Abstract
Branching morphogenesis is thought to be governed by epithelial-stromal interactions, but the mechanisms underlying specification of branch location remain largely unknown. Prompted by the striking absence of Hedgehog (Hh) response at the sites of nascent buds in regenerating tubules of the adult prostate, we investigated the role of Hh signaling in adult prostate branching morphogenesis. We find that pathway activity is localized to stromal cells, and that its attenuation by genetic or pharmacologic manipulation leads to increased branching. Decreased pathway activity correlates with increased stromal production of Hepatocyte growth factor (Hgf), and we show that Hgf induces epithelial tubule branching. Regulation of Hgf expression by Hh signaling is indirect, mediated by Hh-induced expression of microRNAs miR-26a and miR-26b, which in turn down-regulate expression of Hgf. Prostate tubule branching thus may be initiated from regions of low Hh pathway activity, with implications for the prostatic hyperplasia commonly observed in late adulthood.
Introduction
Epithelial-stromal interactions are crucial for normal pattern formation in embryonic organ development and for the homeostatic maintenance of organ integrity in adults. Following bacterial injury of the adult bladder, for example, Sonic hedgehog (Shh) produced in cells of the basal urothelium elicits production of secreted factors from stromal cells, which in turn stimulate proliferation and differentiation of urothelial cells. This epithelial/stromal signal feedback circuit underlies injury-induced regeneration of the urothelium and restoration of its normal function1.
We focus here on growth and branching of the adult prostate. Our experimental system, the mouse prostate, comprises three paired lobes - the ventral, dorsolateral and anterior lobes - each consisting of a highly branched network of ductal tubules2. Castration results in loss of testosterone production and consequent involution of the prostate, largely through loss of distal branches. Testosterone replacement can then induce regeneration of new distal branches3, thus providing a model for adult growth and branching morphogenesis.
Regenerative prostate growth in the adult differs significantly from embryonic and postnatal prostate development, as embryonic epithelium grows and invades an extensive expanse of mesenchyme that surrounds the epithelial ducts. In contrast, adult epithelial ducts are encircled by thin, dense, stromal sheaths which are in turn held together by looser interductal connective tissue2. The importance of understanding adult prostate regeneration is highlighted by the excessive branching and growth associated with benign prostatic hyperplasia, a condition affecting most men over 50 years of age4.
Much previous study of the functional role of Hh signaling in prostate growth has focused on embryonic and early postnatal development5–13 with contradictory reports of inhibitory effects of Hh signaling9–11 or of decreased branching with Hh inhibition12,13, and recent support for a changing role during development8. These studies have been based largely on ex vivo cultures of embryonic or early postnatal prostate from mice or rats treated with recombinant proteins or drugs. These ex vivo conditions do not fully recapitulate in vivo processes, especially responses that may be specifically restricted to epithelial or stromal cells, and the physiological significance of these studies thus requires in vivo validation. A common feature of all these studies is that Hh signal response normally occurs in stromal cells during embryonic5,13 and early postnatal development11, and in adulthood14.
Although potential roles of Hh signaling during development have been suggested5,6,8–13, detailed spatial information regarding Hh signal response in relation to branched outgrowth of adult prostate has not been presented. In addition, evidence from several studies suggests that there may be a shift in prevailing expression from Sonic hedgehog (Shh) in the embryo11–13 to Indian hedgehog (Ihh) after birth6; production of Hh ligands from distinctly regulated genes thus may also contribute to distinct roles for Hh signaling in embryo and adult.
Results
Absence of stromal Hh response at the tips of nascent buds
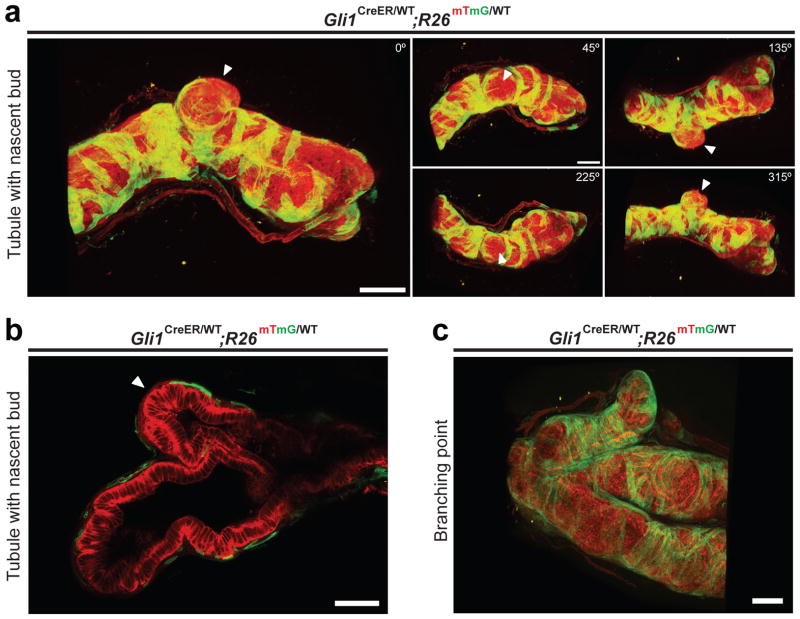
Previous work showed that Hh pathway response, as determined by expression of the Hh pathway target gene Gli1, occurs only in a subset of stromal cells14. To visualize the three-dimensional organization of Hh response during prostate regeneration, Gli1CreER/WT; R26mTmG/WT mice were castrated to cause prostate regression followed two weeks later by insertion of a testosterone pellet to induce regeneration (Supplementary Fig. 1a). Tamoxifen (TM) was administered three days after testosterone replacement for a period of four days to activate CreER and thus induce excision of mT (membrane-targeted tdTomato), leading to permanent marking of Gli1-expressing cells and their progeny by expression of mG (membrane-targeted EGFP). Prostates were harvested three days later and treated with a clearing solution15 to facilitate imaging of entire prostate ducts with two-photon microscopy, thus overcoming the limited imaging depth of confocal microscopy due to photo-bleaching and light scattering. Three-dimensional reconstruction of the two-photon images revealed that Gli1-positive, Hh-responsive cells are entirely stromal and are arranged in bands that enwrap the prostate tubules (Fig. 1a, Supplementary Fig. 1b and Supplementary Videos 1–4). Strikingly, Hh-response was conspicuously absent from stromal cells at the tips of nascent buds (best viewed in Supplementary Videos 1–4; see also Fig. 1a,b, and Supplementary Fig. 1b), but was present in stromal cells along the tubules, at branch points, or surrounding the ends of mature tubules that are not budding (Fig. 1c). These observations suggested that stromal regions lacking Hh pathway activity may promote formation of new prostate branches.
Figure 1. Absence of stromal Hh response at the tips of nascent buds.
(a,c) Three-dimensional reconstruction of two-photon images of a regenerating prostate tubule from a Gli1CreER/WT; R26mTmG prostate. (a) Arrowheads indicate the location of a nascent bud, which lacks Gli1-positive cells. Numbers indicate the degree of rotation of the tubule relative to the starting position. (c) A branch point with three mature tubules. Gli1-positive cells are located at the branch points and along tubules. (b) Optical section through a nascent bud, showing the absence of Gli1-positive stromal cells at the bud tip (arrowhead). Scale bars represent 100 μm.
Reduced Hh pathway activity increases tubulogenesis
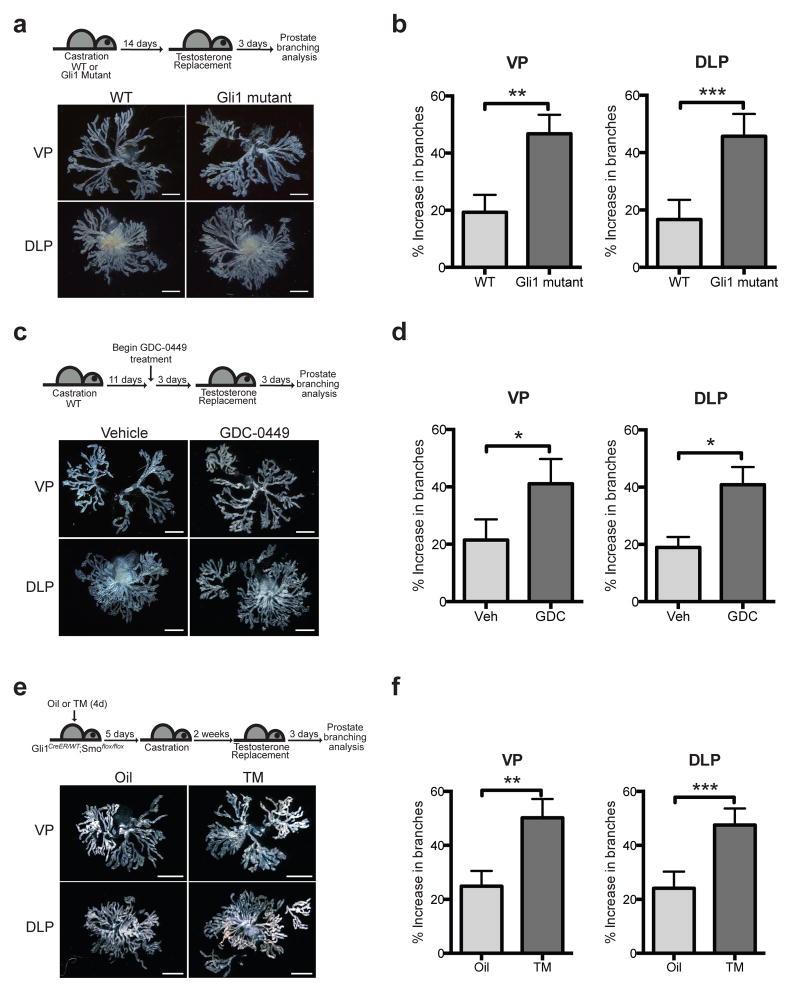
To examine the role of Hh pathway activity in branch formation during prostate regeneration, Gli1 mutant mice and wild type (WT) littermates were subjected to castration and androgen replacement (Fig. 2a), and three days later prostates were harvested. Each lobe was microdissected to display individual branches, which were counted and are presented as the % increase in branches relative to the number of tips that remain in involuted prostates. The % increase in branches upon regeneration more than doubled in Gli1−/− mutants relative to wild-type, with a 2.5-fold increase in the ventral prostate and a 2.9-fold increase in the dorsolateral prostate (Fig. 2b); attenuation of Hh pathway activity thus leads to increased prostate branching. Mice treated with the FDA-approved Smo antagonist, GDC-0449 (Vismodegib) beginning three days prior to androgen replacement and continuing throughout the duration of regeneration (Fig. 2c and Supplementary Fig. 1c) showed a 2.0-fold and 2.2-fold augmentation of the % increase in branches for the ventral and dorsolateral prostate, respectively, as compared to vehicle-treated mice (Fig. 2d).
Figure 2. Reduced Hh pathway activity increases tubulogenesis.
(a) Top: Experimental scheme describing castration and androgen replacement to compare Gli1 mutant and wild type (WT) prostate regeneration. Bottom: Representative images from microdissections of each prostate lobe. Gli1 mutant prostates have more branches than WT prostates. (b) Gli1 mutant ventral prostates (VP) showed a 2.5-fold augmentation in branches relative to WT (47 vs 19 % increase in branches) after testosterone replacement. Gli1 mutant dorsolateral prostates (DLP) showed a 2.9-fold augmentation in branching (43 vs 15 % increase in branches) after testosterone replacement. n = 6 pairs of VP or DLP. (c) Top: Experimental scheme describing the use of Hh pathway antagonist GDC-0449 (GDC) to study the effect of pathway inhibition on prostate branching in adulthood. GDC was administered every 12 hours for 6 consecutive days. Bottom: Representative images from microdissections of each prostate lobe. GDC-treated prostates have more branches than control prostates. (d) GDC-treated VP showed a 2.0-fold augmentation of branching relative to Vehicle (Veh)-treated control (41 vs 21 % increase in branches) after testosterone replacement. GDC-treated DLP showed a 2.2-fold augmentation of branching relative to Veh-treated control (41 vs 19 % increase in branches) after testosterone replacement. n = 3 pairs of VP or DLP. (e) Top: Experimental scheme describing the administration of oil or TM to Gli1CreER/WT; Smoflox/flox mice to ablate Smo in the prostate stroma prior to castration and regeneration. Bottom: Representative images from microdissections of each prostate lobe. TM-treated prostates have more branches than control prostates. (f) VP from TM-treated animals showed a 2.0-fold augmentation of branching relative to Veh-treated animals (50 vs 25 % increase in branches) after testosterone replacement. DLP from TM-treated animals showed a 2.0-fold augmentation of branching relative to Veh-treated animals (48 vs 24 % increase in branches) after testosterone replacement. n = 6 pairs of VP or DLP. (b,d,f) Data are presented as mean ± s.e.m., and significance was calculated by a paired Student’s t-test (*: p<0.05, **: p<0.01, ***: p<0.001). Scale bars represent 2 mm.
Cell layer specificity of Hh action was examined by administering TM to mice expressing CreER under control of the Gli1 promoter, which is active exclusively in stromal cells (see above); these mice also carried homozygous floxed alleles of the essential Hh pathway transductory component Smoothened (Smo) (Gli1CreER/WT; Smoflox/flox). With TM adminsitration prior to castration and testosterone replacement a similar 2.0-fold and 2.0-fold augmentation of the % increase in branching was observed in ventral and dorsolateral prostates respectively as compared to vehicle control mice (Fig. 2e,f and Supplementary Fig. 1d). Each of these three methods of Hh pathway attenuation consistently augments regenerative branching by at least 2-fold in an overall total of 60 samples and in a highly statistically significant manner. Attenuation of Hh pathway activity thus promotes branching, and the critical site of this effect is in the stromal cells that enwrap the growing tubules.
Hh pathway activity in stromal cells reduces Hgf levels
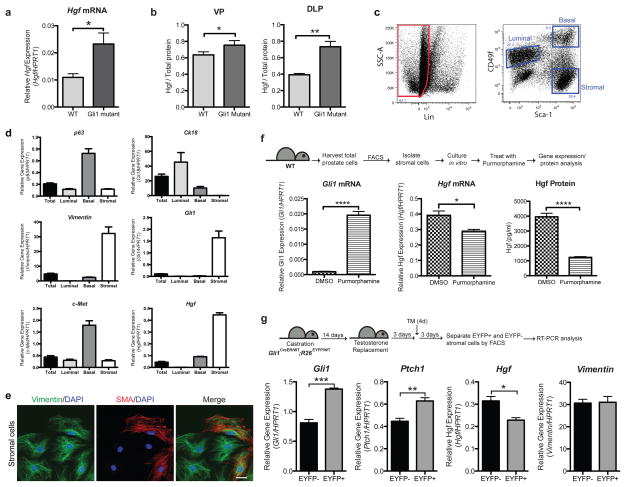
As branching morphogenesis necessarily involves the epithelium, we hypothesized that the effect of stromal Hh pathway response on prostate branching is mediated by a secreted factor. We performed a microarray analysis of RNA extracted from wild type or Gli1 mutant prostates after castration and 3 days of androgen replacement and found that 85 genes were up-regulated by at least 1.4-fold (p<0.01) in regenerating Gli1 mutant prostates as compared to WT. We selected genes annotated with the SP_PIR_Keyword “signal” and “secreted” to identify genes encoding secreted proteins involved in cell signaling (Supplementary Table 1) and found Hepatocyte growth factor (Hgf) as an attractive candidate for a prostate branching factor because it modulates branching of mammary gland16,17, lung18 and kidney19. We confirmed the microarray data by RT-PCR (Fig. 3a), and found that Hgf protein levels were also significantly higher in Gli1 mutant prostates as compared to WT (Fig. 3b).
Figure 3. Hh pathway activity in stromal cells leads to reduced Hgf levels.
(a) Expression of Hgf is 2.1-fold higher in Gli1 mutant compared to WT prostates after 3 days of testosterone replacement following castration. n=3 prostates per genotype. (b) Hgf protein levels in tissue lysates prepared from regenerating prostates, normalized to total lysate protein levels. There is a 1.2-fold increase in Hgf levels in Gli1 mutant compared to WT VP (n=5 prostates per genotype) and a 1.9-fold increase in Hgf levels in Gli1 mutant compared to WT DLP (n=5 prostates per genotype). (c) FACS plot showing the gate drawn for sorting of basal, luminal and stromal cell populations. (d) RT-PCR analysis of RNA from each sorted fraction. Gli1 is only expressed in the stromal cells. Hgf is enriched in the stromal population, and its receptor c-Met is enriched in the basal cell population. CK18, p63 and vimentin are markers for the luminal, basal and stromal populations respectively. ‘Total’ refers to RNA extracted from dissociated unsorted adult prostate cells. (e) Immunostaining of cultured prostate stromal cells with stromal markers vimentin and smooth muscle actin (SMA). Scale bar represents 10 μm. (f) Top: Experimental scheme describing the isolation and culture of prostate stromal cells to test the effect of Hh pathway stimulation on Hgf levels in vitro. Bottom: Treatment of prostate stromal cells with Hh pathway agonist purmorphamine led to an increase in Gli1 transcript levels, a 1.4-fold decrease in Hgf mRNA levels and a 4-fold decrease in secreted Hgf protein levels. n=4 wells per condition, one representative experiment of three is shown. (g) Top: Experimental scheme describing administration of TM to Gli1CreER/WT; R26EYFP/WT mice after testosterone replacement to label Gli1-positive cells during regeneration, allowing isolation of Gli1-positive and negative stromal cells by FACS (gated as in panel c). Bottom: RT-PCR analysis of RNA isolated from EYFP-positive and EYFP-negative stromal cells during prostate regeneration. n=3 technical replicates, one representative experiment of three is shown. (a,b,d,f,g) Data are presented as mean ± s.e.m., and significance was calculated by a unpaired Student’s t-test (*: p<0.05, **: p<0.01,***: p<0.001, ****: p<0.0001).
To investigate the cell layer specificity of gene expression we used an established FACS protocol to isolate epithelial basal and luminal cells as well as stromal cells from WT prostates (Fig. 3c)20 and immediately subjected them to RT-PCR analysis (Fig. 3d); stromal cell identity was confirmed by culture and staining with antibodies against vimentin and smooth muscle actin (SMA) (Fig. 3e). As expected, Gli1 is expressed primarily in stromal cells, which is also the predominant site of Hgf expression (Fig. 3d). Expression of the Hgf receptor c-Met in contrast was highest in epithelial cells, predominantly the basal fraction (Fig. 3d).
To directly determine if Hh pathway activity in stromal cells modulates Hgf levels, cultured stromal cells were treated with the Smoothened agonist, purmorphamine, or DMSO as a control (Fig. 3f). Following 24 hours of purmorphamine treatment we noted an increase in Gli1 transcripts; we also noted a corresponding decrease in levels of Hgf mRNA and in levels of HGF protein secreted into the medium (Fig. 3f). In addition, treatment with purmorphamine of NIH 3T3 cells, a mouse embryo fibroblast line known both to produce Hgf21 and to respond to Hh signaling22, led to decreased activity of conditioned medium in an MDCK cell tubulogenesis assay23,24 (Supplementary Fig. 2), consistent with a negative regulatory effect of Hh signaling on Hgf expression. Taken together, these data indicate an inhibitory relationship between Hh pathway activity and stromal levels of Hgf.
This relationship, in combination with two-photon images of regenerating prostates (Fig. 1a and Supplementary Fig. 1b), raise the interesting possibility that regions where stromal cells lack Hh response may correspond to regions with high levels of Hgf at the locations of new branches. To test this hypothesis, Gli1CreER/WT; R26EYFP/WT mice were castrated, testosterone pellets implanted, and three days later TM was administered on four consecutive days to label Gli1-expressing cells during regeneration. Following an additional three days for completion of TM-induced recombination, prostates were harvested and EYFP-positive and -negative stromal cells isolated by FACS (Fig. 3g). RT-PCR analyses of RNA extracted from these cells showed an increase in Gli1 and Ptch1 mRNA levels in the EYFP-positive cells (Fig. 3g), indicating that the population is enriched for cells that are responding to the Hh ligand. These EYFP-positive cells expressed lower levels of Hgf than EYFP-negative cells (Fig. 3g), suggesting that regions of low Hh response are regions of higher Hgf expression. The level of expression of the stromal marker Vimentin was unchanged between the two populations.
The difference in Gli1 mRNA between EYFP-positive and -negative stromal cells in this experiment, although clear and statistically significant, is not as great as in isolated stromal cells stimulated in vitro, possibly resulting from the 3–6 days between TM administration and prostate harvest. During this elapsed time Hh response patterns may change as branching and maturation of the regenerating prostate tubules proceeds, leading to an underestimate of the difference in pathway activity and in Hgf expression in isolated cells as compared to the actual difference in vivo at the time of recombination and marking.
Hgf induces prostate branching
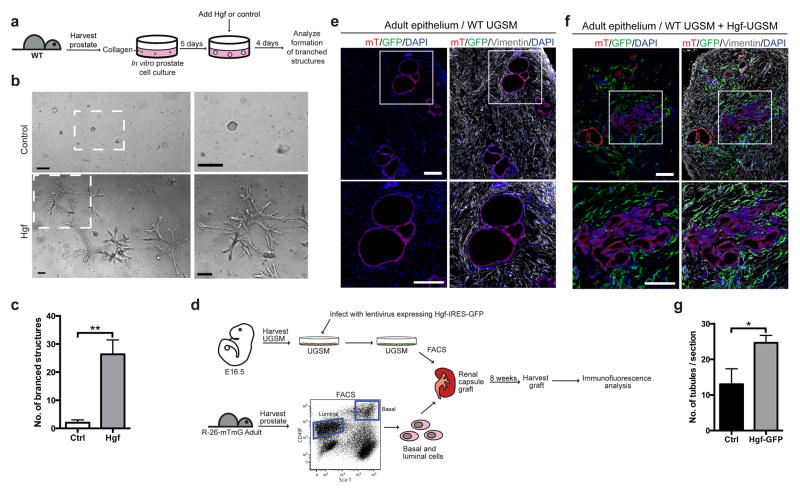
To determine whether Hgf induces prostate branching, dissociated prostate cells were cultured in vitro in three-dimensional cultures for 5 days until they formed spheres (Fig. 4a), after which recombinant Hgf protein was added to the media. Four days later we observed the formation of complex branched structures in Hgf-treated wells, but not in control conditions (Fig. 4b,c and Supplementary Video 5), demonstrating that Hgf acts on the prostate epithelium to induce branching morphogenesis. To investigate the possibility that Hgf induces prostate branching by stimulating cell proliferation, basal prostate epithelial cells were isolated and cultured in vitro until they formed small colonies, at which time recombinant Hgf protein was added. Two days later we found 1.9-fold more Hgf-treated cells, as compared to the control (Supplementary Fig. 3a), suggesting that Hgf indeed stimulates cell proliferation.
Figure 4. Hgf induces prostate branching.
(a) Experimental scheme describing the isolation and culture of prostate cells in vitro until they form spheres, then treatment of the spheres with recombinant Hgf protein or a control to determine if Hgf is able to induce branching. (b) Dissociated adult prostate cells were cultured in vitro for 5 days, then Hgf or a vehicle control was added to the media. 4 days after addition of Hgf, branched structures were observed, but were absent in the control-treated wells. The boxed area is shown enlarged on the right. Scale bars represent 200 μm. One representative of three is shown. (c) Quantification of the total number of branched structures formed in each well. n=3 wells per condition, one representative experiment of three is shown. (d) Experimental scheme to test the ability of Hgf to induce branching in renal capsule prostate reconsitution assays. Urogenital sinus mesenchyme (USGM) was harvested from E16.5 WT embryos, infected with lentivirus expressing Hgf and GFP, GFP-positive and GFP-negative cells isolated by FACS, combined with epithelial cells from R26mTmG mice, and inserted under the kidney capsule of SCID/SCID mice. Grafts were harvested after 8 weeks, and analyzed by immunofluorescence. (e,f) Sections through a renal capsule graft. The boxed regions are shown enlarged at the bottom. Scale bars represent 200 μm. (e) Control graft formed from adult epithelium expressing membrane-tagged tdTomato (mT) and WT uninfected urogenital sinus mesenchyme (UGSM). (f) Graft formed from adult epithelium expressing mT and equal proportions of UGSM infected with lentivirus expressing Hgf-GFP and uninfected UGSM. (g) Quantification of the number of tubules observed in each optical section through a graft. 1.9-fold more tubules were observed in grafts formed from UGSM infected with lentivirus expressing Hgf-GFP as compared to Ctrl. n=3 sections per graft. One representative experiment of two is shown. (c,g) Data are presented as mean ± s.e.m., and significance was calculated by a unpaired Student’s t-test (*: p<0.05)
To further test if stromal Hgf expression induces branching, we utilized a subrenal prostate regeneration assay, which involves engraftment of adult prostate epithelium and embryonic urogenital sinus mesenchyme (UGSM) under the kidney capsule of immunocompromised mice20,25–27. UGSM cells were transduced with a bicistronic lentiviral construct for expression of Hgf, and GFP from an internal ribosome entry site. GFP- positive UGSM cells were isolated by FACS, and combined in equal proportion with adult epithelial cells expressing membrane-tagged tdTomato (Fig. 4d). Examination of tissue sections through these subrenal prostate grafts 8 weeks after implantation revealed a 1.9-fold greater number of tubules in grafts formed from UGSM infected with lentivirus expressing Hgf as compared to the control grafts (Fig. 4e,f,g), indicative of increased branching stimulated by increased Hgf levels.
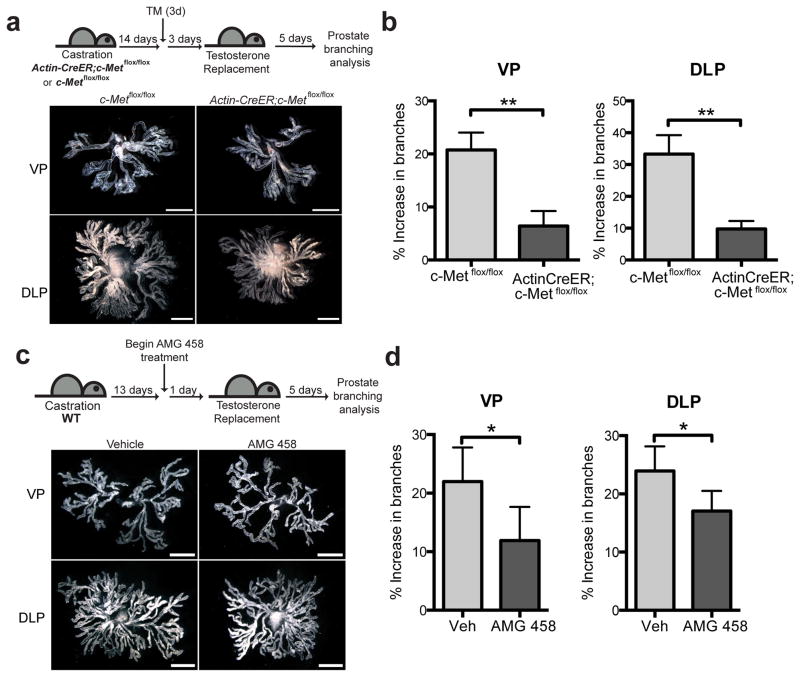
To directly examine the role of Hgf signaling response in prostate branching, we utilized mice expressing CreER under control of the ubiquitously expressed Actin promoter to drive deletion of a conditional c-Met allele28 (ActinCreER; c-Metflox/flox); deletion of c-Met following TM administration was verified by genotyping DNA from prostate lobes (Supplementary Fig. 3b). ActinCreER; c-Metflox/flox or c-Metflox/flox mice were castrated, then treated with TM after prostate regression. Testosterone pellets were implanted three days later to allow completion of TM-induced recombination, and prostates were dissected five days after that (Fig. 5a). As compared to control c-Metflox/flox mice, ActinCreER; c-Metflox/flox mice showed a 3.7-fold and a 3.3-fold attenuation in % increase in branches of ventral and dorsolateral prostate, respectively (Fig. 5b). These results demonstrate that reduction of Hgf response in vivo leads to dramatically decreased branching morphogenesis during regeneration.
Figure 5. Suppression of Hgf response inhibits prostate branching.
(a) Top: Schematic diagram illustrating the experimental strategy used to determine the effect of c-Met ablation on prostate regeneration. Bottom: Representative images from microdissections of each prostate lobe. ActinCreER; c-Metflox/flox prostates have fewer branches than c-Metflox/flox prostates. (b) ActinCreER; c-Metflox/flox VP showed 3.7-fold attenuation relative to c-Metflox/flox VP (6 vs 21 % increase in branches) 5 days after testosterone replacement.. ActinCreER; c-Metflox/flox DLP showed 3.3-fold attenuation relative to c-Metflox/flox DLP (10 vs 33 % increase in branches) 5 days after testosterone replacement. n=6 pairs of VP or DLP. (c) Top: Experimental scheme describing the use of c-Met inhibitor AMG 458 to study the effect of Hgf pathway inhibition on prostate branching. Starting 24 hours before testosterone replacement, AMG 458 was administered every 12 hours for 6 consecutive days. Bottom: Representative images from microdissections of each prostate lobe. AMG 458-treated prostates have more branches than vehicle-treated prostates. (d) AMG 458-treated VP showed 1.8-fold attenuation relative to vehicle (veh)-treated VP (12 vs 22 % increase in branches) 5 days after testosterone replacement. AMG 458-treated DLP showed 1.4-fold attenuation relative to veh-treated DLP (17 vs 24 % increase in branches) 5 days after testosterone replacement. n=5 pairs of VP or DLP. (b,d) Data are presented as mean ± s.e.m., significance was calculated by a paired Student’s t-test (*: p<0.05, **: p<0.01).
To confirm this pharmacologically, WT mice were castrated, and then treated with a c-Met inhibitor, AMG 458, prior to androgen replacement (Fig. 5c). AMG 458 is an orally bioavailable drug that is highly specific for c-Met, showing selectivity for inhibition of c-Met over a panel of tyrosine and serine/threonine kinases29; we indeed noted a reduction of phosphorylated c-Met protein in drug-treated mice as compared to controls (Supplementary Fig. 3c). Five days after androgen replacement, animals treated with AMG 458 showed ventral and dorsolateral prostates with 1.8-fold and 1.4-fold attenuation respectively in % increase in branches as compared to vehicle-treated animals (Fig. 5d). These in vitro and in vivo genetic and pharmacologic data together show that Hgf signaling plays a crucial role in promoting adult prostate branching.
Hh pathway down-regulates Hgf via miR-26a/b
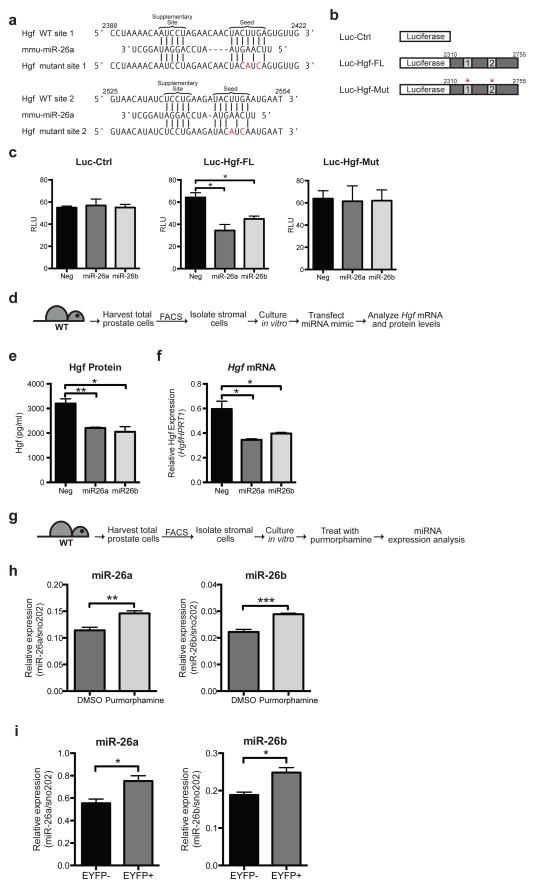
Hh and Hgf pathway activities have opposite effects on prostate branching, and Hh pathway activity is associated with decreased Hgf expression, suggesting a negative regulatory relationship between Hh activity and expression of Hgf. The Hh pathway mediator Gli1, however, functions exclusively as a transcriptional activator30, suggesting an indirect relationship between Gli1 and Hgf expression. To investigate whether this could occur via Hh-pathway activation of microRNA transcription, potential microRNAs that could target Hgf were identified using the TargetScanMouse 6.231 and DIANA-microT 3.032,33 algorithms. Both prediction algorithms had as their top-ranked hits the paralogs miR-26a and miR-26b, which have identical seed sequences (6–8 nucleotides at the 5′ end of the mature miRNA) and may target the same regions on the 3′ UTR of Hgf mRNA. In addition to complementarity of the seed sequence to the Hgf 3′ UTR, both miRNAs also have a supplementary site at the 3′ end of the miRNA, which is complementary to the Hgf UTR and is thought to augment seed pairing34. Both miRNAs are predicted to target two sites on the 3′ UTR of Hgf, with identical seed sequences and slightly different supplementary sequences, and both sites are highly conserved in vertebrate Hgf 3′ UTRs (Fig. 6a and Supplementary Fig. 4).
Figure 6. Hh pathway down-regulates Hgf via miR-26a and miR-26b.
(a) Schematic representation of two predicted miR-26a target sites within the Hgf 3′UTR. Vertical lines indicate Watson-Crick pairing. Two nucleotides were mutated in each target site. Numbers indicate positions of nucleotides in the reference sequence (NM_010427). (b) pMir-Report luciferase vectors used to test if miR-26a/b target the Hgf 3′UTR. Luc-Ctrl contains the luciferase gene only. Luc-Hgf-FL contains the luciferase gene with the entire Hgf 3′UTR. Luc-Hgf-Mut contains the luciferase gene with the entire Hgf 3′UTR, with two nucleotides mutated in both sites (a). (c) Vectors in (b) were transfected into HEK293S cells with miR-26a or miR-26b mimics or a control scrambled (Neg) miRNA, and the pRL-SV40 Renilla luciferase vector. The y-axis shows luciferase normalized to renilla activity. Data are mean ± s.e.m. of separate transfections (n = 3). Transfection of miR-26a or miR-26b led to a 45% and 30% decrease in luciferase activity of Luc-Hgf-FL but not Luc-Ctrl or Luc-Hgf-Mut compared to the control. One representative experiment of three is shown. (d) Experimental scheme to test the effect of miR-26a or miR-26b on Hgf levels in primary prostate stromal cells. (e) Transfection of miR26a or miR26b mimics led to a 30% and 31% decrease in Hgf protein secreted into the media, and a 40% and 30% decrease in Hgf transcripts respectively (f). (e,f) n=3 wells per condition, one representative experiment of three shown. (g) Experimental scheme to test the effect of purmorphamine on miR-26a and miR-26b levels in prostate stromal cells. (h) Treatment with purmorphamine led to a 1.2-fold increase in miR-26a levels and a 1.3-fold increase in miR-26b levels compared to control (DMSO). n=3 wells per condition, one representative experiment of three shown. (i) RT-PCR analysis of RNA isolated from EYFP-positive (EYFP+) and EYFP• negative (EYFP−) stromal cells from Gli1CreER/WT; R26EYFP/WT prostates during regeneration (see Fig. 3g). EYFP+ cells express higher levels of miR-26a and miR-26b than EYFP− cells. n=3 technical replicates, one representative experiment of three is shown. (c,e,f,h,i) Data are presented as mean ± s.e.m., significance was calculated by a unpaired Student’s t-test (*: p<0.05, **: p<0.01,***:p<0.001).
To determine if miR-26a and miR-26b are able to regulate the 3′ UTR of Hgf, luciferase reporter assays were used (Fig. 6b). The 3′ UTR of Hgf was cloned into the pMir-Report vector, downstream of the luciferase gene. HEK293S cells were co-transfected with Renilla luciferase vector and the pMir-Report luciferase vector with or without the Hgf 3′ UTR, and with mature miR-26a or miR-26b mimics, which are chemically modified double-stranded RNAs that mimic endogenous mature miRNAs. Co-transfection of miR-26a or miR-26b mimics suppressed the luciferase activity of the vector with the Hgf 3′UTR by 45% and 30% respectively compared to a negative control scrambled mimic, but did not change the luciferase levels of the control vector (Fig. 6c). Mutation of the seed region in both sites of the Hgf 3′ UTR abrogated the repressive effect of the miRNA mimics, demonstrating the specificity of the miRNA mimics for Hgf (Fig. 6c).
In addition to the Hgf 3′ UTR, the ability of miR-26a and miR-26b to regulate endogenous Hgf mRNA and protein levels in prostate stromal cells was examined. Stromal cells were isolated from WT mouse prostates by FACS and cultured in vitro, transfected with miRNA mimics, and Hgf mRNA and protein levels determined (Fig. 6d). Transfection of either miR-26a or miR-26b led to a decrease in both Hgf mRNA and protein levels as compared to transfection with a scrambled miRNA mimic, demonstrating that both miRNAs are able to regulate endogenous Hgf levels in prostate cells (Fig. 6e,f).
To test the possibility that Hh pathway activity reduces Hgf levels by increasing miR-26a and miR-26b expression, we treated WT prostate stromal cells with purmorphamine and found that levels of both miR-26a and miR-26b increased upon purmorphamine treatment (Fig. 6g,h). We also isolated stromal cells from regenerating prostates based on their status of Hh pathway response, by isolating EYFP-positive and EYFP-negative cells from regenerating Gli1CreER/WT; R26EYFP/WT prostates (Fig. 3g). EYFP expression indicates response to Hh ligand (Fig. 3g), and we find that these cells also express increased levels of miR-26a and miR-26b (Fig. 6i) and decreased levels of Hgf (Fig. 3g), providing further evidence that Hh pathway activity down-regulates Hgf levels via miR-26a and miR-26b.
Ihh expression patterns stromal Hh response
To determine whether expression of Gli1 in a restricted subset of stromal cells results from differential response to the Hh ligand within stromal cells or, alternatively, from restricted Hh ligand expression in epithelial cells, we isolated Gli1-positive and -negative cells from Gli1CreER/WT; R26EYFP/WT mice that were given TM. EYFP-positive and -negative prostate stromal cells were isolated by FACS, cultured separately in vitro, stimulated with purmorphamine, and levels of Gli1 transcript were measured by RT-PCR (Supplementary Fig. 5a). We found that both EYFP-positive and -negative cells responded to purmorphamine by upregulating Gli1 expression to similar extents (Supplementary Fig. 5b), indicating that all stromal cells retain an equivalent capacity to respond to Hh pathway stimulation, and suggesting possible differences in Hh ligand expression.
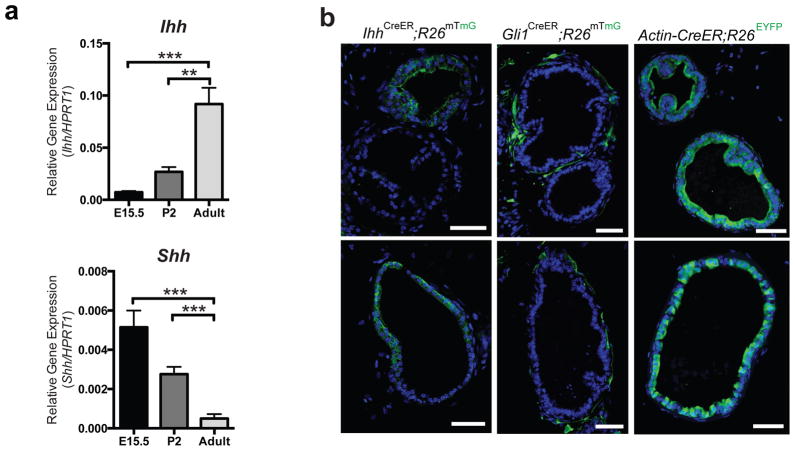
Although Peng et al. showed that basal cells at the proximal end of the prostate dorsal lobe express Shh14, others have shown that Shh expression in the prostate is greatly down-regulated after birth11,13, whereas Ihh expression persists6. Our own RT-PCR analyses of Shh and Ihh mRNA levels in RNA extracted from embryonic (E15.5), early post-natal (P2) and adult ventral and dorsolateral prostate lobes showed that, during development, Shh mRNA levels progressively declined ~10-fold relative to internal controls whereas Ihh mRNA levels progressively increased by ~12-fold. The ~120-fold change in the relative abundance of Ihh mRNA as compared to Shh mRNA from embryo to adult suggests that their relative importance as ligands may shift during development (Fig. 7a and Supplementary Fig. 5c). Indeed, we examined prostates of TM-treated ShhCreER/WT; R26mTmG/WT35 mouse adults and did not detect expression of Shh in the distal regions (Supplementary Fig. 5e). Primary adult prostate stromal cells in culture respond to recombinant Ihh protein by up-regulating expression of Gli1, miR-26a and mir-26b, and down-regulating Hgf expression (Supplementary Fig. 5d), and the increased levels of adult Ihh expression and the prominence of distal prostate in adult regenerative branching morphogenesis together suggest that Ihh may play an important role in patterning Hh pathway response in the stroma, thereby controlling the local levels of stromal Hgf expression.
Figure 7. Ihh expression patterns stromal Hh response.
(a) RT-PCR analysis of Shh or Ihh expression in the E15.5 urogenital sinus (UGS), P2 prostate and adult prostate. Shh is expressed 10-fold lower in adult prostate than in the UGS, and 5.5-fold lower in the adult than in the P2 prostate. Ihh is expressed 12-fold higher in the adult prostate than in the UGS, and 3.4-fold higher in the adult than in the P2 prostate. n=3 prostates per sample (E15.5, P2, or adult). Data are presented as mean ± s.e.m., and significance was calculated by an unpaired Student’s t-test (**: p<0.01, ***:p<0.001). (b) Prostate sections from IhhCreER/WT; R26mTmG/WT or Gli1CreER/WT; R26mTmG/WT or Actin-CreER; R26EYFP/WT mice treated with TM for 4 days, and dissected 5 days later. Scale bars represent 50 μm. One representative experiment of three is shown.
To determine whether the pattern of Ihh expression in the prostate is similar to the pattern of Gli1 expression, we generated an IhhCreER mouse with CreER knocked into the endogenous Ihh locus (Supplementary Fig. 6). TM was administered to IhhCreER/WT; R26mTmG/WT and Gli1CreER/WT; R26mTmG/WT mice, and prostates were harvested after five days. Examination of tissue sections from Gli1CreER/WT; R26mTmG/WT prostates showed ductal regions wholly or partially surrounded by stromal cells that are Gli1-positive and thus are responding to Hh ligand, but also ductal regions surrounded by Gli1-negative stromal cells (Fig. 7b). Tissue sections from IhhCreER/WT; R26mTmG/WT prostates revealed similar patterns of Ihh expression in the epithelium, with some regions entirely or partially Ihh-positive, as well as other regions entirely devoid of Ihh expression. These similarities suggest that the pattern of Gli1 expression in prostate stroma could be accounted for by the pattern of Ihh expression (Fig. 7b). The regional differences in IhhCreER-driven marking are not likely due to incomplete Cre recombination as the dose and duration of TM treatment, when administered to ActinCreER; R26mTmG/WT mice, induced almost 100% recombination in epithelial cells (Fig. 7b).
Discussion
In this study we findthat Hh response in regenerating prostate is present in the stromal cells that enwrap prostate tubules, but is conspicuously absent from stromal cells at the sites of nascent tubule buds. These stromal cells lacking Hh response express higher levels of Hgf than Hh-responsive stromal cells, an effect likely mediated by Hh induction of miR26a and miR26b. We find that Hgf stimulates prostate branching, thus providing a link between reduced Hh response in specific regions of the stroma and the sites of epithelial branching. Finally, we find that the spatial pattern of Hh signal response in stromal cells is strikingly similar to the pattern of Indian hedgehog (Ihh) expression in the epithelium, suggesting that the location of nascent buds during adult prostate branching morphogenesis is specified by spatially restricted Hh signaling from epithelium to stroma.
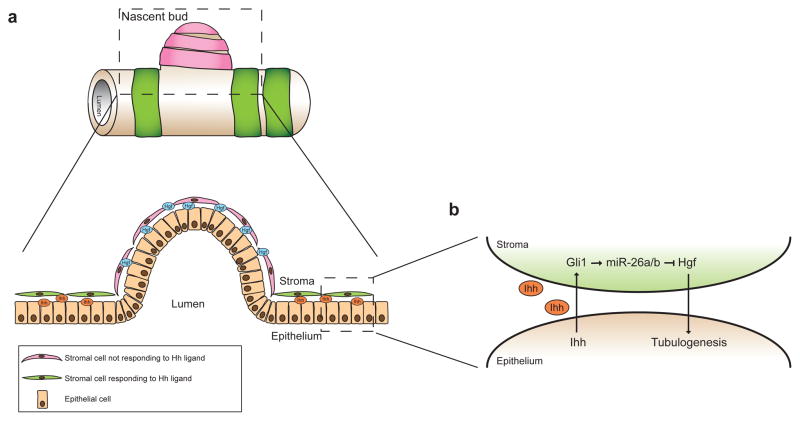
Our data suggest a model (Fig. 8a) in which regional differences in Hh ligand secretion and pathway response determine the location of a nascent bud during adult branching morphogenesis. Thus, in epithelial regions of high Ihh expression, Hh response in adjacent stromal cells down-regulates stromal expression of Hgf by inducing expression of miR-26a and miR-26b (Fig. 8b), leading to low concentrations of Hgf ligand and a consequent lack of branching. Regions of low epithelial Ihh ligand expression in contrast would be associated with low Hh response in adjacent stromal cells, permitting high levels of Hgf secretion that could stimulate formation of nascent buds.
Figure 8. Model of the spatial regulation of branching by Hh and Hgf signaling.
(a) Cartoon of a section of a prostate tubule with a nascent bud. Green bands represent Hh-responsive stromal cells and pink bands represent stromal cells that are not responding to the Hh ligand. A lateral-section through the boxed region is shown below. (b) Enlarged view of the epithelial-stromal interaction in the boxed region. In regions that are not undergoing branching, Ihh secreted by epithelial cells induces expression of Gli1 in the adjacent stromal cells, which increases expression of miR-26a and miR-26b, which inhibit Hgf production, thereby preventing tubulogenesis. In regions lacking Ihh expression, surrounding stromal cells do not respond to the Hh ligand, hence Hgf levels are high and branching is induced.
Although a branched structure is essential for normal prostate function, the occurrence of excessive branching in adulthood is associated with benign prostatic hyperplasia (BPH), a disease that affects 50% of men in their 50s and increases progressively to 90% of men in their 80s4. BPH is characterized by an increase in nodules in the transition zone of the human prostate due to excessive ductal budding and branching36–39. Currently, there are two generally accepted permissive factors for the pathogenesis of BPH, the presence of circulating androgens and increasing age40, but the underlying molecular mechanisms remain poorly understood. The epithelial/stromal interaction described here appears likely to operate in human prostate, as Shh ligand expression in the normal human prostate is localized to the epithelium, whereas pathway response, as determined by Gli1 expression is localized to the stroma41. c-Met expression is localized to the basal cells in both normal human transition zone and in BPH samples42, and cultured prostate stromal cells in vitro secrete Hgf43. The Hh/Hgf circuit we describe thus might be involved in driving the abnormal prostate growth observed in BPH; alternatively, this circuit may simply operate to pattern the branching in the context of growth that is driven by other factors. In either case, manipulation of this epithelial/stromal feedback circuit could prove therapeutically useful.
Methods
Mice
Male mice between 8 and 10 weeks of age were used for all experiments. Gli1LacZ/WT heterozygotes44 from our colony were back-crossed for 10 generations to the FVB strain, then interbred to generate Gli1LacZ/LacZ homozygotes. Wild-type littermates were used as controls. Gli1CreER/WT mice45 were crossed with R26EYFP/EYFP mice46 or R26mTmG/mTmG mice47 or Smoflox/flox mice48 to obtain Gli1CreER/WT; R26EYFP/WT or Gli1CreER/WT; R26mTmG/WT or Gli1CreER/WT; Smoflox/WT mice. Resulting mice were crossed with Smoflox/flox mice to obtain Gli1CreER/WT; Smoflox/flox mice. All mouse strains except as otherwise indicated were obtained from Jackson Laboratories. All castration and testosterone implantation procedures were performed under isoflurane anaesthesia, which was administered in a fume hood with a standard vaporizer (J. B. Baulch and Associates). To induce CreER-mediated recombination in mice harboring the CreER allele, tamoxifen (Sigma, 12 mg per 30 g body weight) was administered by oral gavage. For each experiment, no statistical method was used to predetermine sample size, and mice in each cage were randomly selected for drug/TM or control treatments. The investigators were blinded to allocation during experiments and outcome assessment. Counting of prostate tubules was blinded to ensure that the investigator who assessed the tissues did not know which treatment group each sample belonged to. All procedures were performed under a protocol approved by the Administrative Panel on Laboratory Animal Care at Stanford University. In all experiments, only the ventral and dorsolateral lobes of the prostate were analyzed.
Castration and testosterone replacement
Castration was performed by ligation of the spermatic cord and complete removal of the testes and epididymides through a scrotal approach. 14 days after castration, a pellet of testosterone (Innovative Research of America, SA-151, 10 mg) was implanted subcutaneously in the back of the mouse.
Two-photon imaging
Gli1CreER/WT; R26mTmG/WT mice were castrated and testosterone replaced after 2 weeks. 3 days after testosterone replacement, tamoxifen (Sigma, 12 mg per 30 g body weight) was administered by oral gavage daily for 4 consecutive days. 3 days after the last dose, individual prostate lobes were dissected, fixed in 4% paraformaldehyde for 24 h, washed twice in PBS and incubated in ScaleA2 solution containing 4M urea, 10% (wt/vol) glycerol and 0.1% (wt/vol) Triton X-10015 for 2 weeks at 4°C. Images were captured using the Prairie Ultima IV upright two-photon microscope. Three-dimensional reconstructions of two-photon images were generated using Imaris software (Bitplane Scientific Software).
Branching analysis
Individual prostate lobes were dissected in PBS and incubated in 1% collagenase (Sigma C6885) for 15 minutes at room temperature with gentle agitation. After rinsing in PBS, individual branches of each lobe were separated using fine forceps, and images captured using a Leica M205 FA Stereomicroscope. The number of branch tips in each lobe was counted manually. The difference between the number of branches in a regenerating prostate lobe and the average number of branches in a pool of regressed prostate lobe from equivalent mice (giving the number of new branches) was divided by the number of branches in the regressed lobe and expressed as a percentage increase in the number of new branches. All prostate regeneration experiments involving branching analyses were performed on pairs of mice (control and treatment/mutant) with the same protocol on the same day to reduce variability. Pairs of mice were castrated on the same day, in the same manner, and the prostates allowed to regress. After 2 weeks, testosterone pellets were implanted subcutaneously on the same day for each pair of mice, and the prostates harvested and analyzed together on the same day.
Microarray analysis
Gli1LacZ/LacZand WT littermates were castrated and testosterone replaced after 2 weeks. 3 days after testosterone replacement, the mice were sacrificed, prostate lobes harvested, and total RNA was prepared using the RNeasy Plus Mini Kit (Qiagen). RNA quality was evaluated using the Agilent 2100 Bioanalyzer system. Samples were hybridized to the Affymetrix GeneChip Mouse Exon 1.0 ST microarray chips. Three mice of each genotype were analyzed. After hybridization, expression values were normalized using the RMA function in the Partek Genomics Suite software. Differentially expressed genes were identified using ANOVA, genes showing a fold change greater or equal to 1.4 with P-value < 0.01 were shortlisted for functional annotation analyses. Examination of Swiss-Prot (SP) and Protein Information Resource (PIR) Keywords (SP_PIR_KEYWORDS) was performed using DAVID49 (http://david.abcc.ncifcrf.gov/). Expression changes detected by microarray analysis were validated using quantitative RT-PCR.
Database accession number
The microarray data set is available in the GEO database (GEO accession: GSE59849).
AMG 458 treatment
FVB mice were castrated. After 13 days, a 30mg/kg of body weight dose of AMG 458 in HPMCT (2% hydroxypropylmethylcellulose, 1% Tween-80 in water, adjusted to pH 2.2 with HCl) or vehicle alone was administered by oral gavage 29. A dose of AMG 458 or vehicle was administered 12 h and 24 h later. 6 h after the third dose, a testosterone pellet was implanted subcutaneously and fourth dose was administered 6 h later. AMG 458 or vehicle alone was administered twice daily for 5 d after testosterone implantation. Prostates were harvested 3 h after the last dose, and processed for branching analyses. For protein extraction, a portion of the left lobe of the liver was removed, homogenized in RIPA buffer (50 mM Tris-HCl pH8, 150 mM NaCl, 1% Triton-X 100, 0.5% Sodium deoxycholate, 0.1% SDS, 1 mM EGTA) with Halt Protease and Phosphatase Inhibitor Cocktail (Pierce 78440), incubated at 4°C for 30 min, centrifuged at 10,000g for 30 min and the supernatant removed. 15 μg of total protein was loaded into each lane for SDS/PAGE and Western blotting with a rabbit anti-phospho-c-Met antibody (Invitrogen 44888G, 1:1000) or mouse anti-β-tubulin (Sigma T8660, 1:1000) antibody. Densitometric analysis of Western blot bands was performed using Image J software (NIH).
GDC-0449 treatment
FVB mice were castrated. After 11 days, a 100 mg/kg of body weight dose of GDC-0449 in MCT (0.5% methylcellulose, 0.2% Tween-80)50 or vehicle only control was administered by oral gavage twice a day for 3 d. 6 h after the last dose, testosterone was implanted subcutaneously, and GDC-0449 or vehicle was administered 6 h later, then twice daily for 3 d after testosterone implantation. Prostates were harvested 3 h after the last dose. For each paired lobe of the prostate, one lobe was processed for branching analyses and the other was homogenized for RNA extraction.
Immunofluorescence analysis of tissue sections
Individual prostate lobes were dissected in PBS, fixed in 4% paraformaldehyde for 6 hours, washed 3 times in PBS, incubated in 30% sucrose overnight, then embedded and snap-frozen in OCT compound (Tissue-tek). 12 μm sections were obtained using a Leica cryostat. For immunostaining, tissue sections were rinsed in PBS twice, then blocked in 10% goat serum in PBS containing 2% BSA and 0.25% Triton X-100 for 1 h, and incubated with the following primary antibodies diluted in blocking solution overnight at 4°C in a humidified chamber: rabbit anti-GFP (Invitrogen A11122, 1:500), chicken anti-vimentin (Millipore, 1:600). Sections were washed three times with PBS containing 0.25% Triton X-100, incubated with DAPI and appropriate Alexa fluor -conjugated secondary antibodies diluted 1:1,000 in blocking solution for 1h at 22°C, washed again three times, and mounted on slides with Prolong Gold mounting reagent (Invitrogen). Immunofluorescence images presented are images from one representative experiment of three.
Immunofluorescence analysis of cultured cells
Stromal cells were isolated by FACS and plated onto 18-mm diameter circular coverslips in 12-well plates, and fixed 4% paraformaldehyde for 20 min. Cells were permeabilized with PBS/0.5% Triton X-100 for 3 min and nonspecific binding sites were blocked with 2% BSA in PBST (PBS with 0.5% Tween- 20) for 1 h. Cells were stained with primary antibodies: chicken anti-vimentin (Millipore AB5733, 1:600) and mouse anti-smooth muscle actin (Sigma 1A4, 1:1000) diluted in 2% BSA/PBST for 1 h at room temperature. After washing four times in PBST, cells were incubated for 1 h with appropriate Alexa fluor 488 or 594-conjugated secondary antibodies (Invitrogen, 1:1000) together with 1 g/mL of 4,6-diamidino-2-phenylindole (DAPI) for nuclear staining in 2% BSA/PBST. Cells were washed four times in PBST, then mounted on slides with Prolong Gold mounting reagent (Invitrogen). Immunofluorescence images presented are images from one representative experiment of three.
Fluorescence-activated cell sorting (FACS)
Prostate lobes were dissociated and stained for FACS based on published methods20. Briefly, prostate lobes were dissected, minced, and incubated at 37°C in 10%FBS/DMEM containing 1 mg/ml collagenase (Gibco, cat. no. 17018–029). Further dissociation to a single cell suspension was achieved by incubation in Trypsin/0.05% EDTA (Invitrogen cat. no. 25300) for 5 minutes at 37°C, followed by serially passing through 18-G and 20-G needles and filtering through a nylon mesh filter with a 40 μm pore size. Cells were stained for 20 minutes at 4°C. Antibodies used are: Sca-1-APC (clone D7; eBioscience, cat. no. 17–5981–82), 1:500; Ter119-FITC (clone TER-119; eBioscience, cat. no. 11–5921–85), 1:250; CD31-FITC (clone 390; eBioscience, cat. no. 11–0311–85), 1:250; CD45-FITC (clone 30-F11; eBioscience, cat. no. 11–0451–85), 1:250; CD49f-PE (clone eBioGoH3; eBioscience, cat. no. 12–0495–83) 1:333. Cells were sorted using a FACS AriaII cytometer (BD Biosciences), and analysis of flow cytometry data was performed using FlowJo Software (Treestar). Following FACS, isolated cells were centrifuged at 500g for 5 minutes. The pellet washed in PBS, homogenized in lysis buffer from the RNAqueous-Micro RNA Isolation Kit (Ambion AM1931), the mirVana miRNA Isolation Kit (Ambion AM1560) and RNA extracted following kit instructions.
Stromal cell culture and Hgf ELISA
FACS-isolated stromal cells were cultured in 10% FBS/DMEM in a 48-well tissue culture plate. When the cells were 80% confluent, old media was removed and cells were incubated in 150 μl 10% FBS/DMEM containing 10 μM purmorphamine (Calbiochem 540220) or DMSO control. In separate experiments, cultured stromal cells were treated with 10% FBS/DMEM containing 5 μg/ml recombinant Ihh protein (R&D 1705-HH-025) in 0.1% BSA or a BSA only control. In separate experiments, cells were transfected with miRNA mimics for miR-26a (Ambion, Assay MC10249) or miR-26b (Ambion, Assay MC12899) or a negative control (Ambion 4464076). After 22 h, media was removed and centrifuged at 10,000g for 5 min. To measure the concentration of Hgf in the media, 10 μl of the supernatant was used in each well of the Mouse/Rat HGF Quantikine ELISA Kit (R&D Systems, MHG00) and the plate processed according to manufacturer’s instructions. Each sample was analyzed in duplicate.
Protein extraction from prostate lobes
Individual prostate lobes were dissected in PBS, washed, and homogenized in PBS using a pestle. An equal volume of Lysis Buffer 2 (R&D 895347) was added and the homogenate incubated at room temperature for 30 min with agitation. The lysate was centrifuged at 10,000g for 30 min at 4°C, and the supernatant removed. Protein levels in each extract was determined using the Quick Start Bradford 1x Dye Reagent (Bio-Rad 500–0205), equal amounts of total protein were loaded into each well of the Mouse/Rat HGF Quantikine ELISA Kit (R&D Systems, MHG00) and the plate processed according to manufacturer’s instructions. Each sample was analyzed in duplicate, and Hgf levels for each sample was normalized to total protein in each well.
Quantitative RT-PCR
Prostate lobes were dissected in RNase-free PBS, and placed immediately in lysis buffer from the mirVana miRNA Isolation Kit (Ambion AM1560) or RNeasy Plus Mini Kit (Qiagen 74134). Cells were homogenized in lysis buffer using a pestle, and RNA extracted following respective kit instructions. Colon and bladder samples were snap-frozen in liquid nitrogen, homogenized using a mortar and pestle, and RNA extracted using the RNeasy Plus Mini Kit (Qiagen). For quantitative RT-PCR of mRNA transcripts, first-strand cDNA was made using SuperScript III First-Strand Synthesis SuperMix (Invitrogen 18080–400). Quantitative RT-PCR was performed using iQ SYBR Green Supermix (Bio-Rad 170–8880) and the Bio-Rad iCycler. All assay values were normalized to the HPRT1 internal control.
For quantitative RT-PCR of mature miRNA, cDNA was made using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) and primers from respective TaqMan MicroRNA Assays according to manufacturer’s instructions. Quantitative RT-PCR was performed using the TaqMan Universal PCR Master Mix (Applied Biosystems 4324018), TaqMan MicroRNA Assays for miR-26a (Applied Biosystems, Assay 000405) or miR-26b (Applied Biosystems, Assay 000407) or Sno202 (Applied Biosystems, Assay 001232) and the Applied Biosystems 7300 Real-Time PCR System. All assay values were normalized to the Sno202 internal control.
Conditioned medium from NIH 3T3 fibroblasts
Conditioned media was prepared by incubating 100% confluent NIH 3T3 fibroblasts (ATCC) with 5% BCS containing 10 μM purmorphamine (Calbiochem 540220) or DMSO control. After 72 h, media was collected and passed through a 0.22 μm filter to remove cell debris. 3T3 cells were washed in PBS and RNA was extracted using the RNeasy Plus Mini Kit (Qiagen).
MDCK tubulogenesis
Madin-Darby canine kidney (MDCKII) cells (ATCC) were mixed with 170 μl ice cold Geltrex Matrix (Gibco A1413201), layered onto 12-mm Transwell clear filters and allowed to solidify at 37°C. Pre-warmed DMEM containing 10% FBS was added above and below the wells. Media was changed every 3 d. When cysts were formed (7 d after plating), tubulogenesis was induced using a 1:1 mix of 5% FBS and conditioned media from NIH 3T3 cells treated with DMSO or purmorphamine. Media was changed daily, and cysts were analyzed after 6 d. The percentage of cysts that formed extensions in 10 fields of view per well was determined, and 4 wells were analyzed for each type of conditioned medium. For staining of the cysts, cysts were recovered from the Geltrex Matrix by incubating in Cell Recovery Solution (BD Biosciences 354253) on ice for 1 h. After centrifugation at 300g for 5 min, the pellet containing the cysts was washed with PBS and fixed with 4% paraformaldehyde for 20 min at 22°C. After washing in PBS, cysts were stained with Alexa Fluor 594 phalloidin (Invitrogen A12381, 1:25) and DAPI in 10% goat serum in PBS containing 2% BSA and 0.25% Triton X-100 at 4°C overnight. Cysts were washed in PBS containing 0.25% Triton X-100 thrice and mounted on slides in Cytoseal mounting reagent (Thermo Scientific 23–244257). Immunofluorescence images presented are images from one representative experiment of three.
Confocal microscopy
Immunofluorescence images were obtained using a Zeiss LSM 510 inverted confocal microscope and prepared for publication with Zeiss LSM 5 Image Browser software and Adobe Photoshop CS4.
Generation of miRNA target reporter constructs
The full length 3′ UTR of Hgf (corresponding to position 2310–2755 of the RefSeq sequence NM_010427) and both fragments of 3′UTR each containing a putative miR-26a/b target sequence (corresponding to positions 2363–2428 and 2508–2588 of the RefSeq sequence NM_010427) were amplified by PCR using cDNA from adult murine prostate cells as a template and primers that introduced a SpeI and HindIII cut site at the 5′ and 3′ end of the PCR product respectively. Each PCR product was inserted downstream of the luciferase gene in the pMir-Report vector using the SpeI and HindIII cut sites. All products were sequenced. Mutation of the putative miR-26a/b target sequence within the 3′UTR of Hgf was generated using the QuikChange Site-Directed Mutagenesis kit (Stratagene).
Luciferase reporter assays
HEK293S cells were seeded at 1 × 105 cells per well in 24-well plates the day prior to transfection. All transfections were carried out with Lipofectamine 2000 (Invitrogen), according to the manufacturer’s instructions. Cells were transfected with 80 ng of pMir-Report luciferase expression construct containing the 3′UTR of murine Hgf, 20 ng pRL-SV40 Renilla luciferase vector (Promega E2231) and 100 nM miR-26a mirVana miRNA mimic (Ambion, Assay MC10249) or miR-26b mimic (Ambion, Assay MC12899) or negative control miRNA mimic (Ambion 4464076). 24 h after transfection, luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega) and normalized to Renilla luciferase activity. All experiments were performed in triplicate.
In vitro tubulogenesis assay
Adult prostates from WT or R26mTmG mice were harvested, dissociated, mixed with neutralized collagen (BD Biosciences), plated out in 48 well plates (5×104 cells per well), and 500 μl of PrEGM (Lonza) was added. Media was changed after 3 days. 5 days from the start of culture, PrEGM was replaced with PrEBM (Lonza) containing hydrocortisone (5 μg/ml) and insulin/transferrin/selenium (Gibco) with or without Hgf (50 ng/ml). Media was changed after 3 days and cells were imaged the following day. One representative experiment of three is presented.
In vitro cell proliferation assay
Adult prostates from WT were harvested, dissociated and plated out in 96 well plates (104 cells per well), and 500ul of PrEGM (Lonza) was added. After 3 days, PrEGM was replaced with PrEBM (Lonza) containing hydrocortisone (5 μg/ml) and insulin/transferrin/selenium (Gibco) with or without Hgf (50 ng/ml). Cells were imaged after 3 days. To quantify the number of cells in each well, cells were trypsinized and counted using a haemocytometer.
Subrenal capsule prostate grafts
Subrenal capsule graft experiments were performed as previously described20. Briefly, adult prostates from R26mTmG mice were harvested, dissociated and basal and luminal cells isolated by FACS. Urogenital sinus mesenchyme (UGSM) was harvested from E16.5 embryos, passaged twice in vitro, then infected with lentivirus expressing Hgf-IRES-GFP. 24 hours after infection, UGSM were washed, trypsinized and GFP-positive and GFP-negative cells were isolated by FACS. For the control grafts, 2×105 GFP-negative cells were combined with a total of 2×105 basal and luminal cells in equal proportion in neutralized collagen. For experimental grafts, 105 GFP-negative and 105 GFP-positive UGSM were combined with total of 2×105 basal and luminal cells in equal proportion. Grafts were implanted into the renal capsule of anesthetized SCID/SCID mice and a testosterone pellet (Innovative Research of America, 12.5 mg) was inserted subcutaneously. Grafts were harvested 8 weeks later for analysis. One representative experiment of two is presented.
Generation of the IhhCreER mouse strain
A bacterial artificial chromosome (BAC) clone containing the Ihh gene locus was obtained, and genetic modifications of the clone were performed using the Gene Bridges Quick and Easy BAC Modification kit (Cat #K001) according to the manufacturer’s protocol. A CreER-FRT-Neo-FRT cassette was inserted into the locus, replacing the ATG start codon of Ihh with the ATG codon of CreER. Mutation-free, successfully modified BAC clones were subcloned into a minimal vector, linearized by digestion with ScaI, then electroporated into R1 mouse embryonic stem cells (ESCs) Correctly targeted clones were identified by Southern blotting, karyotyped, and microinjected into 2–8 cell stage embryos. Heterozygous progeny from germ-line transmitting chimeric mice were mated with R26-FlpO mice in order to excise the FRT-flanked Neo cassette in the targeted Ihh allele to generate the final IhhCreER strain.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software v.5. All data are presented as mean ± s.e.m., and two group comparisons were done with a two-tailed Student’s t-test. A value of P < 0.05 was taken as statistically significant. Paired t-tests were used to compare regenerating prostates because pairs of age-matched and weight-matched test and control animals were analyzed on the same day, and only one pair of animals was analyzed per day. All other t-tests were unpaired.
Supplementary Material
Acknowledgments
This research was supported in part by grants from the National Institutes of Health to P.A.B. and a Pathway to Independence Award (K99/R00) to K.S. P.A.B. is an investigator of the Howard Hughes Medical Institute. We acknowledge the Stanford Neuroscience Imaging Facility for use of the 2-Photon microscope. We thank Owen Witte for advice on subrenal prostate regeneration experiments.
Footnotes
Author Contributions
A.L., K.S and P.A.B. conceived ideas and experimental design. A.L. and K.S. performed the experiments. C.Z. assisted with subrenal capsule prostate grafting experiments. S.K. genotyped the animals. A.L., K.S. and P.A.B. wrote the manuscript.
References
- 1.Shin K, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34:961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- 3.Sugimura Y, Cunha G, Donjacour A. Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol Reprod. 1986;34:973. doi: 10.1095/biolreprod34.5.973. [DOI] [PubMed] [Google Scholar]
- 4.Paolone DR. Benign prostatic hyperplasia. Clinics in geriatric medicine. 2010;26:223–239. doi: 10.1016/j.cger.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Berman DM, et al. Roles for Hedgehog signaling in androgen production and prostate ductal morphogenesis. Dev Biol. 2004;267:387–398. doi: 10.1016/j.ydbio.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Doles J, et al. Functional compensation in Hedgehog signaling during mouse prostate development. Dev Biol. 2006;295:13–25. doi: 10.1016/j.ydbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Karhadkar SS, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 8.Yu M, Bushman W. Differential stage-dependent regulation of prostatic epithelial morphogenesis by Hedgehog signaling. Dev Biol. 2013;380:87–98. doi: 10.1016/j.ydbio.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freestone S, et al. Sonic hedgehog regulates prostatic growth and epithelial differentiation. Dev Biol. 2003;264:352–362. doi: 10.1016/j.ydbio.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Wang BE, et al. Inhibition of epithelial ductal branching in the prostate by sonic hedgehog is indirectly mediated by stromal cells. J Biol Chem. 2003;278:18506–18513. doi: 10.1074/jbc.M300968200. [DOI] [PubMed] [Google Scholar]
- 11.Pu Y, Huang L, Prins G. Sonic hedgehog-patched Gli signaling in the developing rat prostate gland: lobe-specific suppression by neonatal estrogens reduces ductal growth and branching. Dev Biol. 2004;273:257–275. doi: 10.1016/j.ydbio.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podlasek C, Barnett D, Clemens J, Bak P, Bushman W. Prostate development requires Sonic hedgehog expressed by the urogenital sinus epithelium. Dev Biol. 1999;209:28–39. doi: 10.1006/dbio.1999.9229. [DOI] [PubMed] [Google Scholar]
- 13.Lamm M, et al. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev Biol. 2002;249:349–366. doi: 10.1006/dbio.2002.0774. [DOI] [PubMed] [Google Scholar]
- 14.Peng YC, Levine CM, Zahid S, Wilson EL, Joyner AL. Sonic hedgehog signals to multiple prostate stromal stem cells that replenish distinct stromal subtypes during regeneration. Proceedings of the National Academy of Sciences. 2013;110:20611–20616. doi: 10.1073/pnas.1315729110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hama H, et al. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci. 2011;14:1481–1488. doi: 10.1038/nn.2928. [DOI] [PubMed] [Google Scholar]
- 16.Niranjan B, et al. HGF/SF: a potent cytokine for mammary growth, morphogenesis and development. Development. 1995;121:2897–2908. doi: 10.1242/dev.121.9.2897. [DOI] [PubMed] [Google Scholar]
- 17.Soriano JV, Pepper MS, Nakamura T, Orci L, Montesano R. Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. Journal of Cell Science. 1995;108(Pt 2):413–430. doi: 10.1242/jcs.108.2.413. [DOI] [PubMed] [Google Scholar]
- 18.Ohmichi H, Koshimizu U, Matsumoto K, Nakamura T. Hepatocyte growth factor (HGF) acts as a mesenchyme-derived morphogenic factor during fetal lung development. Development. 1998;125:1315–1324. doi: 10.1242/dev.125.7.1315. [DOI] [PubMed] [Google Scholar]
- 19.Santos OFP, et al. Involvement of Hepatocyte Growth Factor in Kidney Development. Dev Biol. 1994;163:525–529. doi: 10.1006/dbio.1994.1169. [DOI] [PubMed] [Google Scholar]
- 20.Lukacs RU, Goldstein AS, Lawson DA, Cheng D, Witte ON. Isolation, cultivation and characterization of adult murine prostate stem cells. Nature Protocols. 2010;5:702–713. doi: 10.1038/nprot.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 22.Taipale J, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 23.Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- 24.Montesano R, Schaller G, Orci L. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell. 1991;66:697–711. doi: 10.1016/0092-8674(91)90115-f. [DOI] [PubMed] [Google Scholar]
- 25.Lawson D, Xin L, Lukacs R, Cheng D, Witte O. Isolation and functional characterization of murine prostate stem cells. Proceedings of the National Academy of Sciences. 2007;104:181. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein AS, et al. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proceedings of the National Academy of Sciences. 2008;105:20882–20887. doi: 10.1073/pnas.0811411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huh CG. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA. 2004;101:4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, et al. Discovery of a Potent, Selective, and Orally Bioavailable c-Met Inhibitor: 1-(2-Hydroxy-2-methylpropyl)- N-(5-(7-methoxyquinolin-4-yloxy)pyridin-2-yl)-5-methyl-3-oxo-2-phenyl-2,3-dihydro-1 H-pyrazole-4-carboxamide (AMG 458) J Med Chem. 2008;51:3688–3691. doi: 10.1021/jm800401t. [DOI] [PubMed] [Google Scholar]
- 30.Dai P, et al. Sonic Hedgehog-induced Activation of the Gli1Promoter Is Mediated by GLI3. J Biol Chem. 1999;274:8143–8152. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- 31.Lewis BP, Burge CB, Bartel DP. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates that Thousands of Human Genes are MicroRNA Targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Maragkakis M, et al. Accurate microRNA target prediction correlates with protein repression levels. BMC Bioinformatics. 2009;10:295. doi: 10.1186/1471-2105-10-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maragkakis M, et al. DIANA-microT web server: elucidating microRNA functions through target prediction. Nucleic Acids Research. 2009;37:W273–W276. doi: 10.1093/nar/gkp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harfe B, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Mcneal JE. Pathology of benign prostatic hyperplasia. Insight into etiology. Urol Clin North Am. 1990;17:477–486. [PubMed] [Google Scholar]
- 37.McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15:340–345. [PubMed] [Google Scholar]
- 38.Price H, Mcneal JE, Stamey TA. Evolving patterns of tissue composition in benign prostatic hyperplasia as a function of specimen size. Human Pathology. 1990;21:578–585. doi: 10.1016/s0046-8177(96)90002-7. [DOI] [PubMed] [Google Scholar]
- 39.Lee KL, Peehl DM. Molecular and cellular pathogenesis of benign prostatic hyperplasia. J Urol. 2004;172:1784–1791. doi: 10.1097/01.ju.0000133655.71782.14. [DOI] [PubMed] [Google Scholar]
- 40.Untergasser G, Madersbacher S, Berger P. Benign prostatic hyperplasia: age-related tissue-remodeling. Exp Gerontol. 2005;40:121–128. doi: 10.1016/j.exger.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Fan L, et al. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 2004;145:3961. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- 42.Pisters LL, et al. c-met proto-oncogene expression in benign and malignant human prostate tissues. J Urol. 1995;154:293–298. [PubMed] [Google Scholar]
- 43.Nakashiro K, Okamoto M, Hayashi Y, Oyasu R. Hepatocyte growth factor secreted by prostate-derived stromal cells stimulates growth of androgen-independent human prostatic carcinoma cells. American Journal of Pathology. 2000;157:795. doi: 10.1016/s0002-9440(10)64593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai C, Auerbach W, Lee J, Stephen D, Joyner A. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 45.Ahn S, Joyner A. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 46.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Developmental Biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 48.Long F, Zhang XM, Karp S, Yang Y, Mcmahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- 49.Huang DW, et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Research. 2007;35:W169–W175. doi: 10.1093/nar/gkm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robarge K, et al. GDC-0449--A potent inhibitor of the hedgehog pathway. Bioorganic & medicinal chemistry letters. 2009;19:5576–5581. doi: 10.1016/j.bmcl.2009.08.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.