less than 18 years old at time of transplantation) kidney-only, liver-only, and SLK recipients between October 1987 through February 2012 were identified in the SRTR. Based on clinical knowledge and precedent set by the SRTR program-specific regression models (available at www.srtr.org), etiology of renal disease was categorized as either focal segmental glomerular sclerosis (FSGS), other glomerular diseases, congenital anomalies of the kidney and urinary tract (CAKUT), or other/missing diagnosis. Etiology of liver disease was categorized as either biliary atresia, metabolic disease, acute hepatic necrosis, malignancy, or other/missing diagnosis.
Outcome Ascertainment
All-cause graft survival was defined as the time between transplantation and either graft loss (marked by either retransplanatation or additionally for KT recipients, a return to dialysis) or death, censoring for administrative end of study. All-cause graft survival was examined except where specified. In a sensitivity analysis, death-censored graft survival, defined as the time between transplantation and either graft loss (marked by retransplantation or a return to dialysis) or last date of follow-up with a functioning graft, with censoring for death and administrative end of study, was also examined. Death ascertainment was supplemented by linkage to the Social Security Death Master File; death and graft loss ascertainment were also supplemented by linkage to data from the Centers for Medicare and Medicaid Services.
Hazard Plots and Models
Survival analyses were conditioned on a minimum graft survival of six months to exclude the increased risk of graft loss in the immediate post-operative period. A sensitivity analysis without this six month conditioning was also performed. Graft failure rates were analyzed within various time intervals; specifically, seven-year graft failure rates were calculated to determine the likelihood of graft survival to age 24 given a functioning graft at age 17.
As previously reported (9), the risk of graft loss across age (in other words, the graft failure rate at a recipient’s current age) was graphically explored by plotting hazard functions against current recipient age (rather than the conventional post-transplant follow-up time). Age 0 (rather than date of transplantation) served as the time origin, with late entries into the risk set at each age of transplantation. The hazard function then provided the current graft failure rate at a given age conditional on graft survival up to that age. Stratified analyses were performed across donor type and age at transplantation to explore the possibility that differences in kidney and liver graft loss across age could be due to differences in donor type and timing of transplantation.
A piecewise-constant hazard rate model was used to quantify the hazard of graft loss across post-transplant age. This model is an exponential hazard model that assumes a constant hazard within pre-defined time segments and then estimates variation in hazard between time segments (28). Time segments were chosen as previously described (9). Given that the time axis in the analysis was current recipient age, the time segments therefore consisted of periods of age (rather than follow-up time), thus enabling a closer examination of the high-risk age window of ages 17–24 in comparison to the ages before and after this window.
A multivariable parameterization of the piecewise-constant hazard rate model was used to compare hazard between post-transplant age categories while adjusting for potential recipient (sex, race, insurance, diagnosis, previous transplant history), donor (living versus deceased, age, and race), and center-level (pediatric transplant volume) confounders, as well as the year of transplantation. Dialysis history, peak PRA, and HLA mismatch were additionally included in KT models. An unadjusted model was used to compare hazard in the SLK recipient group given the small sample size. All tests were two-sided with statistical significance set at α = 0.05. Analyses were performed using STATA 12.1/SE (College Station, Texas).
RESULTS
Study Population
A total of 17,446 KT recipients, 12,161 LT recipients, and 224 SLK recipients underwent transplantation during the study period (Table 1). The mean age at transplantation was older for KT recipients (11.6 years) compared to LT (5.4 years) and SLK (9.6 years) recipients. The most common known etiologies of disease were CAKUT for KT recipients and biliary atresia for LT recipients. Nearly half of the KT recipients received a living donor transplant (49.3%), while the proportion of LT recipients receiving a living donor transplant was only 11.6%. HLA mismatch was greater in LT and SLK recipients (80.2% and 87.2% having 4–6 mismatches, respectively) compared to KT recipients (55.6% having 3 or fewer mismatches).
Table 1.
Recipient, donor, transplant, and center characteristics for pediatric (<18 years old) kidney, liver, and simultaneous liver-kidney (SLK) transplants performed between 1987–2012.
| RECIPIENT | KIDNEY n= 17,446 |
LIVER n= 12,161 |
SLK n=224 |
|
|---|---|---|---|---|
| Mean Age (years) (SD) | 11.6 (5.1) | 5.4 (5.6) | 9.6 (5.3) | |
| Female Sex | 40.8% | 52.2% | 52.7% | |
| Race | ||||
| Caucasian | 57.7% | 57.7% | 63.8% | |
| African American | 18.6% | 17.9% | 9.8% | |
| Other | 23.7% | 24.3% | 26.4% | |
| Public Insurance | 64.1% | 56.1% | 54.3% | |
| Etiology of Disease | Kidney | Liver | ||
| FSGS | 10.4% | 1.3% | ||
| Other glomerular diseases | 14.3% | 3.1% | ||
| CAKUT | 34.9% | 25.9% | ||
| Other/missing | 40.4% | 27.9% | 69.6% | 45.5% |
| Biliary atresia | 42.9% | 4.0% | ||
| Metabolic disease | 11.9% | 45.5% | ||
| Acute hepatic necrosis | 12.6% | 2.7% | ||
| Malignancy | 4.7% | 2.2% | ||
| Previous Transplant | ||||
| Kidney | 11.4% | 0.2% | 15.6% | |
| Liver | 0.4% | 14.5% | 8.5% | |
| Simultaneous Kidney Liver | 0.1% | 0.1% | 2.7% | |
| DONOR | ||||
| Mean Age (years) (SD) | 29.8 (12.7) | 14.6 (14.7) | 14.5 (12.4) | |
| Race | ||||
| Caucasian | 68.5% | 66.5% | 66.5% | |
| African American | 12.7% | 15.8% | 16.1% | |
| Other | 18.8% | 17.7% | 17.4% | |
| Living Donor | 48.7% | 11.6% | 0.0% | |
| TRANSPLANT | ||||
| HLA Mismatch | ||||
| 0 | 4.2% | 0.5% | 0.0% | |
| 1–3 | 51.4% | 19.3% | 12.8% | |
| 4–6 | 44.4% | 80.2% | 87.2% | |
SD=standard deviation; FSGS=focal segmental glomerulosclerosis; CAKUT=congenital anomalies of the kidneys and urinary tract; HLA=human leukocyte antigen
Graft Loss in Kidney versus Liver Recipients
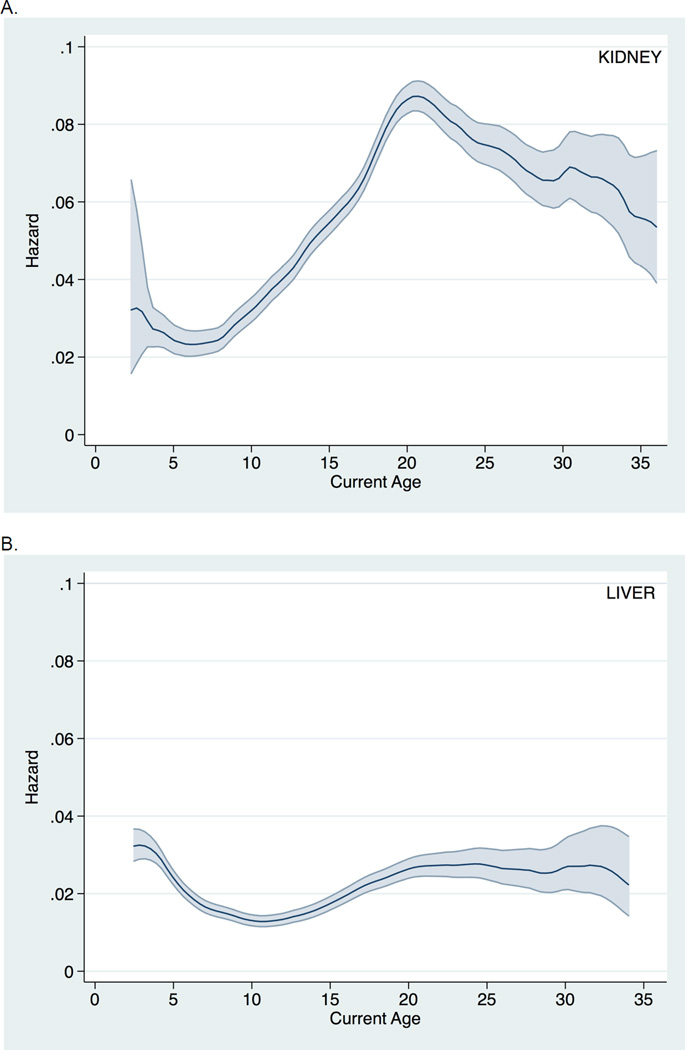
Graft loss among pediatric KT recipients (Figure 1A) was markedly elevated during late adolescence and early adulthood. In fact, in patients with a functioning graft at age 17, 43.3% were expected to lose the graft by age 24. Graft loss among pediatric LT recipients (Figure 1B) was less pronounced during late adolescence and early adulthood. In patients with a functioning graft at age 17, only 15.9% were expected to lose the graft by age 24.
Figure 1.
Hazard of all-cause graft loss across current recipient age (with 95% confidence interval) among pediatric A. kidney transplant recipients and B. liver transplant recipients.
After adjusting for recipient, donor, and transplant characteristics, the hazard of graft loss in KT recipients during ages 17–24 was significantly greater than that during ages 0–17 (aHR: 1.79, 95% CI: 1.69–1.89; p<0.001) and ages >24 (aHR 1.11, 95% CI: 1.03–1.20; p=0.005). However, the hazard of graft loss in LT recipients during ages 17–24 was not significantly different compared to ages 0–17 (aHR 1.03, 95% CI: 0.92–1.16; p=0.6) or ages >24 (aHR 1.18, 95% CI: 0.98–1.42; p=0.1).
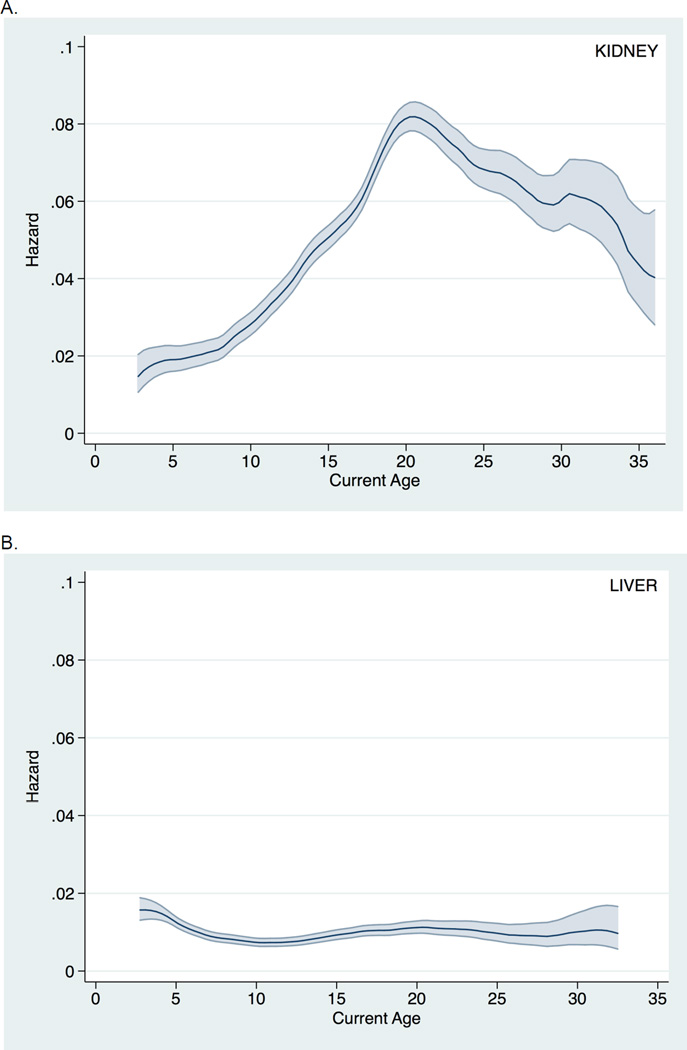
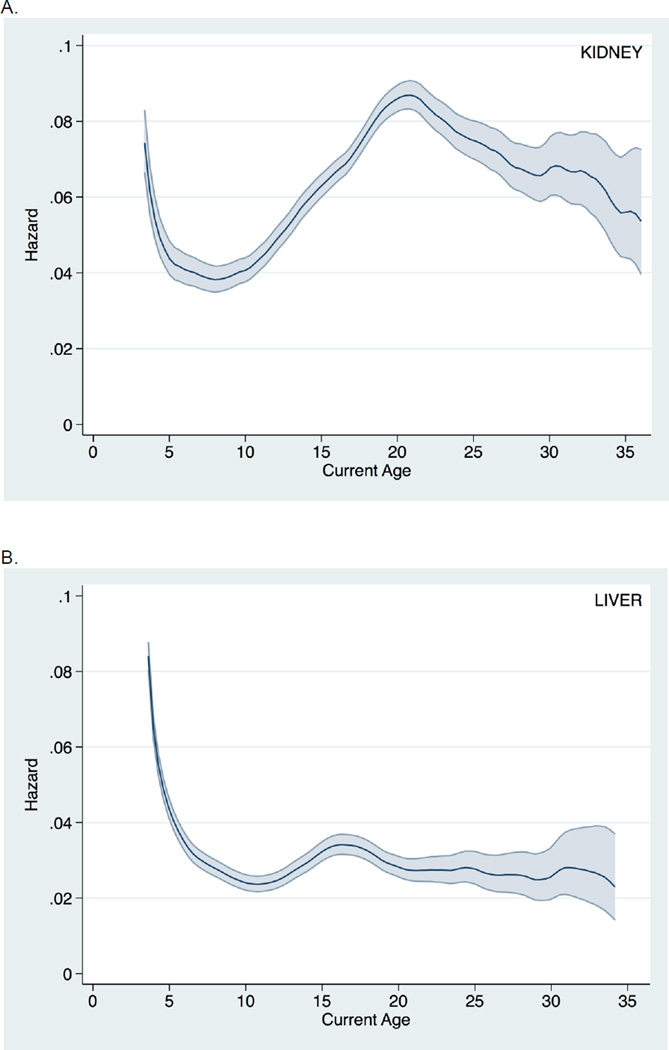
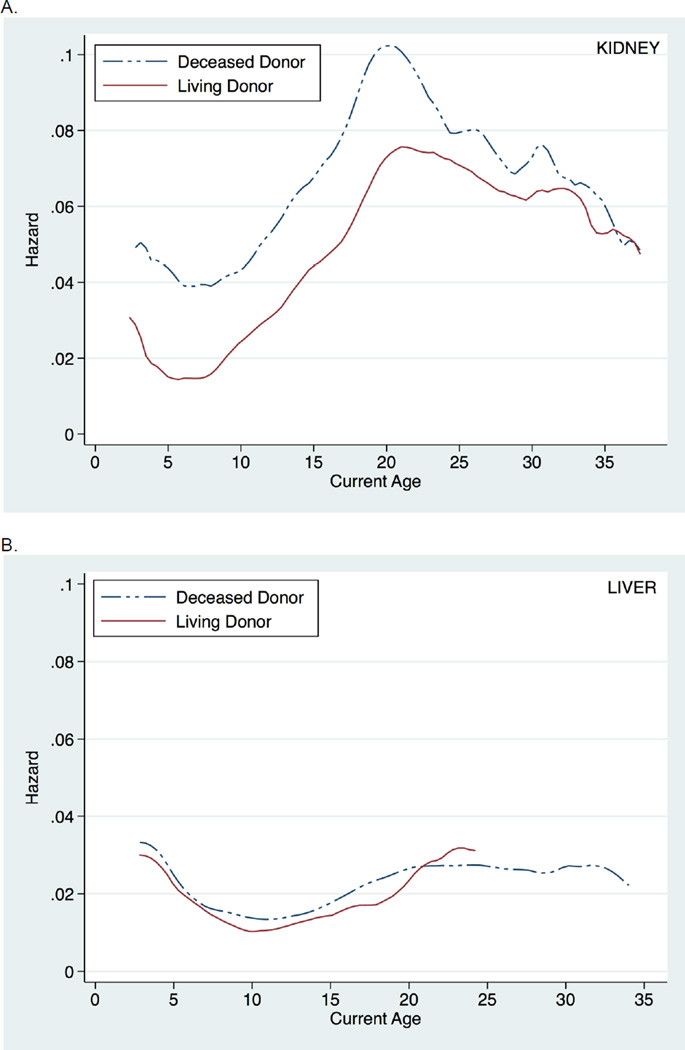
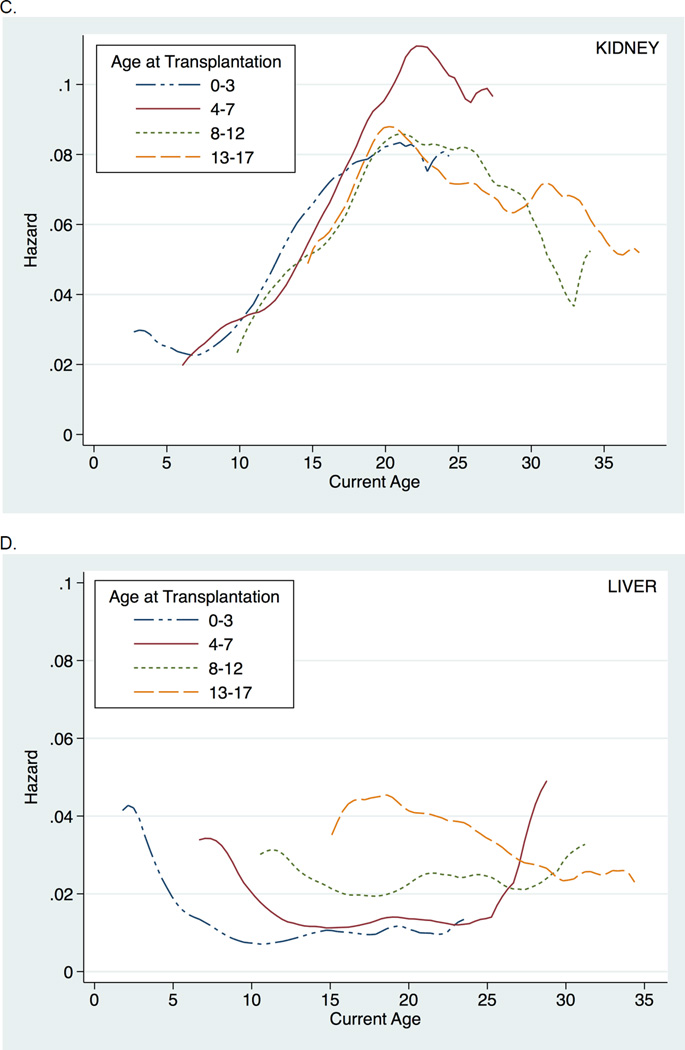
Similar findings were seen when death-censored graft survival was instead examined (Figure 2). Likewise, inferences were unchanged when examining all post-transplant follow-up time (i.e., without conditioning on a minimum graft survival of six months) (Figure 3). Finally, findings were consistent across donor type and recipient age at transplantation (Figure 4).
Figure 2.
Hazard of death-censored graft loss across current recipient age (with 95% confidence interval) among pediatric A. kidney transplant recipients and B. liver transplant recipients.
Figure 3.
Hazard of all-cause graft loss (without conditioning on a minimum graft survival of six months) across current recipient age (with 95% confidence interval) among pediatric A. kidney transplant recipients and B. liver transplant recipients.
Figure 4.
Hazard of all-cause graft loss across current recipient age, stratified by donor type and age at transplantation, among pediatric kidney (A. and C.) and liver (B. and D.) transplant recipients.
Graft Loss in Simultaneous Liver-Kidney Recipients
For graft loss among SLK recipients, kidney graft loss during ages 17–24 appeared to be accentuated compared to liver graft loss. In patients with a functioning kidney at age 17, 19.1% were expected to lose the kidney graft by age 24, and in patients with a functioning liver at age 17, 10.5% were expected to lose the liver graft by age 24. The hazard of kidney graft loss for ages 17–24 appeared to be greater (although not statistically so) compared to ages 0–17 (HR 1.47, 95% CI: 0.65–3.32; p=0.4) and was similar to ages >24 (HR 0.82, 95% CI: 0.26–2.58; p=0.7). The hazard of liver graft loss for ages 17–24 was similar to both ages 0–17 (HR 0.98, 95% CI: 0.40–2.36; p=0.9) and ages >24 (HR 0.86, 95% CI: 0.23–3.24; p=0.8).
DISCUSSION
In this national study of nearly 30,000 pediatric KT, LT, and SLK recipients, we found a significant difference in the rate at which kidney and liver grafts are lost during the high-risk age window of late adolescence and early adulthood (8, 9). Graft loss sharply peaks during this time period among KT recipients; however there is little change in the rate of graft loss across recipient age among LT recipients. Importantly, this finding was consistent in sensitivity analyses examining both death-censored graft survival (to focus specifically on graft failure given the suspected mechanism of non-adherence) and all post-transplant follow-up time (to avoid potential selection bias in examining only those with graft survival of at least six months). Among pediatric SLK recipients, a trend toward higher rates of kidney graft loss, compared to liver graft loss, was also observed during the high-risk age window, although the small sample size limited definitive conclusions.
The increased risk of graft loss during late adolescence and early adulthood among pediatric KT recipients has been attributed to lack of adherence to immunosuppression (10–16), alterations in health insurance coverage (17–19), and transitions from pediatric to adult care (14, 20–24). These issues are not unique to pediatric KT recipients, however. Pediatric LT recipients, as expected, have been found to have similar difficulties with immunosuppression adherence, insurance, and care transitions (29–32). While non-adherence between kidney and liver recipients may not be identical given the different immunosuppression regimens that are required, one would still expect to find at least some level of a high-risk age window among pediatric LT recipients, even if attenuated. However, no statistically appreciable increased risk of graft loss during these ages was identified among LT recipients.
The absence of a high-risk age window in pediatric LT recipients may be related to the biologic differences between kidney and liver grafts. The period of increased growth during adolescence could lead to hyperfiltration injury within kidney grafts, and thereby subsequent increased rates of graft loss, similar to the hyperfiltration injury thought to occur when kidneys from small donors are transplanted into large recipients (26). On the other hand, liver grafts may be able to sustain this period of growth without injury given the concurrent growth of liver grafts within pediatric recipients as they grow (33, 34). Liver grafts have been shown to develop significant histologic changes over time though, including signs of chronic hepatitis and fibrosis (35, 36). The exact clinical relevance of these histologic changes is not entirely clear, however; nor is it known if the incidence of these changes peaks within any particular age group, or rather if the changes just accumulate with a longer duration of time since transplantation.
The difference in kidney and liver graft loss during the high-risk age window may also be related to a reduced susceptibility to immunosuppression withdrawal (or sporadic usage) among liver grafts. Indeed, recent success has been found with early immunosuppression withdrawal among pediatric LT recipients (37, 38). In this way, our study could indirectly lend additional support to the promise of achieving tolerance within pediatric LT recipients. Finally, and perhaps most likely, the explanation for the difference in kidney and liver graft loss during the high-risk age window may be an interaction between any of the aforementioned potential mechanisms.
Pediatric KT and LT recipients have two other notable differences that may contribute to the difference in kidney and liver graft loss seen during the high-risk age window. Specifically, pediatric LT recipients more frequently receive deceased donor organs and are generally transplanted at younger ages compared to pediatric KT recipients, highlighting the importance of our sensitivity analyses showing that inferences were consistent across donor type and recipient age at transplantation. In examining graft loss risk stratified by donor type, living donor organs among pediatric LT recipients, in contrast to among pediatric KT recipients, appeared to have little benefit in terms of graft survival compared to deceased donor organs, consistent with other studies showing similar graft survival between deceased donor and living donor LT in pediatric recipients (39, 40). This may reflect differential sensitivity to cold ischemia, importance of HLA matching, or importance of technical issues related to graft types or other differences between LT and KT. Also, stratification by age at transplantation showed that despite transplantation at different ages, pediatric KT grafts are most at risk during late adolescence and early adulthood, in contrast to the relatively stable risk seen among pediatric LT grafts. This finding further supports the hypothesis that kidney grafts, regardless of the time since transplantation, may be especially vulnerable to the increasing physiologic demands placed on the graft with the significant growth and maturation that occurs during late adolescence and early adulthood, a vulnerability that may be absent or diminished among liver grafts.
Pediatric SLK recipients provide a unique study population to compare the differential kidney and liver graft loss during the high-risk age window. SLK recipients essentially provide the desired counterfactual comparison between kidney and liver grafts; in other words, SLK recipients enable comparison of two organs within an otherwise identical patient in an otherwise identical behavioral, environmental, and social setting (in fact, the same patient in the same setting). If differential kidney and liver graft loss within the same patient were to be observed, it would provide strong evidence that biologic mechanisms in addition to social mechanisms are likely involved in creating the high-risk age window seen among pediatric KT recipients. Although the sample size for SLK recipients in this study was quite small, a trend toward increased kidney graft loss compared to liver graft loss during the high-risk age window did appear.
This study is limited by the small sample size of the SLK recipient group and the resulting difficulty in providing a robust statistical comparison between kidney and liver graft loss in these recipients. However, the large sample sizes for the KT and LT recipient groups still provide ample and robust evidence of a clear difference in pediatric kidney and liver graft loss during the high-risk age window. In addition, the study is limited by the variables available in the SRTR. Ideally, graft loss during the high-risk age window could be compared both across organ type and across varying degrees of immunosuppression adherence and insurance coverage. The SRTR unfortunately lacks granularity with respect to both adherence and insurance status and their changes over time, as well as other important factors such as the timing of the care transition process, the specific immunosuppression regimens used, and recipient cognitive function.
In conclusion, our comparison of graft loss in pediatric KT, LT, and SLK recipients found that the high-risk age window of late adolescence and early adulthood is significantly less detrimental in terms of liver graft loss compared to kidney graft loss. This finding suggests that sociobehavioral mechanisms alone may be insufficient to create a high-risk age window after pediatric transplantation. Instead an interaction between the previously-suggested sociobehavioral mechanisms and other biologic mechanisms, such as differential susceptibility to immunosuppression withdrawal, may be responsible for the exceptionally high rate of graft loss seen among pediatric KT recipients during late adolescence and early adulthood.
ACKNOWLEDGMENTS
This work was supported in part by grant number K24DK101828 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This research was presented in preliminary form as an oral abstract at the 2013 American Society of Transplant Surgeons State of the Art Winter Symposium and the 2013 American Transplant Congress. The Minneapolis Medical Research Foundation as the contractor for the SRTR has supplied the data reported here. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Abbreviations
- KT
Kidney Transplant
- SLK
Simultaneous Liver Kidney
- LT
Liver Transplant
- SRTR
Scientific Registry of Transplant Recipients
- OPTN
Organ Procurement and Transplantation Network
- FSGS
Focal Segmental Glomerular Sclerosis
- CAKUT
Congenital Anomalies of the Kidney and Urinary Tract
- PRA
Panel Reactive Antibody
- HLA
Human Leukocyte Antigen
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Gjertson DW, Cecka JM. Determinants of long-term survival of pediatric kidney grafts reported to the United Network for Organ Sharing kidney transplant registry. Pediatric transplantation. 2001;5(1):5–15. doi: 10.1034/j.1399-3046.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- 2.Cecka JM, Gjertson DW, Terasaki PI. Pediatric renal transplantation: a review of the UNOS data. United Network for Organ Sharing. Pediatr Transplant. 1997;1(1):55–64. [PubMed] [Google Scholar]
- 3.Smith JM, Ho PL, McDonald RA. Renal transplant outcomes in adolescents: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatric transplantation. 2002;6(6):493–499. doi: 10.1034/j.1399-3046.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 4.Hwang AH, Cho YW, Cicciarelli J, Mentser M, Iwaki Y, Hardy BE. Risk factors for short- and long-term survival of primary cadaveric renal allografts in pediatric recipients: a UNOS analysis. Transplantation. 2005;80(4):466–470. doi: 10.1097/01.tp.0000168090.19875.b0. [DOI] [PubMed] [Google Scholar]
- 5.Kiberd JA, Acott P, Kiberd BA. Kidney Transplant Survival in Pediatric and Young Adults. BMC Nephrol. 2011;12(1):54. doi: 10.1186/1471-2369-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magee JC, Bucuvalas JC, Farmer DG, Harmon WE, Hulbert-Shearon TE, Mendeloff EN. Pediatric transplantation. Am J Transplant. 2004;4(Suppl 9):54–71. doi: 10.1111/j.1600-6143.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 7.Harmon WE, McDonald RA, Reyes JD, Bridges ND, Sweet SC, Sommers CM, et al. Pediatric transplantation, 1994–2003. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(4 Pt 2):887–903. doi: 10.1111/j.1600-6135.2005.00834.x. [DOI] [PubMed] [Google Scholar]
- 8.Foster BJ, Dahhou M, Zhang X, Platt RW, Samuel SM, Hanley JA. Association between age and graft failure rates in young kidney transplant recipients. Transplantation. 2011;92(11):1237–1243. doi: 10.1097/TP.0b013e31823411d7. [DOI] [PubMed] [Google Scholar]
- 9.Van Arendonk KJ, James NT, Boyarsky BJ, Garonzik-Wang JM, Orandi BJ, Magee JC, et al. Age at graft loss after pediatric kidney transplantation: exploring the high-risk age window. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(6):1019–1026. doi: 10.2215/CJN.10311012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dew MA, Dabbs AD, Myaskovsky L, Shyu S, Shellmer DA, DiMartini AF, et al. Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation. 2009;88(5):736–746. doi: 10.1097/TP.0b013e3181b2a0e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rianthavorn P, Ettenger RB. Medication non-adherence in the adolescent renal transplant recipient: a clinician's viewpoint. Pediatr Transplant. 2005;9(3):398–407. doi: 10.1111/j.1399-3046.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 12.Shellmer DA, Dabbs AD, Dew MA. Medical adherence in pediatric organ transplantation: what are the next steps? Curr Opin Organ Transplant. 2011;16(5):509–514. doi: 10.1097/MOT.0b013e32834a8c89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant. 2010;14(5):603–613. doi: 10.1111/j.1399-3046.2010.01299.x. [DOI] [PubMed] [Google Scholar]
- 14.Watson AR. Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol. 2000;14(6):469–472. doi: 10.1007/s004670050794. [DOI] [PubMed] [Google Scholar]
- 15.Wolff G, Strecker K, Vester U, Latta K, Ehrich JH. Non-compliance following renal transplantation in children and adolescents. Pediatr Nephrol. 1998;12(9):703–708. doi: 10.1007/s004670050531. [DOI] [PubMed] [Google Scholar]
- 16.Dobbels F, Van Damme-Lombaert R, Vanhaecke J, De Geest S. Growing pains: non-adherence with the immunosuppressive regimen in adolescent transplant recipients. Pediatric transplantation. 2005;9(3):381–390. doi: 10.1111/j.1399-3046.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 17.Willoughby LM, Fukami S, Bunnapradist S, Gavard JA, Lentine KL, Hardinger KL, et al. Health insurance considerations for adolescent transplant recipients as they transition to adulthood. Pediatr Transplant. 2007;11(2):127–131. doi: 10.1111/j.1399-3046.2006.00639.x. [DOI] [PubMed] [Google Scholar]
- 18.White PH. Access to health care: health insurance considerations for young adults with special health care needs/disabilities. Pediatrics. 2002;110(6 Pt 2):1328–1335. [PubMed] [Google Scholar]
- 19.Schnitzler MA, Lentine KL, Burroughs TE, Irish WD, Brennan DC, Woodward RS. Consequences of the end of medicare coverage in pediatric renal transplant recipients. Am J Transplant. 2005;5:563–563. [Google Scholar]
- 20.Chaturvedi S, Jones CL, Walker RG, Sawyer SM. The transition of kidney transplant recipients: a work in progress. Pediatr Nephrol. 2009;24(5):1055–1060. doi: 10.1007/s00467-009-1124-y. [DOI] [PubMed] [Google Scholar]
- 21.Magee JC, Thomas SE, Fredericks EM, Guidinger MK, Port FK, Kalbfleisch JD, et al. Effect of Recipient Age and "Transition" on Graft Loss in Pediatric Transplant Recipients. Transplantation. 2006;82(1):214. [Google Scholar]
- 22.Samuel SM, Nettel-Aguirre A, Hemmelgarn BR, Tonelli MA, Soo A, Clark C, et al. Graft failure and adaptation period to adult healthcare centers in pediatric renal transplant patients. Transplantation. 2011;91(12):1380–1385. doi: 10.1097/TP.0b013e31821b2f4b. [DOI] [PubMed] [Google Scholar]
- 23.Watson AR, Harden P, Ferris M, Kerr PG, Mahan J, Ramzy MF. Transition from pediatric to adult renal services: a consensus statement by the International Society of Nephrology (ISN) and the International Pediatric Nephrology Association (IPNA) Pediatr Nephrol. 2011;26(10):1753–1757. doi: 10.1007/s00467-011-1981-z. [DOI] [PubMed] [Google Scholar]
- 24.Foster BJ, Platt RW, Dahhou M, Zhang X, Bell LE, Hanley JA. The impact of age at transfer from pediatric to adult-oriented care on renal allograft survival. Pediatr Transplant. 2011;15(7):750–759. doi: 10.1111/j.1399-3046.2011.01567.x. [DOI] [PubMed] [Google Scholar]
- 25.Harden PN, Walsh G, Bandler N, Bradley S, Lonsdale D, Taylor J, et al. Bridging the gap: an integrated paediatric to adult clinical service for young adults with kidney failure. BMJ. 2012;344:e3718. doi: 10.1136/bmj.e3718. [DOI] [PubMed] [Google Scholar]
- 26.el-Agroudy AE, Hassan NA, Bakr MA, Foda MA, Shokeir AA, Shehab el-Dein AB. Effect of donor/recipient body weight mismatch on patient and graft outcome in living-donor kidney transplantation. American journal of nephrology. 2003;23(5):294–299. doi: 10.1159/000072819. [DOI] [PubMed] [Google Scholar]
- 27.OPTN / SRTR 2010 Annual Data Report. Rockville, MD: Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR); 2011. [Google Scholar]
- 28.Sorensen JB. STPIECE: Stata module to estimate piecewise-constant hazard rate models. Boston, MA: Statistical Software Components, Boston College Department of Economics; 1999. [Google Scholar]
- 29.Annunziato RA, Emre S, Shneider B, Barton C, Dugan CA, Shemesh E. Adherence and medical outcomes in pediatric liver transplant recipients who transition to adult services. Pediatric transplantation. 2007;11(6):608–614. doi: 10.1111/j.1399-3046.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 30.Annunziato RA, Parkar S, Dugan CA, Barsade S, Arnon R, Miloh T, et al. Brief report: Deficits in health care management skills among adolescent and young adult liver transplant recipients transitioning to adult care settings. Journal of pediatric psychology. 2011;36(2):155–159. doi: 10.1093/jpepsy/jsp110. [DOI] [PubMed] [Google Scholar]
- 31.Fredericks EM, Magee JC, Opipari-Arrigan L, Shieck V, Well A, Lopez MJ. Adherence and health-related quality of life in adolescent liver transplant recipients. Pediatric transplantation. 2008;12(3):289–299. doi: 10.1111/j.1399-3046.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- 32.Pollock-Barziv SM, Finkelstein Y, Manlhiot C, Dipchand AI, Hebert D, Ng VL, et al. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatric transplantation. 2010;14(8):968–975. doi: 10.1111/j.1399-3046.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki S, Makuuchi M, Ishizone S, Matsunami H, Terada M, Kawarazaki H. Liver regeneration in recipients and donors after transplantation. Lancet. 1992;339(8793):580–581. doi: 10.1016/0140-6736(92)90867-3. [DOI] [PubMed] [Google Scholar]
- 34.Uslu Tutar N, Kirbas I, Ozturk A, Sevmis S, Kayahan Ulu EM, Coskun M, et al. Computed tomography volumetric follow-up of graft volume in living related liver recipients. Transplantation proceedings. 2007;39(4):1175–1177. doi: 10.1016/j.transproceed.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Ekong UD, Melin-Aldana H, Seshadri R, Lokar J, Harris D, Whitington PF, et al. Graft histology characteristics in long-term survivors of pediatric liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2008;14(11):1582–1587. doi: 10.1002/lt.21549. [DOI] [PubMed] [Google Scholar]
- 36.Evans HM, Kelly DA, McKiernan PJ, Hubscher S. Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology. 2006;43(5):1109–1117. doi: 10.1002/hep.21152. [DOI] [PubMed] [Google Scholar]
- 37.Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA : the journal of the American Medical Association. 2012;307(3):283–293. doi: 10.1001/jama.2011.2014. [DOI] [PubMed] [Google Scholar]
- 38.Koshiba T, Li Y, Takemura M, Wu Y, Sakaguchi S, Minato N, et al. Clinical, immunological, and pathological aspects of operational tolerance after pediatric living-donor liver transplantation. Transplant immunology. 2007;17(2):94–97. doi: 10.1016/j.trim.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Diamond IR, Fecteau A, Millis JM, Losanoff JE, Ng V, Anand R, et al. Impact of graft type on outcome in pediatric liver transplantation: a report From Studies of Pediatric Liver Transplantation (SPLIT) Annals of surgery. 2007;246(2):301–310. doi: 10.1097/SLA.0b013e3180caa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burgos L, Hernandez F, Barrena S, Andres AM, Encinas JL, Leal N, et al. Variant techniques for liver transplantation in pediatric programs. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie. 2008;18(6):372–374. doi: 10.1055/s-2008-1038900. [DOI] [PubMed] [Google Scholar]