Abstract
Alignment-free methods are increasingly used to calculate evolutionary distances between DNA and protein sequences as a basis of phylogeny reconstruction. Most of these methods, however, use heuristic distance functions that are not based on any explicit model of molecular evolution. Herein, we propose a simple estimator dN of the evolutionary distance between two DNA sequences that is calculated from the number N of (spaced) word matches between them. We show that this distance function is more accurate than other distance measures that are used by alignment-free methods. In addition, we calculate the variance of the normalized number N of (spaced) word matches. We show that the variance of N is smaller for spaced words than for contiguous words, and that the variance is further reduced if our spaced-words approach is used with multiple patterns of ‘match positions’ and ‘don’t care positions’. Our software is available online and as downloadable source code at: http://spaced.gobics.de/.
Keywords: k-mers, Spaced words, Alignment-free, Phylogeny, Word frequency, Distance estimation, Variance, Genome comparison
Background
Alignment-free methods are increasingly used for DNA and protein sequence comparison since they are much faster than traditional alignment-based approaches [1]. Applications of alignment-free approaches include protein classification [2-5], read alignment [6-8], isoform quantification from RNAseq reads [9], sequence assembly [10], read-binning in metagenomics [11-16] or analysis of regulatory elements [17-20]. Most alignment-free algorithms are based on the word or k-mer composition of the sequences under study [21]. To measure pairwise distances between genomic or protein sequences, it is common practice to apply standard metrics such as the Euclidean or the Jensen-Shannon (JS) distance [22] to the relative word frequency vectors of the sequences.
Recently, we proposed an alternative approach to alignment-free sequence comparison. Instead of considering contiguous subwords of the input sequences, our approach considers spaced words, i.e. words containing wildcard or don’t care characters at positions defined by a pre-defined patternP. This is similar as in the spaced-seeds approach that is used in database searching [23]. As in existing alignment-free methods, we compared the (relative) frequencies of these spaced words using standard distance measures [24]. In [25], we extended this approach by using whole sets of patterns and calculating the spaced-word frequencies with respect to all patterns in 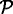 . In this multiple-pattern approach, the distance between two sequences is defined as the average of the distances based on the individual patterns , see also [26]. ‘Spaced words’ have been proposed simultaneously by Onodera and Shibuya for protein classification [27] and by Ghandi et al. to study regulatory elements [28,29].
. In this multiple-pattern approach, the distance between two sequences is defined as the average of the distances based on the individual patterns , see also [26]. ‘Spaced words’ have been proposed simultaneously by Onodera and Shibuya for protein classification [27] and by Ghandi et al. to study regulatory elements [28,29].
Phylogeny reconstruction is an important application of alignment-free sequence comparison. Consequently, most alignment-free methods were benchmarked by applying them to phylogeny problems [30-35]. The distance metrics used by these methods, however, are only rough measures of dissimilarity, not derived from any explicit model of molecular evolution. This may be one reason why distances calculated by alignment-free algorithms are usually not directly evaluated, but are used as input for distance-based phylogeny methods such as Neighbour-Joining [36]. The resulting tree topologies are then compared to trusted reference topologies. For applications to genomic sequences, we modified our distance dN by taking into account that sequences can contain repeats and homologies on different strands. Obviously, this is only a very rough way of evaluating sequence-comparison methods, since the resulting tree topologies not only depend on the distance values calculated by the evaluated methods, but also on the tree-reconstruction method that is applied to them. Also, comparing topologies ignores branch lengths, so the results of these benchmark studies depend only indirectly on the distance values calculated by the alignment-free methods that are to be evaluated.
Three remarkable exceptions are the papers describing Kr [37], Co-phylog [38] and andi [39]. Kr estimates evolutionary distances based on shortest unique substrings, Co-phylog uses so-called microalignments defined by spaced-word matches and considers the don’t care positions to estimate distances, while andi uses gap-free local alignments bounded by maximal unique matches. To our knowledge, these approaches are the only alignment-free methods so far that try to estimate phylogenetic distances in a rigorous way, based on a probabilistic model of evolution. Consequently, the authors of Kr, Co-phylog and andy compared the distance values calculated by their methods directly to reference distances. Haubold et al. could show that Kr can correctly estimate evolutionary distances between DNA sequences up to around 0.5 mutations per site [37].
In previous papers, we have shown, that our spaced-word approach is useful for phylogeny reconstruction. Tree topologies calculated with Neighbour-Joining based on spaced-word frequency vectors are usually superior to topologies calculated from the contiguous word frequency vectors that are used by traditional alignment-free methods [25]. Moreover, we could show that the ‘multiple-pattern approach’ leads to much better results than the ‘single-pattern approach’; these results were confirmed by Noé and Martin [40]. We also showed experimentally that distance values and tree topologies produced from spaced-word frequencies are statistically more stable than those based on contiguous words. In fact, the main difference between our spaced words and the commonly used contiguous words is that spaced-word matches at neighbouring positions are statistically less dependent on each other.
Since the aim of our previous papers was to compare (multiple) spaced-word frequencies to contiguous word frequencies, we applied the same distance metrics to our spaced-word frequencies that are applied by standard methods to k-mer frequencies, namely Jensen-Shannon and the Euclidean distance. In the present paper, we propose a new pairwise distance measure based on a probabilistic model of DNA evolution. We estimate the evolutionary distance between two nucleic-acid sequences based on the number N of space-word matches between them. We show that this distance measure is more accurate and works for more distantly related sequences than existing alignment-free distance measures. In addition, we calculate the variance of N for contiguousk-mers, as well as for spaced words using our single and multiple pattern approaches. We show that the variance of N is lower for spaced words than for contiguous words and that the variance is further reduced if multiple patterns are used.
This paper is an extended version of a manuscript that was first published in the proceedings of Workshop on Algorithms in Bioinformatics (WABI) 2013 in Wroclaw, Poland [41]. We added two extensions to our WABI paper that are crucial if our method is to be applied to real-world genomic sequences. (a) While the original version of our distance function assumed that homologies are located on the same strand of two genomes under comparison, we modified our original distance measure to account for homologies that are on different strands. (b) The number N of spaced-word matches is highly sensitive to repeats in the compared sequences, and our previously defined distance function could grossly under-estimate phylogenetic distances in the present of repeats. We therefore propose a simple modification of this distance function that is insensitive to repeats. Finally, we added more test data sets to evaluate our method.
Motifs and spaced words
As usual, for an alphabet Σ and ℓ∈N, Σℓ denotes the set of all sequences of length ℓ over Σ. For a sequence S∈Σℓ and 0<i≤ℓ, S[i] denotes the i-th character of S. A pattern of length ℓ is a word P∈{0,1}ℓ, i.e. a sequence over {0,1} of length ℓ. In the context of our work, a position i with P[i]=1 is called a match position while a position i with P[i]=0 is called a don’t care position. The number of all match positions in a patterns P is called the weight of P. For a pattern P of weight k, denotes the i-th match position and denotes the set of all match positions.
A spaced wordw of weight k over an alphabet Σ is a pair (P,w′) such that P is a pattern of weight k and w′ is a word of length k over Σ. We say that a spaced word (P,w′) occurs at position i in a sequence S over Σ, if for all 1≤r≤k. For example, for
we have , and the spaced word w=(P,w′) occurs at position 2 in sequence S=CACGTCA since
A pattern is called contiguous if it consists of match positions only, a spaced word is called contiguous if the underlying pattern is contiguous. So a ‘contiguous spaced word’ is just a ‘word’ in the usual sense.
For a pattern P of weight k and two sequences S1 and S2 over an alphabet Σ, we say that there is a spaced-word match with respect to P – or a P-match – at (i,j) if
holds for all 1≤r≤k. For example, for sequences
and P as above, there is a P-match at (3,1) since one has S1[3]=S2[1],S1[4]=S2[2] and S1[6]=S2[4]. For a set of patterns, we say that there is a 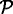 -match at (i,j) if there is some such that there is a P-match at (i,j).
-match at (i,j) if there is some such that there is a P-match at (i,j).
The number N of spaced-word matches for a pair of sequences with respect to a set 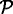 of patterns
of patterns
We consider sequences S1 and S2 as above and a fixed set of patterns. For simplicity, we assume that all patterns in 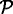 have the same length ℓ and the same weight k. For now, we use a simplified model of sequence evolution without insertions and deletions, with a constant mutation rate and with different sequence positions independent of each other. Moreover, we assume that we have the same substitution rates for all substitutions a→b,a≠b. We therefore consider two sequences S1 and S2 of the same length L with match probabilities
have the same length ℓ and the same weight k. For now, we use a simplified model of sequence evolution without insertions and deletions, with a constant mutation rate and with different sequence positions independent of each other. Moreover, we assume that we have the same substitution rates for all substitutions a→b,a≠b. We therefore consider two sequences S1 and S2 of the same length L with match probabilities
If qa is the relative frequency of a single character , is the background match probability, and p≥q is the match probability for a pair of ‘homologous’ positions.
For a pattern P, let N(S1,S2,P) be the number of pairs of positions (i,j) where there is a P-match between S1 and S2. We then define
to be the sum of all P-matches for patterns . Note that for two sequences, there can be P-matches for different patterns P at the same pair of positions (i,j). In the definition of N, we count not only the positions (i,j) where there is some P-match, but we count allP-matches with respect to all patterns in 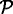 .
.
N can be seen as the inner product of -dimensional count vectors for spaced words with respect to the set of patterns 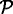 . In the special case where
. In the special case where 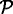 consists of a single contiguous pattern, i.e. for k=ℓ and m=1, N is also called the D2 score [42]. The statistical behaviour of the D2 score has been studied under the null model where S1 and S2 are unrelated [18,43]. In contrast to these studies, we want to investigate the number N of spaced-word matches for evolutionarily related sequence pairs under a model as specified above. To this end, we define to be the Bernoulli random variable that is 1 if there is a P-match between S1 and S2 at (i,j), , and 0 otherwise, so N can be written as
consists of a single contiguous pattern, i.e. for k=ℓ and m=1, N is also called the D2 score [42]. The statistical behaviour of the D2 score has been studied under the null model where S1 and S2 are unrelated [18,43]. In contrast to these studies, we want to investigate the number N of spaced-word matches for evolutionarily related sequence pairs under a model as specified above. To this end, we define to be the Bernoulli random variable that is 1 if there is a P-match between S1 and S2 at (i,j), , and 0 otherwise, so N can be written as
If we want to calculate the expectation value and variance of N, we have to distinguish between ‘homologous’ spaced-word matches, that is matches that are due do ‘common ancestry’ and ‘background matches’ due to chance. In our model where we do not consider insertions and deletions, a P-match at (i,j) is ‘homologous’ if and only if i=j holds. So in this special case, we can define
Note that if sequences do not contain insertions and deletions, every spaced-word match is either entirely a homologous match or entirely a background match. If indels are considered, a spaced-word match may involve both, homologous and background regions, and the above definitions need to be adapted. The set 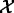 of all random variables can be written as , the total sum N of spaced-word matches with respect to the set
of all random variables can be written as , the total sum N of spaced-word matches with respect to the set 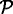 of patterns is
of patterns is
and the expected number of spaced-word matches is
where the expectation value of a single random variable is
| (1) |
There are L−ℓ+1 positions (i,i) and (L−ℓ)·(L−ℓ+1) positions (i,j),i≠j where spaced-word matches can occur, so we obtain
| (2) |
Estimating evolutionary distances from the number N of spaced-word matches
If the weight of the patterns – i.e. the number of match positions – in the spaced-words approach is sufficiently large, random space-word matches can be ignored. In this case, the Jensen-Shannon distance between two DNA sequences approximates the number of (spaced) words that occur in one of the compared sequences, but not in the other one. Thus, if two sequences of length L are compared and N is the number of (spaced) words that two sequences have in common, their Jenson-Shannon distance can be approximated by L−N. Accordingly, the Euclidean distances between two sequences can be approximated by the square root of this value if the distance is small and k is large enough. For small evolutionary distances, the Jensen-Shannon distance grows therefore roughly linearly with the distance between two sequences, and this explains why it is possible to produce reasonable phylogenies based on this metric. It is clear, however, that the Jensen-Shannon distance is far from linear to the real distance for larger distances. We therefore propose an alternative estimator of the evolutionary distance between two sequences in terms of the number N of spaced-word matches between them.
Again, we first consider sequences without insertions and deletions. From the expected number E(N) of spaced words shared by sequences S1 and S2 with respect to a set of patterns 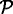 as given in equation (2), we obtain
as given in equation (2), we obtain
| (3) |
as an estimator for the match probability p for sequences without indels, and with Jukes-Cantor [44] we obtain
| (4) |
as an estimator for the distance d between the sequences S1 and S2. Note that for a model without insertions and deletions, it is, of course, not necessary to estimate the mismatch probability p from the number N of spaced-word matches. In this case, one could simply count the mismatches between two sequences and use their relative frequency as an estimator for p. The reason why we want to estimate p based on the number of spaced-word matches is that this estimate can be easily adapted to a model with insertions and deletions.
Local homologies, homologies on different strands and repeats
Next, we consider the case where S1 and S2 may have different lengths and share one region of local homology. Again, we assume again that there are no insertions and deletions within this homologous region. Let LHom be the length of the local homology; we assume that LHom is known. Equation (2) for the expected number N of spaced-word matches between two sequences S1 and S2 can be easily generalized to the case of local homology. If L1 and L2 are the lengths of S1 and S2, respectively, we define
to be the (approximate) number of positions (i,j) where a background match can occur (L∗ is only an approximation since we ignore spaced-word matches that involve both, homologous and background regions of the sequences). Then, we can the estimate the expected number of spaced-word matches as
and we obtain
| (5) |
as an estimator for the distance between S1 and S2. It is straight-forward, though somewhat cumbersome, to extend this estimator to the case where the homologous region contains insertions and deletions.
Note that, if local homologies between input sequences are known to the user, the best thing would be to remove the non-homologous regions of the sequences and to use the distance dN defined by equation (4) to the remaining homologous regions (which are then ‘globally’ related to each other). Nevertheless, the distance dloc might be useful in situations where the extent of homology between genomic sequences can be estimated, even though the precise location and boundaries of these homologies are unknown.
So far, we considered the case where homologies between two genomic sequences S1 and S2 are located on the same respective strand. For realistic applications, we have to take into account that homologies can occur on both strands of the DNA double helix. More importantly, we have to consider the case where a region of homology is located on one strand of S1, but on the reverse strand on S2. Let L1 and L2 be the lengths of S1 and S2, respectively, with L1≤L2. For simplicity, we assume that the entire sequence S1 is homologous to a contiguous segment of S2 and we ignore insertions and deletions. We now assume, however, that some segments of S1 may align to their homologous counterpart in S2 while other segments of S2 may align to the reverse complement of their counterparts in S2. The more general situation involving local homology and indels can be accounted for as discussed above.
The simplest way to capture homologies between S1 and S2 regardless of their orientation is to concatenate one of the sequences, say S2 with its reverse complement and to compare S1 to this concatenated sequence. So in this case, we would consider all spaced-word matches between S1 and where is the concatenation of S2 and its reverse complement. To estimate the expected number of spaced-word matches in this situation, we can homologous spaced-word matches can be locate and ≈2·(L1−ℓ+1)·(L2−ℓ) positions where background matches can occur. By adapting Formulae (2) to (4) accordingly and ignoring fringe effects, we obtain
| (6) |
| (7) |
as an estimator for the match probability p for sequences without indels, and with Jukes-Cantor [44] we obtain
| (8) |
as an estimator for the distance d between the sequences S1 and S2 if homologies on the reverse complement are taken into account.
Finally, we consider the case where sequences contain repeats. A direct application of the distance functions discussed so far would be highly sensitive to repeats in the input sequences, since repeats can drastically increase the number N of (spaced) word matches. This can even lead to negative distance values if the number N of 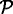 matches between two sequences with repeats exceeds the expected number of
matches between two sequences with repeats exceeds the expected number of 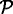 matches between a non-repetitive sequence of the same length to itself. A simple but efficient way of dealing with repeats is to use binary variables Nbin(S1,S2,P) that are one if there are one or several P matches between sequences S1 and S2, and zero if there is no such match. Instead of using the number N of
matches between a non-repetitive sequence of the same length to itself. A simple but efficient way of dealing with repeats is to use binary variables Nbin(S1,S2,P) that are one if there are one or several P matches between sequences S1 and S2, and zero if there is no such match. Instead of using the number N of  matches for a set
matches for a set  of patterns, we then consider
of patterns, we then consider
and distances , and , respectively, can be defined as in equations (4), (5) and (8), but with N replaced by Nbin.
The variance of N
Our new distance measure and other word-based distance measures depend on the number N of (spaced) word matches between sequences. To study the stability of these measures, we want to calculate the variance of N. To do so, we adapt results on the occurrence of words in a sequence as outlined in [45]. Since N can be written as the sum of all random variables , we need to calculate the covariances of these random variables. To simplify this, we make a further assumption on our sequence model: we assume that the four nucleotides occur with the same probability 0.25. In this case, the covariance of two random variables and can be non-zero only if i′−i=j′−j holds (note that this is not true if nucleotides have different probabilities to occur). In particular, for random variables and , their covariance is zero. Thus, we only need to consider covariances of pairs of random variables and .
For patterns P,P′ and s∈N we define n(P,P′,s) to be the number of integers that are match positions of P or match positions of P′ shifted by s positions to the right (or both). Formally, if
denotes the set of match positions of a pattern P shifted by s positions to the right, we define
For example, for P=101011,P′=111001 and s=2, there are 6 positions that are match positions of P or of P′ shifted by 2 positions to the right, namely positions 1, 3, 4, 5, 6, 8:
so one has n(P,P′,s)=6. In particular, one has n(P,P,0)=k for all patterns P of weight k, and
for all contiguous patterns P of weight (or length) k. With this notation, we can write
| (9) |
for all
To calculate the covariance of two random variables from  , we distinguish again between homologous and random matches. We first consider ’homologous’ pairs . Here, we obtain with (9)
, we distinguish again between homologous and random matches. We first consider ’homologous’ pairs . Here, we obtain with (9)
| (10) |
Similarly, for a pair of ‘background’ variables , one obtains
| (11) |
Since ‘homologous’ and ‘background’ variables are uncorrelated, the variance of N can be written as
We express the variance of these sums of random variable as the sum of all of their covariances, so for the ’homologous’ random variables we can write
Since the covariance for non-correlated random variables vanishes, we can ignore the covariances of all pairs with |i−i′|≥l so, ignoring side effects, we can write the above sum as
and since the above covariances depend only on s but not on i, we can use (9) and (11) and obtain
and similarly
Together, we get
| (12) |
Test results
Simulated DNA sequences
To evaluate the distance function dN defined by equation (4), we simulated pairs of DNA sequences with an (average) length of 100,000 and with an average of d substitutions per sequence position. More precisely, we generated sequence pairs by generating a first sequence using a Bernoulli model with probability 0.25 for each nucleotide. A second sequence was then generated from the first sequence by substituting nucleotides with a probability corresponding to the substitution frequency d, as calculated with Jukes-Cantor. We varied d between 0 and 1 and compared the distances estimated by our distance measure and by various other alignment-free programs to the ‘real’ distance d. We performed these experiments for sequence pairs without insertions and deletions and for sequence pairs where we included insertions and deletions with a probability of 1% at every position. The length of indels was randomly chosen between 1 and 50 with uniform probability.
Figure 1 shows the results of these experiments. Our new distance measure dN applied to spaced-word frequencies is well in accordance with the real distances d for values of d≤0.8 on sequence pairs without insertions and deletions if the single-pattern version of our program is used. For the multiple-pattern version, our distance function estimates the real distances correctly for all values of d≤1. If indels are added as specified above, our distance functions slightly overestimates the real distance d. By contrast, the Jensen-Shannon distance applied to the same spaced-word frequencies increased non-linearly with d and flattened for values of around d≥0.4.
Figure 1.
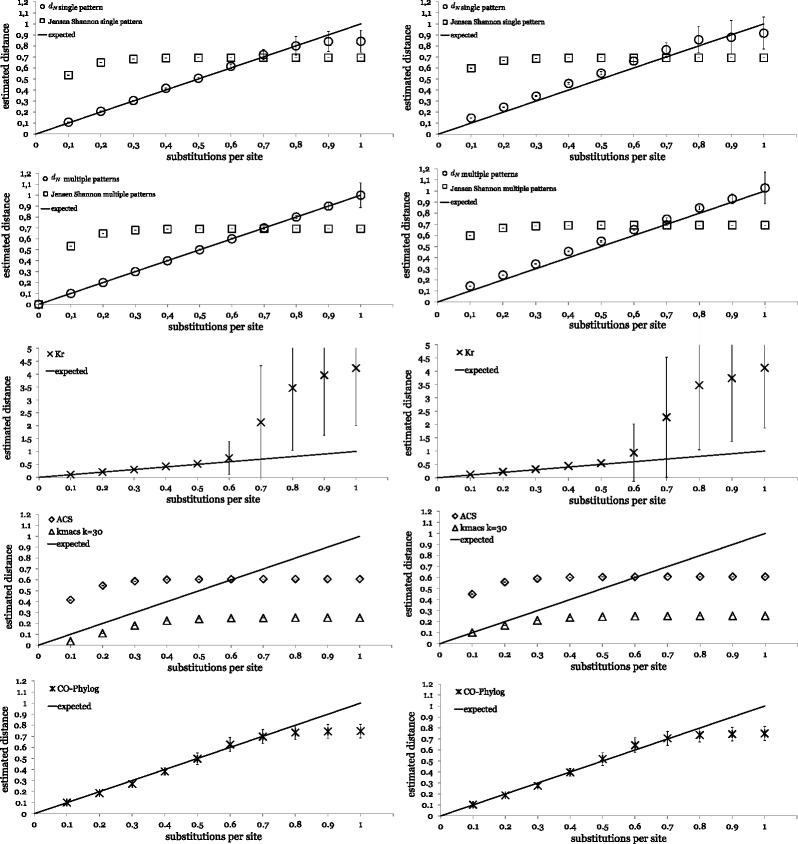
Distances calculated by different alignment-free methods. Distances were calculated for pairs of simulated DNA sequences and plotted against their ‘real’ distances d measured in substitutions per site. Plots on the left-hand side are for sequence pairs without insertions and deletions, on the right-hand side the corresponding results are shown for sequences with an indel probability of 1% for each site and an average indel length of 25. From top to bottom, the applied methods were: 1. spaced words with the single-pattern approach and the Jensen-Shannon distance (squares) and the distance d
N defined in equation (4) in this paper (circles), 2. the multiple-pattern version of Spaced Words using sets 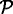 of m=100 patterns with the same distance functions, 3. distances calculated with K
r [37], 4. with kmacs [47] and ACS [30] and 5. with Co-phylog [38].
of m=100 patterns with the same distance functions, 3. distances calculated with K
r [37], 4. with kmacs [47] and ACS [30] and 5. with Co-phylog [38].
As mentioned, Kr [46] estimates evolutionary distances on the basis of a probabilistic model of evolution. In our study, Kr correctly estimated the true distance d for values of around d≤0.6, this precisely corresponds to the results reported by the authors of the program. For larger distances, Kr grossly overestimates the distance d, though, and the variance strongly increases. The distances estimated by Co-phylog [38] nearly coincide with the substitution rate d for values d≤0.7, then the curve flattens. Moreover, it appears that the distances calculated with Co-phylog are not much affected by indels. The distance values calculated by the program k mismatch average common substring (kmacs) that we previously developed [47] are roughly linear to the real distances d for values of up to around d=0.3. From around d=0.5 on, the curve becomes flat. With k=30 mismatches, the performance of kmacs was better than with k=0, in which case kmacs corresponds to the Average Common Substring (ACS) approach [30].
Real-world genomes
Next, we applied various distance measures to a set of 27 mitochondrial genomes from different primates that were previously used by [46] as a benchmark data set for alignment-free approaches. We used our multiple spaced-words approach with the parameters that we used in [25], that is with a pattern weight (number of match positions) of k=9 and with pattern lengths ℓ between 9 and 39, i.e. with up to 30 don’t-care positions in the patterns. For each value of ℓ, we randomly generated sets  of m=100 patterns. For this data set, we used the distance dRC defined in equation (8) that takes the reverse complement of the input sequences into account. (We did not use the ‘binary’ version of our distance function, since these sequences do not contain major repeats). In addition, we used the competing approaches FFP [32], CVTree [48], Kr [37], kmacs [47], ACS [30] and Co-phylog [38]. For some of these methods, program parameters need to be defined, e.g. a predefined word length or the number of allowed mismatches. For these methods we tested various parameters and used the best performing values.
of m=100 patterns. For this data set, we used the distance dRC defined in equation (8) that takes the reverse complement of the input sequences into account. (We did not use the ‘binary’ version of our distance function, since these sequences do not contain major repeats). In addition, we used the competing approaches FFP [32], CVTree [48], Kr [37], kmacs [47], ACS [30] and Co-phylog [38]. For some of these methods, program parameters need to be defined, e.g. a predefined word length or the number of allowed mismatches. For these methods we tested various parameters and used the best performing values.
With each method, we calculated a distance matrix for the input sequences, and we compared this matrix to a reference distance matrix that we calculated with the program Dnadist from the PHYLIP package [49] based on a reference multiple alignment. For comparison with the reference matrix, we used a software program based on the Mantel test [50] that was also used by Didier et al. [51]. Figure 2 shows the results of this comparison. As can be seen, our new distance measure dRC, applied to multiple spaced-word frequencies, produced distance matrices close to the reference matrix and outperformed the Jenson-Shannon distance for all pattern lengths ℓ that we tested. The distance function dRC also outperformed some of the existing alignment-free methods, with the exception of Kr and kmacs.
Figure 2.
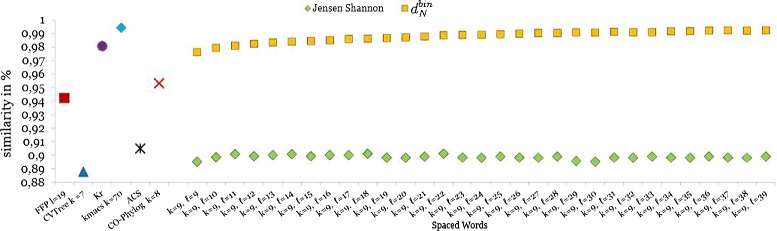
Comparison of distance matrices for primate mitochondrial genomes. We applied various alignment-free methods to a set of 27 mitochondrial genomes from different primates and compared the resulting distance matrices to a trusted reference distance matrix using the Mantel test. The similarity between the calculated matrices and the reference matrix is plotted. We applied our Spaced-Words approach using sets 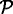 of 100 randomly calculated patterns with weight k=9 and length ℓ between 9 and 39, i.e. with 9 match positions and up to 30 don’t care positions. Yellow squares are the results for the ‘binary’ version of new distance measure . We did not use the reverse-complement option on these data, since genes in the compared genomes are known to be on the same strand. Green diamonds are the results for the Jensen-Shannon distance applied to the same spaced-word frequency vectors as explained in [25]. In addition, distances calculated by six other alignment-free methods were evaluated.
of 100 randomly calculated patterns with weight k=9 and length ℓ between 9 and 39, i.e. with 9 match positions and up to 30 don’t care positions. Yellow squares are the results for the ‘binary’ version of new distance measure . We did not use the reverse-complement option on these data, since genes in the compared genomes are known to be on the same strand. Green diamonds are the results for the Jensen-Shannon distance applied to the same spaced-word frequency vectors as explained in [25]. In addition, distances calculated by six other alignment-free methods were evaluated.
In addition to this direct distance comparison we performed an indirect evaluation by phylogeny analysis. To do so, we applied Neighbor-Joining [36] to the distance matrices and compared the resulting trees to the corresponding reference tree, using the Robinson-Foulds (RF) metric [52]. The results for the mitochondrial genomes are shown in Figure 3. The outcome of this evaluation is partially in contradiction to the results of the direct comparison of the distances. Figure 2 shows that the distance matrices produced by Spaced-Words with the Jensen-Shannon divergence are worse than the distance matrices produced by most other methods, if these matrices are directly compared to the reference distance matrix. However, Spaced Words with Jensen-Shannon led to better tree topologies than most other methods in our study, as shown in Figure 3. A similar contradictory result is observed for Kr. While the distance matrix produced by Kr is similar to the reference matrix, the tree topology produced with these distances is further away from the reference topology than the trees computed by the other alignment-free approaches in our study.
Figure 3.
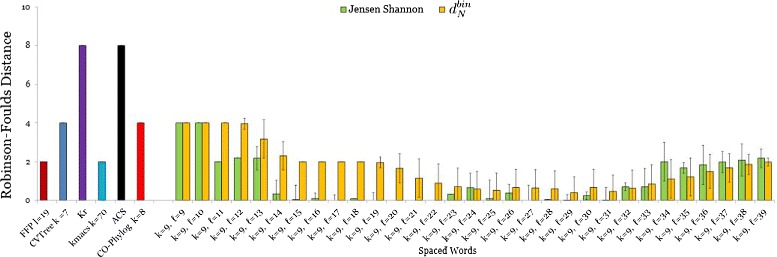
RF distances for primate mitochondrial genomes. Performance of various alignment-free methods on the same set of 27 primate mitochondrial genomes as in Figure 2. Neighbour-Joining was applied to the calculated distance matrices, the resulting tree topologies were compared with the Robinson-Foulds metric. Parameters for Spaced Words and colour coding as in Figure 2.
To evaluate our new method for larger sequences we used two prokaryotic data sets. The first data set consists of 26 E. coli and Shigella genomes, which are very closely related and the second data set consist of 32 Roseobacter genomes, which are far more divergent. For these sequences, we used our ‘repeat-aware’ distance function . As for the primate mitochondrial genomes, we calculated distance matrices using the same alignment-free methods, constructed trees with Neighbor-Joining and compared the resulting tree topologies to a benchmark tree using the RF metric. For the E.coli/Shigella genomes we used the tree proposed by [53] as reference which is based on concatenated alignments of the 2034 core genes. For the Roseobacter genomes we used the tree by [54] as reference. This benchmark tree was constructed based on alignments of 70 universal single-copy genes.
The results for the E.coli/Shigella are shown in Figure 4. The best result was achieved by Co-phylog with a RF distance of only 4, followed by our new distance dRC with a RF distance of 10, which is a huge improvement compared to the previously described version of Spaced Words where we used the Jensen-Shannon divergence. Kr performed slightly worse than our new estimator with a RF distance of 12. The other alignment-free methods performed relatively poorly. For the Spaced Words approach we performed 25 runs with m=100 patterns that were randomly generated. For this data set, all sets of patterns led to the same tree topology. Additionally the results are also not influenced by the number of don’t-care positions, which can be explained by the very small number of substitutions between these genomes.
Figure 4.
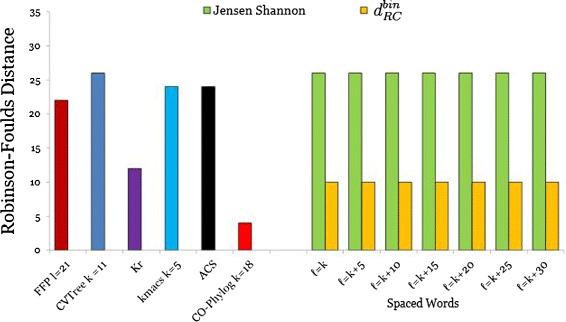
RF distances for E.coli/Shigella genomes. Performance of various alignment-free methods on a set of 26 E.coli/Shigella genomes. Robinson-Foulds distances to the reference tree are shown. For Spaced Words, we used a weight of k=17 and applied the ‘binary’ distance function . Colour coding as in Figure 2.
For the Roseobacter genomes we used the same evaluation procedure as for the E. coli/Shigella genomes. Here, our new evolutionary distance dRC outperformed the other alignment-free methods, if don’t-care positions are incorporated in the patterns, and the performance increased with the number of don’t-care positions, as shown in Figure 5. (Without don’t-care positions, i.e. if classical word-matches are counted, dRC was slightly outperformed by Co-phylog, but was still better than all other methods in our comparison). The RF distance to the benchmark tree varied between 24 and 28. Co-phylog ranked second in this evaluation with a RF distance of 28. All other methods achieved a RF distance of greater or equal to 30. Surprisingly, Kr performs worse than other programs on these sequences.
Figure 5.
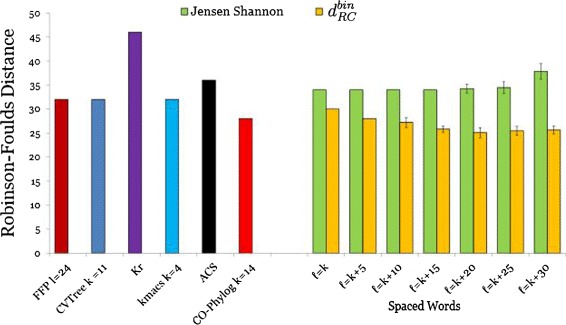
RF distances for Roseobacter genomes. Performance of various alignment-free methods on a set of 32 Roseobacter genomes. Robinson-Foulds distances to the reference tree are shown. Spaced Words was used with parameters as in Figure 4, colour coding is as in Figure 2.
The variance of N: experimental results
Figure 1 shows not only striking differences in the shape of the distance functions used by various alignment-free programs. There are also remarkable differences in the variance of the distances calculated with our new distance measure dN that we defined in equation (4). The distance DN is defined in terms of the number N of (spaced) word matches between two sequences. As mentioned above, the established Jensen-Shannon and Euclidean distances on (spaced) word frequency vectors also depend on N, for small distances, they can be approximated by L−N and , respectively. Thus, the variances of these three distance measures directly depend on the variance of N. As can be seen in Figure 1, the variance of dN increases with the frequency of substitutions. Also, the variance is higher for the single-pattern approach than for the multiple-pattern approach. To explain this observation, we calculated the variance of the normalized number N/m of spaced-word matches using equation 12. Figure 6 summarizes the results for a sequence length of L=16.000 and mismatch frequencies of 0.7 and 0.25, respectively. As can be seen, for single spaced words the variance of N/m is far smaller than for contiguous words, and for multiple spaced words, the variance is further reduced.
Figure 6.
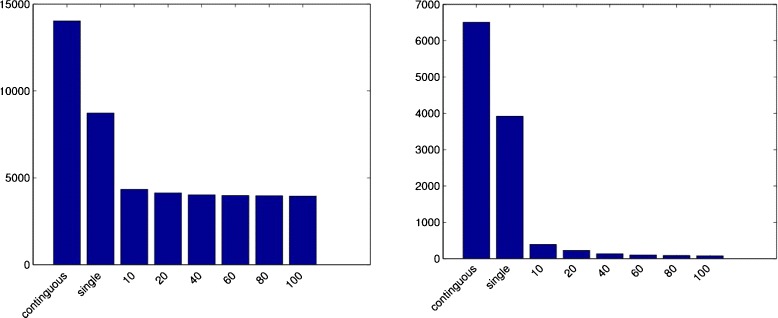
Variance of the number of spaced-word matches. Variance of the normalized number of spaced-word matches where is the number of patterns in the multiple-pattern approach. Formula (12) was applied to contiguous words and to single and multiple spaced words for un-gapped sequence pairs of length 16,000 nt with a mismatch frequency of 0.7 (left) and 0.25 (right).
Discussion
In this paper, we proposed a new estimator dN for the evolutionary distance between two DNA sequences that is based on the number N of spaced-word matches between them. While most alignment-free methods use ad-hoc distance measures, the distance function that we defined is based on a probabilistic model of evolution and seems to be a good estimator for the number of substitutions per site that have occurred since two sequences have evolved separately. For simplicity, we used a model of evolution without insertions and deletions. Nevertheless, our test results show that our distance function is still a reasonable estimator if the input sequences contain a moderate number of insertions and deletions although, in this case, distances between the input sequences are overestimated since the number N of spaced-word matches is smaller than it would be for sequences without indels.
The model that we used to derive our distance dN assumes that two sequences are globally related. If sequences share only local homology, the number N of spaced-word matches would be smaller than for globally related sequences with the same length and rate of mismatches, so their distance would be over-estimated by our distance measure dN. This is clearly a limitation of our approach. However, as indicated in section Estimating evolutionary distances from the number N of spaced-word matches, our distance function can be adapted to the case of local homologies if the length of these homologies and the number of gaps in the homologous regions can be estimated. In principle, it should therefore be possible to apply our method to locally related sequences by first estimating the extent of their shared (local) homology and then using the distance dloc defined in equation (5) instead of dN.
The distance measures introduced in this paper and other distances that we previously used for our spaced words approach depend on the number N of space-word matches between two sequences with respect to a set 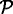 of patterns of ‘match’ and ‘don’t care’ positions. This is similar for more traditional alignment-free methods that calculated distances based on k-mer frequencies. While the expected number of (spaced) word matches is essentially the same for contiguous words and for spaced words of the corresponding weight, we have showed that the variance of N is considerably lower for spaced-words than for the traditionally used contiguous words. Moreover, with our multiple-pattern approach the variance of the normalized number of spaced-word matches is further reduced. This seems to be the main reason why our multiple spaced words approach outperforms the single-pattern approach that we previously introduced as well as the classical k-mer approach when used for phylogeny reconstruction.
of patterns of ‘match’ and ‘don’t care’ positions. This is similar for more traditional alignment-free methods that calculated distances based on k-mer frequencies. While the expected number of (spaced) word matches is essentially the same for contiguous words and for spaced words of the corresponding weight, we have showed that the variance of N is considerably lower for spaced-words than for the traditionally used contiguous words. Moreover, with our multiple-pattern approach the variance of the normalized number of spaced-word matches is further reduced. This seems to be the main reason why our multiple spaced words approach outperforms the single-pattern approach that we previously introduced as well as the classical k-mer approach when used for phylogeny reconstruction.
As we have shown, the variance of N depends on the number of overlapping ‘match’ positions if patterns from 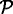 are shifted against each other. Consequently, in our single-pattern approach, the variance of N is higher for periodic patterns than for non-periodic patterns. For example, for the periodic pattern 101010…, the variance is equal to the variance of the contiguous pattern of the corresponding weight. In our previous benchmark studies, we could experimentally confirm that spaced words performs better with non-periodic patterns than with periodic patterns. The theoretical results of this study may be useful to find patterns or sets of patterns that minimize the variance of N to further improve our spaced-words approach.
are shifted against each other. Consequently, in our single-pattern approach, the variance of N is higher for periodic patterns than for non-periodic patterns. For example, for the periodic pattern 101010…, the variance is equal to the variance of the contiguous pattern of the corresponding weight. In our previous benchmark studies, we could experimentally confirm that spaced words performs better with non-periodic patterns than with periodic patterns. The theoretical results of this study may be useful to find patterns or sets of patterns that minimize the variance of N to further improve our spaced-words approach.
Acknowledgements
We would like to thank Marcus Boden, Sebastian Lindner, Alec Guyomard and Claudine Devauchelle for help with the program evaluation and Gilles Didier for help with the software to compare distance matrices. We thank Matteo Comin, Ruth Kantorovitz, Saurabh Sinha and an unknown WABI reviewer for pointing out an an error regarding the covariance of spaced-word matches in the previous version of this manuscript that was published at WABI 2014.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BM conceived the new distance measures and the theoretical results on the variance of N and wrote most of the manuscripts. CAL implemented Spaced Words, did most of the program evaluation and wrote parts of the manuscript. BZ and SH contributed to the program evaluation. All authors read and approved the final manuscript.
Contributor Information
Burkhard Morgenstern, Email: bmorgen@gwdg.de.
Bingyao Zhu, Email: lucil_zby@hotmail.com.
Sebastian Horwege, Email: sebastian.horwege@stud.uni-goettingen.de.
Chris André Leimeister, Email: chris.leimeister@stud.uni-goettingen.de.
References
- 1.Vinga S. Editorial: Alignment-free methods in computational biology. Briefings Bioinf. 2014;15:341–2. doi: 10.1093/bib/bbu005. [DOI] [PubMed] [Google Scholar]
- 2.Leslie C, Eskin E, Noble WSS. Pacific Symposium on Biocomputing. Singapore: World Scientific Publishing; 2002. The spectrum kernel: a string kernel for SVM protein classification. [PubMed] [Google Scholar]
- 3.Lingner T, Meinicke P. Remote homology detection based on oligomer distances. Bioinformatics. 2006;22:2224–31. doi: 10.1093/bioinformatics/btl376. [DOI] [PubMed] [Google Scholar]
- 4.Lingner T, Meinicke P. Word correlation matrices for protein sequence analysis and remote homology detection. BMC Bioinf. 2008;9:259. doi: 10.1186/1471-2105-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comin M, Verzotto D. The irredundant class method for remote homology detection of protein sequences. J Comput Biol. 2011;18:1819–29. doi: 10.1089/cmb.2010.0171. [DOI] [PubMed] [Google Scholar]
- 6.Li R, Li Y, Kristiansen K, Wang J. SOAP: short oligonucleotide alignment program. Bioinformatics. 2008;24:713–4. doi: 10.1093/bioinformatics/btn025. [DOI] [PubMed] [Google Scholar]
- 7.Langmead B, Trapnell C, Pop M, Salzberg S. Ultrafast and memory-efficient alignment of short dna sequences to the human genome. Genome Biol. 2009;10:25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmadi A, Behm A, Honnalli N, Li C, Weng L, Xie X. Hobbes: optimized gram-based methods for efficient read alignment. Nucleic Acids Res. 2011;40:1. doi: 10.1093/nar/gkr1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patro R, Mount SM, Kingsford C. Sailfish enables alignment-free isoform quantification from RNA-seq reads using lightweight algorithms. Nat Biotechnol. 2014;32:462–4. doi: 10.1038/nbt.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–9. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teeling H, Waldmann J, Lombardot T, Bauer M, Glockner F. Tetra: a web-service and a stand-alone program for the analysis and comparison of tetranucleotide usage patterns in dna sequences. BMC Bioinf. 2004;5:163. doi: 10.1186/1471-2105-5-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterji S, Yamazaki I, Bai Z, Eisen JA. Research in Computational Molecular Biology, 12th Annual International Conference, RECOMB 2008, Singapore, March 30 - April 2, 2008. Proceedings. Berlin, Heidelberg: Springer; 2008. Compostbin: A DNA composition-based algorithm for binning environmental shotgun reads. [Google Scholar]
- 13.Wu Y-W, Ye Y. A novel abundance-based algorithm for binning metagenomic sequences using l-tuples. J Comput Biol. 2011;18:523–34. doi: 10.1089/cmb.2010.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaseichuk O, Borneman J, Jiang T. Separating metagenomic short reads into genomes via clustering. Algorithms Mol Biol. 2012;7:27. doi: 10.1186/1748-7188-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung HCM, Yiu SM, Yang B, Peng Y, Wang Y, Liu Z, et al. A robust and accurate binning algorithm for metagenomic sequences with arbitrary species abundance ratio. Bioinformatics. 2011;27:1489–95. doi: 10.1093/bioinformatics/btr186. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Leung HCM, Yiu SM, Chin FYL. Metacluster 5.0: a two-round binning approach for metagenomic data for low-abundance species in a noisy sample. Bioinformatics. 2012;28:356–62. doi: 10.1093/bioinformatics/bts397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meinicke P, Tech M, Morgenstern B, Merkl R. Oligo kernels for datamining on biological sequences: a case study on prokaryotic translation initiation sites. BMC Bioinf. 2004;5:169. doi: 10.1186/1471-2105-5-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantorovitz M, Robinson G, Sinha S. A statistical method for alignment-free comparison of regulatory sequences. Bioinformatics. 2007;23:249–55. doi: 10.1093/bioinformatics/btm211. [DOI] [PubMed] [Google Scholar]
- 19.Leung G, Eisen MB. Identifying cis-regulatory sequences by word profile similarity. PloS one. 2009;4(9):6901. doi: 10.1371/journal.pone.0006901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Federico M, Leoncini M, Montangero M, Valente P. Direct vs 2-stage approaches to structured motif finding. Algorithms Mol Biol. 2012;7:20. doi: 10.1186/1748-7188-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blaisdell BE. A measure of the similarity of sets of sequences not requiring sequence alignment. Proc Nat Acad Sci USA. 1986;83:5155–9. doi: 10.1073/pnas.83.14.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J. Divergence measures based on the shannon entropy. IEEE Trans Inf theory. 1991;37:145–51. doi: 10.1109/18.61115. [DOI] [Google Scholar]
- 23.Ma B, Tromp J, Li M. PatternHunter: faster and more sensitive homology search. Bioinformatics. 2002;18:440–5. doi: 10.1093/bioinformatics/18.3.440. [DOI] [PubMed] [Google Scholar]
- 24.Boden M, Schöneich M, Horwege S, Lindner S, Leimeister C-A, Morgenstern B. German Conference on Bioinformatics 2013. In: Beißbarth T, Kollmar M, Leha A, Morgenstern B, Schultz A-K, Waack S, Wingender E, editors. OpenAccess Series in Informatics (OASIcs) Dagstuhl, Germany: Schloss Dagstuhl–Leibniz-Zentrum fuer Informatik; 2013. [Google Scholar]
- 25.Leimeister C-A, Boden M, Horwege S, Lindner S, Morgenstern B. Fast alignment-free sequence comparison using spaced-word frequencies. Bioinformatics. 2014;30:1991–9. doi: 10.1093/bioinformatics/btu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwege S, Lindner S, Boden M, Hatje K, Kollmar M, et al. Spaced words and kmacs: fast alignment-free sequence comparison based on inexact word matches. Nucleic Acids Res. 2014;42:W7–W11. doi: 10.1093/nar/gku398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onodera T, Shibuya T. The gapped spectrum kernel for support vector machines. In: Perner P, editor. Machine Learning and Data Mining in Pattern Recognition, Lecture Notes in Computer Science. Berlin,Heidelberg: Springer; 2013. [Google Scholar]
- 28.Ghandi M, Mohammad-Noori M, Beer MA. Robust k-mer frequency estimation using gapped k-mers. J Math Biol. 2014;69:469–500. doi: 10.1007/s00285-013-0705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghandi M, Lee D, Mohammad-Noori M, Beer MA. Enhanced regulatory sequence prediction using gapped k-mer features. PLoS Comput Biol. 2014;10(7):1003711. doi: 10.1371/journal.pcbi.1003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulitsky I, Burstein D, Tuller T, Chor B. The average common substring approach to phylogenomic reconstruction. J Comput Biol. 2006;13:336–50. doi: 10.1089/cmb.2006.13.336. [DOI] [PubMed] [Google Scholar]
- 31.Didier G, Debomy L, Pupin M, Zhang M, Grossmann A, Devauchelle C, et al. Comparing sequences without using alignments: application to HIV/SIV subtyping. BMC Bioinf. 2007;8:1. doi: 10.1186/1471-2105-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sims GE, Jun S-R, Wu GA, Kim S-H. Alignment-free genome comparison with feature frequency profiles (FFP) and optimal resolutions. Proc Nat Acad Sci. 2009;106:2677–82. doi: 10.1073/pnas.0813249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domazet-Loso M, Haubold B. Alignment-free detection of local similarity among viral and bacterial genomes. Bioinformatics. 2011;27(11):1466–72. doi: 10.1093/bioinformatics/btr176. [DOI] [PubMed] [Google Scholar]
- 34.Haubold B, Reed FA, Pfaffelhuber P. Alignment-free estimation of nucleotide diversity. Bioinformatics. 2011;27:449–55. doi: 10.1093/bioinformatics/btq689. [DOI] [PubMed] [Google Scholar]
- 35.Comin M, Verzotto D. Alignment-free phylogeny of whole genomes using underlying subwords. Algorithms Mol Biol. 2012;7:34. doi: 10.1186/1748-7188-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 37.Haubold B, Pierstorff N, Möller F, Wiehe T. Genome comparison without alignment using shortest unique substrings. BMC Bioinf. 2005;6:123. doi: 10.1186/1471-2105-6-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi H, Jin L. Co-phylog: an assembly-free phylogenomic approach for closely related organisms. Nucleic Acids Res. 2013;41:75. doi: 10.1093/nar/gkt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haubold B, Klötzl F, Pfaffelhuber P. andi: Fast and accurate estimation of evolutionary distances between closely related genomes. Bioinformatics.doi:10.1093/bioinformatics/btu815. [DOI] [PubMed]
- 40.Noé L, Martin DEK. A coverage criterion for spaced seeds and its applications to SVM string-kernels and k-mer distances. J Comput Biol. 2014;12:947–63. doi: 10.1089/cmb.2014.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgenstern B, Zhu B, Horwege S, Leimeister C. Proc. Workshop on Algorithms in Bioinformatics (WABI’14). Lecture Notes in Bioinformatics. Berlin Heidelberg.: Springer; 2014. Estimating evolutionary distances from spaced-word matches. [Google Scholar]
- 42.Lippert RA, Huang H, Waterman MS. Distributional regimes for the number of k-word matches between two random sequences. Proc Nat Acad Sci. 2002;99:13980–9. doi: 10.1073/pnas.202468099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinert G, Chew D, Sun F, Waterman MS. Alignment-free sequence comparison (i): Statistics and power. J Comput Biol. 2009;16:1615–34. doi: 10.1089/cmb.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jukes TH, Cantor CR. Evolution of Protein Molecules: Academy Press, NY; 1969.
- 45.Robin S, Rodolphe F, Schbath S. DNA, Words and Models: Statistics of Exceptional Words. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- 46.Haubold B, Pfaffelhuber P, Domazet-Loso M, Wiehe T. Estimating mutation distances from unaligned genomes. J Comput Biol. 2009;16:1487–500. doi: 10.1089/cmb.2009.0106. [DOI] [PubMed] [Google Scholar]
- 47.Leimeister C-A, Morgenstern B. kmacs: the k-mismatch average common substring approach to alignment-free sequence comparison. Bioinformatics. 2014;30:2000–8. doi: 10.1093/bioinformatics/btu331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi J, Luo H, Hao B. CVTree: a phylogenetic tree reconstruction tool based on whole genomes. Nucleic Acids Res. 2004;32(suppl 2):45–7. doi: 10.1093/nar/gkh362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Felsenstein J. PHYLIP - Phylogeny Inference Package (Version 3.2) Cladistics. 1989;5:164–6. [Google Scholar]
- 50.Bonnet E, de Peer YV. zt: A sofware tool for simple and partial mantel tests. J Stat Software. 2002;7:1–12. [Google Scholar]
- 51.Didier G, Laprevotte I, Pupin M, Hénaut A. Local decoding of sequences and alignment-free comparison. J Comput Biol. 2006;13:1465–76. doi: 10.1089/cmb.2006.13.1465. [DOI] [PubMed] [Google Scholar]
- 52.Robinson D, Foulds L. Comparison of phylogenetic trees. Mathematical Biosciences. 1981;53:131–47. doi: 10.1016/0025-5564(81)90043-2. [DOI] [Google Scholar]
- 53.Zhou Z, Li X, Liu B, Beutin L, Xu J, Ren Y, et al. Derivation of Escherichia coli O157:H7 from Its O55:H7 Precursor. PLOS One. 2010;5:8700. doi: 10.1371/journal.pone.0008700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newton RJ, Griffin LE, Bowles KM, Meile C, Gifford S, Givens CE, et al. Genome characteristics of a generalist marine bacterial lineage. ISME J. 2010;4:784–98. doi: 10.1038/ismej.2009.150. [DOI] [PubMed] [Google Scholar]


