Abstract
This study investigates how collectively moving epithelial cells function to coordinate their collective movement to repair a wound. Using a lens ex vivo mock cataract surgery model we show that region-specific reorganization of cell-cell junctions, cytoskeletal networks and myosin function along apical and basal domains of an epithelium mediates the process of collective migration. An apical junctional complex composed of N-cadherin/ZO-1/myosin II linked to a cortical actin cytoskeleton network maintains integrity of the tissue during the healing process. These cells’ basal domains often preceded their apical domains in the direction of movement, where an atypical N-cadherin/ZO-1 junction, linked to an actin stress fiber network rich in phosphomyosin, was prominent in cryptic lamellipodia. These junctions joined the protruding forward-moving lamellipodia to the back end of the cell moving directly in front of it. These were the only junctions detected in cryptic lamellipodia of lens epithelia migrating in response to wounding that could transmit the protrusive forces that drive collective movement. Both integrity of the epithelium and ability to effectively heal the wound was found to depend on myosin mechanical cues.
Keywords: Collective migration, Wound healing, cell-cell junctions, myosin II, injury-repair, cadherin, sheet movement, lens
Introduction
Collective migration involves the coordinated movement of groups, strands or sheets of cells (Friedl and Gilmour, 2009; Ilina and Friedl, 2009; Rorth, 2009). It is a process essential to development, wound repair and pathogenic disease (Montell, 2008; Rorth, 2009; Weijer, 2009). Cell-cell junctions are a pivotal feature of collectively migrating cells, as they provide the mechanism by which cells associate and communicate with one another. The mechanisms that coordinate cell movement, particularly how groups of cells are able to maintain their collectivity while at the same time altering their interactions with each other and their environment as they move together to repair a wound, remains an area in need of further exploration.
Cadherin cell-cell junctions physically connect to the actin cytoskeleton through protein intermediates including catenins and actin binding proteins (Yonemura, 2011). This linkage to the cytoskeleton provides a mechanism by which these receptors can interconnect and “hardwire” cells together, integrate mechanical cues to and from the environment, couple the physical properties of cells and coordinate cell behavior. As mechanosensors, adhesion receptor complexes are poised as essential responders to and mediators of force (Bershadsky et al., 2003; Borghi et al., 2012; le Duc et al., 2010; Leckband et al., 2011; Tabdanov et al., 2009). Force generation through the actomyosin cytoskeleton is necessary to drive individual cell movement (Vicente-Manzanares and Horwitz, 2011; Vicente-Manzanares et al., 2009), but there is less evidence as the how these forces coordinate the cohesive and protrusive function of cells to regulate collective movement. In individual mesendoderm cells the cadherin complex has a mechanosensing function associated with directing both protrusive behavior and migration, with important implications for collective migration. In these cells, application of mechanical force to a cadherin-coated magnetic bead induced both polarized cell protrusion and persistent migration of single cells (Weber et al., 2012). Myosin II is activated in a front-to-back gradient within a collectively moving epithelial sheet of MCF10A cells. Transmission of this mechanosensing signal, important to polarized cell movement, is dependent on cadherin junctions that couple forces between these cells. (Ng et al., 2012). In addition, modeling studies support the importance of cell-cell adhesion to directed collective migration (Vitorino et al., 2011). Mechanosensing through cell-cell junctions is an important feature in the regulation of collective migration, and the mechanisms by which actomyosin dynamics are coordinated with cadherin junctions to regulate collective cell migration are investigated here.
Cell-cell interactions can also provide spatial inputs to cells that can affect cell behavior on a local level. In response to a scratch wound, epithelial sheets of migrating MDCK cells exhibit regionally-specific organization. The cells a few rows behind the leader cells at the wound edge maintain contact with one another apically, while at the same time they extend the cryptic lamellipodia along the substrate that drive cell sheet movement (Farooqui and Fenteany, 2005). Spatial differences in cell structure and function are likely essential to regulating the behavior of interacting cells to maintain collectivity and control collective movement, but is there distinct regulation of cell-cell junctions at the apical and basal domains of migrating epithelial?
To better understand the mechanisms involved in regulating the coordinate movement of an epithelium in response to injury we have performed our studies with an ex vivo lens mock cataract surgery wound healing model. This model makes it possible to study the mechanisms of collective migration of an epithelium in response to injury in the context of a clinically relevant wounding. Epithelial wound healing in this model involves the cooperative movement of a sheet of epithelial cells, directed at the wound edge by a distinct population of repair cells that serve as leader cells in the wound-healing process (Walker et al., 2010). Unique to these cultures is the ability to follow the collective movement of a wounded epithelium, in situ, on their endogenous ECM substrate. Here, we have examined in situ how the dynamic regulation of cell-cell junctions at the cells’ apical and basal domains, and the regional action of actomyosin at these junctions, coordinates the complex movements necessary for effective wound repair.
We identified distinctly organized cytoskeletal networks and junctional types at the apical and basal domains of lens epithelial cells responding to a mock cataract surgery wounding, each with distinct roles in coordinating collective migration. The apical junctional complex was rich in N-cadherin and ZO-1 linked to a cortical actin cytoskeleton that maintains the cohesivity of the moving monolayer. At the basal domains of the cells we identified a previously unreported type of N-cadherin/ZO-1 adhesion junction located within the cryptic lamellipodia, linked to a contiguous stress fiber network that both interconnected and mechanically coupled the cells.
This novel junction was also rich in phosphomyosin, which would provide it with the ability to transmit the protrusive forces necessary to drive the forward movement of the lamellipodia. Active myosin was also localized to discrete regions at the cells’ apical junctions where it is poised to regulate force generation required to maintain collectivity of the monolayer. Loss of myosin function removed organization constraints on collective movement. The cells acquired a mesenchymal-like morphology, the ability to migrate over one another, and disorganized, but increased rate of migration. Here we identified mechanisms that can be regulated in a spatial-temporal manner to modulate junctional interactions and mechanical inputs/outputs to coordinate both the collectivity and movement of epithelial cells during wound repair.
Materials and Methods
Preparation of Ex Vivo Mock Cataract Surgery Wounded Explants
Ex vivo mock cataract surgery injured lens epithelial cultures were prepared from lenses removed from embryonic day (E)15 chicken embryos (B&E Eggs) (Walker et al., 2007; Walker et al., 2010) modified from a human lens capsular bag model (Liu et al., 1996) (modeled in Fig. 1), and cultured in Media 199 (Invitrogen, Carlsbad, CA) containing 1% pen-strep (Mediatech-Cellgro, Manassas, VA) and 1% L-glutamine (Mediatech-Cellgro, Manassas, VA) with 10% FCS (Invitrogen, Carlsbad, CA). Myosin function was blocked in the wounded cultures by exposure to 50μM Blebbistatin (Enzo Life Sciences, Farmingdale, NY) while control cultures were exposed to the vehicle, DMSO.
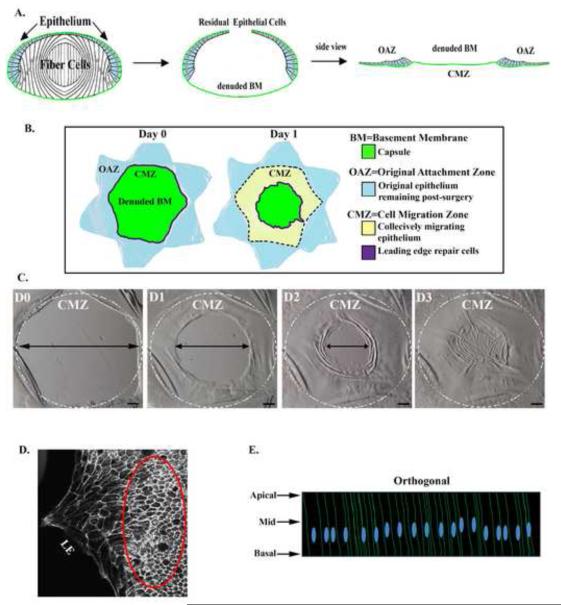
Figure 1. System for studying collective migration during wound healing.
(A) Mock cataract surgery was performed on isolated E15 chick embryo lenses by making a small incision in the anterior-most region of the lens capsule (green) and removing the lens fiber cell mass by hydroelution. (B) Cuts were made in the anterior capsule to flatten the wounded epithelium, still attached to its endogenous basement-membrane (BM) on the culture dish, creating a star shaped ex vivo wound-repair model where the wounded epithelium surrounds the cell-denuded area of the capsule. This region of the wounded epithelium is referred to as the original attachment zone (OAZ). The area of the BM from where the lens fiber cells were removed, and onto which the epithelium will migrate to repair the wound is called the central migration zone (CMZ). Regions of the cultures at Time 0 (D0) and Day 1 (D1) post-injury are depicted as follows: the original wounded epithelium (OAZ, blue); mesenchymal repair cells, immediate responders to injury located at the wound edge (purple); the cell denuded BM (CMZ, green), and the collective migration of lens epithelial cells repairing the wound at D1 (yellow). (C) Phase contrast images in the area of the CMZ of a wounded lens epithelium from D0 through day 3 (D3) when wound repair is complete. Dashed circle - OAZ and CMZ border. Mag. Bar = 500μm. (D) An ex vivo wounded culture at D1 stained for F-actin, with the typical area examined in this study, the collectively migrating epithelial cells located a few rows behind the leading edge located within the red ellipse. LE – leading edge. (E) A model representing an orthogonal (z-plane) view of the lens epithelium demonstrating the major areas of interest along the cells’ apical to basal domains in this study. Studies are representative of at least three independent experiments.
Quantification of migration, cell height and cell breadth
Collective migration of the lens epithelium was imaged on an AZ100 Nikon microscope (Tokyo, Japan) using a Nikon Digital Sight camera and NIS Elements software (Tokyo, Japan). Wound area (μm2) was calculated in NIS Elements and exported to and graphed in Microsoft Excel. For cell morphology measurements, wounded cultures were labeled for F-actin and images acquired on the LSM510 confocal microscope. LSM software was used to perform measurements of the area, based on the outer perimeter of the cells in an individual optical plane and of the cell height, measured on orthogonal (z) cuts of z-stacks collected from the cells’ apical to basal domains. Height of the monolayer was calculated from at least 4 individual wounded cultures at D0 and D1 and presented as μm ± standard error of the mean (SEM). The cell area of 5 follower cells was measured at their apical domains from 6 different wounded epithelia for a total of 30 cells. The mean area (μm2) is presented ± the (SEM). Similar methods were used to determine the height of the monolayer and mean basal area of cells in the myosin inhibition study. The height of the monolayer was determined by measuring 4 individual wounded epithelial in control and blebbistatin treatments. To calculate the basal cell area 10 cells were measured in at least 4 different wounded epithelia for a total of 40 cell areas in both blebbistatin and control treated cultures. To determine whether basal regions of cells were migrating at a greater distance compared to their apical regions, the angles of 3 individual cells were calculated at D0 and D1, as a deviation from 90° relative to the substratum from a 3D reconstruction using Imaris software (Zurich, Switzerland).
Immunofluorescence
Wounded lens epithelial cultures were immunostained as described previously (Walker et al., 2007; Walker et al., 2010). Briefly, cultures were fixed in 3.7% formaldehyde in PBS and permeabilized in 0.25% Triton X-100 (Sigma-Aldrich, St. Louis, MO) in PBS prior to immunostaining. Cells were incubated with primary antiserum followed by rhodamine (Jackson ImmunoResearch Laboratories, West Grove, PA and Millipore, Billerica, MA), fluorescein (Jackson ImmunoResearch Laboratories, West Grove, PA), or Alexa Fluor 488 (Invitrogen-Molecular Probes, Carlsbad, CA) conjugated secondary antibodies. Primary antibodies included β-catenin (BD Transduction Laboratories, San Jose, CA), 4G10 (pY, Millipore, Billerica, MA), cortactin (Millipore, Billerica, MA), myosin IIA (polyclonal, Sigma-Aldrich, St. Louis, MO), myosin IIB (mAb; Developmental Studies Hybridoma Bank, Iowa City, IA), phospho-Myosin Light Chain 2 (Thr18/Ser19) (Cell Signaling, Danvers, MA), ZO-1 (Invitrogen, Carlsbad, CA), NCD-2 (mAb; Invitrogen), and β1 integrin (JG22; mAb; Developmental Studies Hybridoma Bank, Iowa City, IA). F-actin was labeled with Alexa Fluor 488- or Alexa Fluor 633-conjugated phalloidin. Labeling was imaged with a confocal microscope (LSM 510; Zeiss, Thornwood, NY). Z-stacks were collected and shown as either representative optical planes or as orthogonal sections.
Results
Modeling a system to study mechanisms that regulate the collective movement of an epithelium in response to injury
An ex vivo lens mock cataract surgery model (Walker et al., 2007; Walker et al., 2010) was adapted to investigate the mechanisms involved in regulating the coordinate movement of an epithelium in response to injury. Certain intrinsic features of the lens make it a particularly appealing model for these studies, including that it is a self-contained organ surrounded by a thick basement membrane capsule, avascular, not innervated and free of associated stroma. To create this injury-repair model the lens is removed from chicken embryos at day 15 and a clinically relevant wound is created by removing the lens fiber cell mass through a small incision in the anterior capsule (Fig. 1A), using the classical hydroelution approach to cataract surgery. This procedure leaves behind the lens epithelial cells, which are tightly adherent to the lens basement membrane capsule (Fig. 1A and (Walker et al., 2010)). In order to directly image the wound healing response of the epithelium additional incisions are made in the anterior lens capsule, and the lens capsule with attached epithelium is flattened on the substrate, resulting in a star shaped ex vivo explant (Fig 1A,B). At the time of the mock cataract surgery lens epithelial cells are still attached to the basement membrane capsule as they were in vivo and occupy only the points of the star in the wounded explant (Fig. 1B, blue), an area referred to as the original attachment zone (OAZ). The cell-denuded area of the endogenous basement membrane left behind by the mock cataract surgery (Fig 1B, green) onto which the epithelial cells migrate to heal the wound, is called the cell migration zone (CMZ). The lens epithelium is induced to collectively migrate across the CMZ (Fig. 1B, green, Fig 1C), led by an innate subpopulation of mesenchymal repair cells (Fig. 1B (purple), C and (Walker et al., 2010)). At D1 post-injury the epithelial cells have migrated, collectively, across a significant area of the CMZ, and wound healing typically was completed by day 3 in culture (Fig. 1C).
An important feature of the ex vivo mock cataract surgery model is the ability to study functional changes in cellular structure and organization in situ as the epithelial cells coordinately migrate on their endogenous basement membrane. In the studies that follow, the wounded explants were fixed at time 0 (D0) and day 1 (D1) post-injury. These time points provided the opportunity to examine the dynamic regulation of adhesion junctions and the cytoskeleton during the collective movement of the epithelium into the CMZ (Fig. 1C). Throughout this study we examined the lens epithelial cells behind the leading edge of the wound (denoted in Fig. 1D). For each study multiple optical planes were collected by confocal microscopy imaging as Z-stacks along the apical to basal aspects of the cells (modeled as an orthogonal view of a Z-stack in Fig. 1E). With this approach we were able to determine how distinct areas of cell-cell junctional contact, and the cytoskeleton to which these junctions are linked, coordinate both cohesiveness and collective movement of the epithelium in response to injury.
Alterations in epithelial cell morphology and organization/position within the monolayer as they transition from a stationary to a migratory phenotype in response to wounding
We investigated the changes in cell shape, organization and the apical to basal position of cells within the monolayer in the transition from a stable epithelium to a migratory phenotype. For these studies we compared cell structure and position along multiple planes of the cells’ apical-basal axis at both D0 and one day post-injury (D1) following labeling for F-actin. At D0, prior to movement into the CMZ, the newly wounded lens epithelial cells were organized as a tightly packed cuboidal epithelium (cell area, 25.86±2.08μm2), of significant height (z-axis, 40.13±4.00μm), and alignment of their apical and basal domains (Fig. 2A, E) along their z axis, as is typical of a stable epithelium.
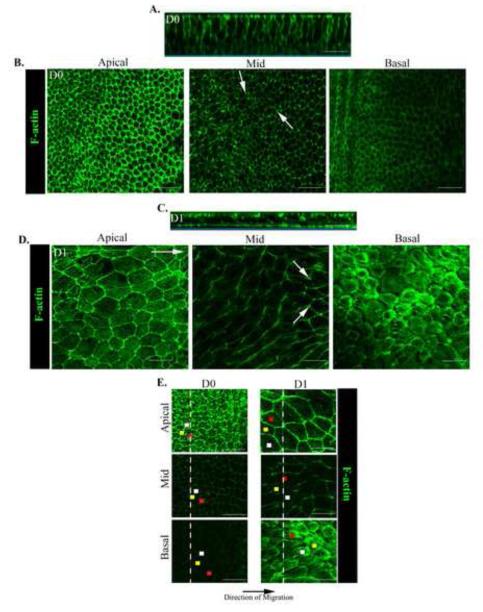
Figure 2. Alterations in cell morphology, cell positioning and organization of actin cytoskeleton accompany the transition from a stationary to a migratory epithelial phenotype.
Ex vivo wounded epithelial cultures at D0 and D1 post-injury labeled with fluorescent-conjugated phalloidin to examine cell structure, position, and F-actin organization were examined by confocal microscopy imaging. Images were collected as z-stacks at multiple planes along the epithelial cells’ apical-basal axis. Shown are (B, D, E) single optical planes at the cells’ apical, mid, and basal regions and (A, C) an orthogonal view of the collected z-stack. (E) To follow the alignment of cells within the monolayer from their apical to basal domains each cell’s position is marked by a different color dot in the apical, mid, and basal planes of the epithelium. (A, E) At D0 the cells were organized as a tightly packed cuboidal epithelium linearly aligned from their apical to basal domains. As cells entered the CMZ they dramatically altered their morphology decreasing in height (z) while increasing their spread in the x-y plane. (C, E) Cells moved at angles to one another, their basal regions typically preceding their apical domains in the direction of migration. (A,B,E) At D0 F-actin was organized as cortical actin filaments most concentrated in the cells apical domains. (C,D,E) At D1 actin remained cortical in the cells apico- and mid-lateral domains but assembled de novo lamellipodia actin filaments and stress fibers along the cells’ basal surfaces. White arrows denote F-actin concentrated at cell vertices. White long arrow indicates the direction of migration. Mag. Bar = 20 μm. Studies are representative of at least three independent experiments.
In response to injury the epithelial cells migrated into the CMZ as a collective sheet (D1), still maintaining a cuboidal morphology but not the tight packing, height, or linear alignment of the epithelium at D0, shown here as both single xy optical planes (Fig. 2D,E) and in an orthogonal view (Fig. 2C). Migrating epithelial cells were dramatically decreased in height (z-axis - 25.89±2.32μm) and increased in area along the xy plane (136.26±11.00μm2). This result demonstrated that the epithelial monolayer had spread and flattened as it migrated collectively to repair the wounded area. Cells within the moving epithelium were positioned at different angles to the direction of movement and relative to one another (Fig. 2C). Consistent with the different positions of their apical and basal domains in the z-plane, the mid-lateral aspects of these migrating epithelial cells appeared elongated, reflecting that we are looking at an oblique view of the mid-lateral regions of cells moving at an angle (Fig. 2D, mid). Tracking the position of epithelial cells in the CMZ along their apical-basal axis revealed that the basal domains of many cells within the monolayer significantly preceded their apical domain in the direction of migration (Fig. 2E, D1). The angle of the cells relative to the substratum at D1 (16.23° +/− 2.4°) greatly decreased when compared to D0 (63.03° +/− 4.4°), further evidence that the basal regions of epithelial cells at D1 are migrating a greater distance then at D0. Although most cells were positioned in this manner, some cells were also be detected in the reverse, highlighting the dynamics of these cells within the moving monolayer. These changes in cell shape and cellular organization within the monolayer were a significant characteristic of the transition from a stationary to a migratory epithelial phenotype.
Distinct actin filament networks at the apical and basal domains of epithelial cells migrating in response to wounding
F-actin organization was examined along the apical-basal axis of wounded lens epithelial cells to determine if changes in F-actin dynamics occurred with movement of the epithelium into the CMZ (D1). At D0 actin filaments were exclusively cortical, most concentrated in the cells’ apical domains (Fig. 2A,B). This F-actin organization is typical of mature polarized epithelia, and consistent with F-actin’s role in maintaining epithelial morphology and integrity of an epithelial sheet. At mid-lateral regions of the cells cortical F-actin was concentrated at the cell vertices (Fig. 2B, mid). This distribution of F-actin was highly similar to that reported for the uninjured lens epithelium (Leonard et al., 2011; Weber and Menko, 2006)
Movement of the epithelium into the CMZ was accompanied by striking alterations in F-actin dynamics at both the apical and basal domains of the cells. In contrast to D0, a rich concentration of actin filaments was now present at the cells’ basal aspects with a distinct pattern of organization from apical F-actin (Fig. 2C,D). At their apical domains, the migrating cells maintained a cortical actin cytoskeleton with a fine actin filament network added just under the cells’ apical surfaces. This apical cytoskeleton likely is necessary for coordinated cell movement (Fig. 2D, apical). The cortical actin filaments extended along the lateral borders of the moving epithelial cells with a lower staining intensity than at the cells’ apical domains (Fig. 2C,D). These cortical filaments likely allow for the maintenance of close cell-cell interactions as the cells move together in response to injury. Similar to D0, F-actin filaments were concentrated at the cell vertices along lateral contacts (Fig. 2D, mid). The distribution of cortactin, an actin binding molecule that signals polymerization of cortical actin filaments (Weed and Parsons, 2001), closely followed that of F-actin along apical-lateral borders of the migrating epithelial cells (Suppl. Fig. 1, apical, mid). In response to injury, and quite distinct from the static lens epithelium, is the assembly of an actin filament network along the cells’ basal surfaces (Fig. 2D, basal). Here, actin filaments in the form of stress fibers formed a contiguous network, linking each cell to the next as they moved along their endogenous basement membrane to heal the wound (Fig. 2D). These cells also extended cryptic lamellipodia rich in F-actin (Fig. 2D, basal), typical of cell structures required for protrusive cell movement. Cryptic refers to the fact that these lamellipodial extensions are hidden from view by the cells themselves. Cortactin co-localized with F-actin at the cells’ basal surfaces specifically in these cryptic lamellipodia structures (Supplemental Fig. 1C, basal; Fig. 4J,L). Together these results revealed the presence of discrete F-actin cytoskeletal networks in collectively moving cells that may function on a supracellular level (an organization shared among many cells that can function as single unit) to spatiotemporally and independently regulate the function of the epithelial cells’ apical and basal domains for cooperative cell movement.
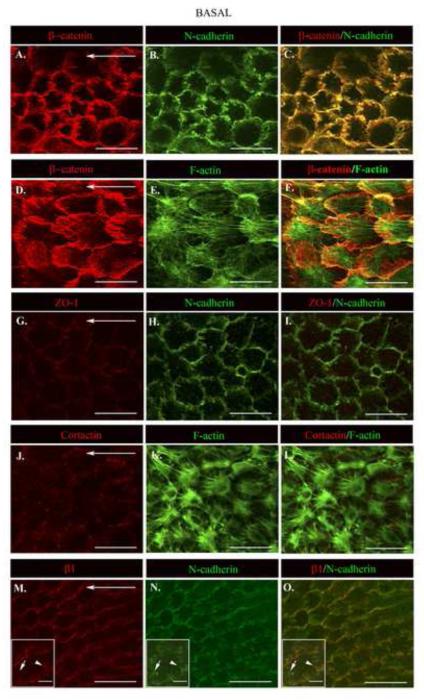
Figure 4. Atypical N-cadherin/ZO-1 junctions in the cryptic lamellipodia of collectively migrating epithelia.
The basal surfaces of lens epithelial cells migrating in response to wounding are distinguished by cryptic lamellipodia that contained discrete adhesion structures comprised of (A,D) β-catenin, (B,H,N) N-cadherin, (G) ZO-1, and (J) cortactin. (E,K) F-actin stress fibers appear to insert directly into these atypical N-cadherin/ZO-1 junctions. Right panels depict antibody overlays. (M) β1 integrin localizes to the cryptic lamellipodia and also exhibits a distinct distribution from N-cadherin junctions (indicated by arrow and arrowhead, insets M-O).. Horizontal arrows specify the direction of migration. All images are shown as single optical sections. Mag. Bar = 20 μm, inset Mag. Bar = 5μm. Studies are representative of at least three independent experiments.
The apical junctional complex is maintained for collective migration of an epithelium in response to wounding
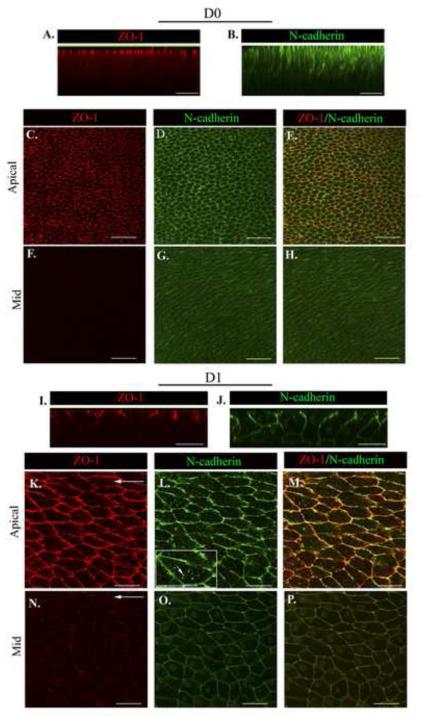
We investigated whether the apical junctions of epithelial cells, which can transmit and respond to mechanical signals and mediate cohesive cell-cell interactions, were retained following wounding for a role in collective sheet movement. For these studies the apical junctional complexes of lens epithelial cells were examined before (D0) and during (D1) migration of the epithelium into the CMZ following wounding. These studies focused on the localization and state of organization of the apical tight junction protein, ZO-1, and the adherens junction protein N-cadherin. Focus was placed here on N-cadherin because in lens epithelial cells this cadherin is a principal component of the adherens junction (Hatta and Takeichi, 1986; Leong et al., 2000). The apical junctional complex of lens epithelial cells at the time of injury (Fig, 3A-E, D0) was quite similar to that previously reported for the epithelial cells of the normal, uninjured lens (Leonard et al., 2011). At D0 ZO-1 was limited to the apical-most cell-cell borders (Fig. 3A, C, F). N-cadherin co-localized with ZO-1 in this domain, but also extended basally along the lateral interfaces, its intensity diminishing in an apical to basal direction (Fig. 3 B,D,G). The composition of the apical junctions remained remarkably unchanged as the cells responded to injury, even as they changed their morphology (see Fig. 2) as they migrated collectively across the basement membrane to heal the wound (Fig. 3I-M, D1). As at D0, at D1 post-injury ZO-1 was highly concentrated at the cells’ apical domains (Fig. 3I, K,), and the cadherin junctions co-localized with the apical ZO-1 junctions but also extended laterally towards the cells’ basal aspects (Fig. 3J,L,O). The apical N-cadherin/ZO-1 junctions likely are linked to the apical perijunctional cortical actin belt involved in interconnecting cells within the monolayer (see Fig. 2D, apical). At D1, as cells moved into the CMZ, ZO-1 remained exclusively along cell-cell borders, but N-cadherin also appeared in discrete puncta not localized to cell-cell interfaces (Fig. 3 K-M, see inset). This finding suggested that N-cadherin junctions are undergoing dynamic remodeling. Note that these N-cadherin puncta were limited to the most apical regions of the cells. An optical section through the mid-lateral zone of the migrating lens epithelium revealed a limited redistribution of ZO-1 to lateral cell-cell borders (Fig. 3N) that were even more enriched in N-cadherin junctions (Fig. 3O). The ZO-1/N-cadherin junctions along these lateral borders were linked to the actin cytoskeleton (Fig.2D, mid), and are likely to ensure collectivity to the movement of the epithelium across the wounded area.
Figure 3. The apical junctional complex is maintained as cells migrate into the CMZ.
Wounded epithelial cultures were imaged at D0 and D1 after injury following immunostaining for (A,C,F,I,K, N) ZO-1 and (B,D,G,J,L,O) N-cadherin. (E,H,M,P) ZO-1/N-cadherin overlays. Z-stacks were collected and shown as (A,B,I,J) orthogonal cuts or (C-H, K-P) single optical planes at the cells’ apical and mid-lateral domains. At D0 ZO-1 is largely restricted to the cells’ apical domains (A,C). N-cadherin is similarly localized but also extends basally along the cells’ lateral borders, although at diminishing concentrations (B,D,G). At D1 there was little change in the distribution of ZO-1 (I,K) but increased levels of N-cadherin junctions in the cells’ mid-lateral zones (J,O). A higher magnification image included as an inset in (L), short white arrow indicates N-cadherin puncta. Arrows indicate the direction of migration. Mag. Bar = 20 μm. Studies are representative of at least three independent experiments.
Novel junctional complexes identified in cryptic lamellipodia at the basal surfaces of the migrating wounded epithelia
To further elucidate how epithelial cells interact with each other and their environment and move coordinately in the direction of wound closure we investigated the type of adhesion junctions present in the basal regions of the collectively moving epithelium. While the apical cell-cell junctions of collectively moving lens epithelial cells remained remarkably similar to those of an uninjured lens epithelium the type of junctions at their basal surfaces were significantly altered in response to wounding. The most notable change was the appearance of an atypical N-cadherin/β-catenin cell-cell junction in the protrusive extensions of the newly formed cryptic lamellipodia (Fig. 4A-F). This atypical junction had a discrete, adhesion plaque-like structure and was oriented anti-parallel to the lamellipodial edge (Fig 4A-C). These junctions were concentrated in regions of significant cell-cell overlap between the forward moving cryptic lamellipodia of one cell and the back of the cell just in front of it. Their organizational state was evocative of nascent cadherin junctions (Leonard et al., 2011; Vaezi et al., 2002), but unlike nascent junctions these N-cadherin-rich junctions also contained ZO-1 (Fig.4 G-I). Note that the basal cell-cell junctions at D0 had diminished levels of N-cadherin but no detectable ZO-1co-localizing with cortical F-actin, (Supplemental Fig. 3). To our knowledge this is the first time ZO-1 and N-cadherin/β-catenin have been identified in junctions of the cryptic lamellipodia of a migrating epithelium. The F-actin filaments at the basal surfaces of these migrating cells were of two types, intense, diffuse labeling specific to the cryptic lamellipodia that is typical of branched actin filaments, and actin stress fibers that extended across the cell. These stress fibers appeared to link the front end of one cell to the back end of the cell directly in front of it by inserting into the atypical N-cadherin junctions (Fig 4E, F), providing a mechanism to transmit mechanosensing signals and coordinate cell movement. Cortactin, a molecule able to bind actin (Wu and Parsons, 1993), the actin nucleator Arp2/3 (Weed et al., 2000), and ZO-1 (Katsube et al., 1998) also localized to the atypical N-cadherin junction (Fig. 4J, and supplemental Fig. 1A, basal). While β1 integrin, a subunit of the principal receptors of cell-matrix adhesion contacts, localized near the lamellipodial edge (Fig. 4M), this receptor also exhibited distinct patterns of localization from the atypical N-cadherin junction (Fig. 4O, see inset M-O). The N-cadherin junctions of the cryptic lamellipodia likely provide the mechanism whereby the moving epithelial cells interconnect, exchange signals, and coordinate motile forces with their near neighbors.
Tyrosine phosphorylation, a mechanism to spatially regulate junctional remodeling during collective migration
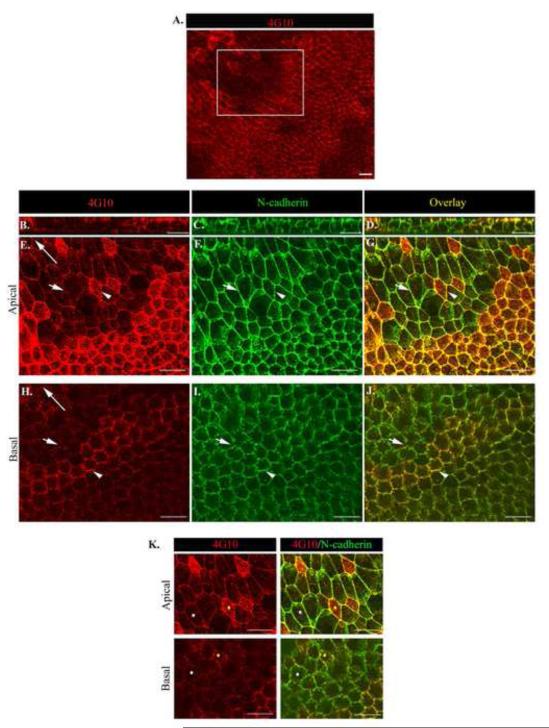
Phosphorylation is the principal mechanism by which adhesion junction assembly and disassembly is regulated (Daniel and Reynolds, 1997; Lilien and Balsamo, 2005), providing a reversible way to dynamically regulate junctional integrity during collective movement. The cadherin-catenin complex is subject to regulation by tyrosine phosphorylation (pY) (Daniel and Reynolds, 1997; Lilien and Balsamo, 2005) and increased pY at these adhesion structures is associated with their disassembly (Daniel and Reynolds, 1997; Lilien and Balsamo, 2005; Matsuyoshi et al., 1992). Therefore, we investigated if cell-cell adhesion junctions along the apical and basal domains of epithelial cells moving in response to injury were targeted sites of tyrosine phosphorylation. For these studies ex vivo wounded cultures at D1 were co-immunostained for the anti-phosphotyrosine (pY) antibody 4G10 and N-cadherin. The results revealed that tyrosine phosphorylation of cell-cell junctions was spatially regulated (Fig. 5 A-J). An orthogonal view showed pY staining was greatest at, but not limited to, the cells’ apical domains (Fig. 5B). However, the most interesting finding was that increased pY staining in the region of N-cadherin cell-cell junctions, at both apical and basal surfaces, occurred in a spatially restricted manner, with groups of cells enriched for pY next to groups of cells with little pY labeling at cell-cell junctions (Fig. 5E-J, arrow heads vs. arrows). Increased tyrosine phosphorylation occurred concurrently at apical and basal junctions (Fig. 5K, yellow dot follows cell with increased pY staining at apical and basal junctions). In unwounded lens epithelium, pY staining appeared uniformly distributed and restricted to apical cell-cell borders (Supplemental Fig. 4). These findings suggested that junctional remodeling by pY during wound repair is a dynamic and discretely regulated process and that there is communication between the apical and basal domains so that their junctions are remodeled simultaneously.
Figure 5. Tyrosine phosphorylation at junctional interfaces provide a mechanism to spatially regulate distinct apical/basal junctional remodeling during collective migration.
Wounded lens cultures were imaged by confocal microscopy at one day after injury following co-immunostaining for (A,B,E,H,K left panel) phosphotryosine (pY, 4G10) and (C,F I) N-cadherin and (D,G,J,K right panel) pY/N-cadherin overlay. Z-stacks were collected and shown as (B-D) orthogonal cuts or (A, E-K) single optical planes at the cells’ apical and basal domains. Low magnification image of the wounded epithelium (A) boxed region indicates the area depicted in (E-K). The cell-cell junctions of groups of neighboring cells were independently targeted by pY (E-K). White arrow head indicates an N-cad junction with increased pY staining vs. short white arrow depicting N-cadherin junction with less pY staining. To determine if cadherin junctions at the apical and basal domains were being targeted by pY at the same time, cells were marked by different color dots and followed in the apical to basal planes of the epithelium. Results reveal simultaneous increased pY targeting of apical and basal N-cad junctions (K, yellow dot). White dot follows N-cad junctions with less pY staining. Long arrows indicate the direction of migration. Mag. Bar = 20 μm. Studies are representative of at least three independent experiments.
Myosin IIA and myosin IIB are functionally poised to regulate the collectivity and movement of the epithelium
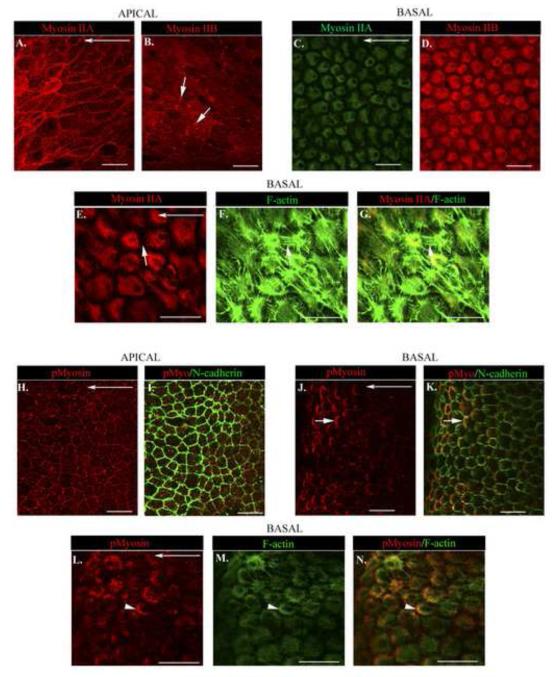
Next we sought to determine if the mechanoenzyme myosin II, which functions in concert with the F-actin cytoskeleton to generate force, is functionally poised to regulate both collectivity and migration of an epithelial sheet in response to injury. Both myosin II isoforms, myosin IIA and IIB, were examined, each which can have distinct cellular distributions and functions (Betapudi et al., 2006; Kolega, 1998; Maupin et al., 1994; Rochlin et al., 1995; Smutny et al., 2010; Vicente-Manzanares et al., 2009; Vicente-Manzanares et al., 2007). At D1 post-injury, along the cells’ apical domains, myosin IIA had a distinct cortical distribution (Fig. 6A), paralleling the organization of apical actin (Fig. 2D). In contrast, the organization of myosin IIB at cell-cell borders was discontinuous and often highly enriched at the cell vertices (Fig. 6B). These distinct patterns of localization suggest there are different roles for myosin II isoforms in maintaining the apical junctional complex through force-generation. In contrast, at the basal aspects of the migrating epithelial cells, myosin IIA and IIB had a similar distribution (Fig. 6C,D). Basally, myosin II was highly co-distributed with F-actin (Fig. 6E,G); however, there was a surprising low level of co-localization of myosin II with F-actin stress fibers (Fig. 6E-G, see white arrow). Actomyosin-rich structures at the basal substrate likely participate in generating forces to regulate cell-cell connections and the protrusive forces needed to promote migration.
Figure 6. Myosin IIA and myosin IIB have distinct distributions at apico-lateral borders of a collectively migrating epithelium and different functions at the cells’ apical and basal domains.
(A-N) Wounded lens epithelia in culture at D1 post-injury were labeled and imaged as a single optical plane by confocal microscopy. (A,C,E,G) Myosin IIA; (B,D) Myosin IIB; (H,I, J,K, L,N) activated, dually phosphorylated (Thr18/Ser19) myosin II regulatory light chain – pMyosin; (I,K) N-cadherin; or (F,G,M,N) F-actin. The wounded epithelial were imaged at their apical (A,B,H,I) or basal (C,D,E-G,J-N) domains. The following groupings: (C,D), (E-G), (H K), and (L-N) represent labeling of the identical region of cells. While apical myosin IIA and myosin IIB were both cortically distributed, myosin IIB was most concentrated at cell vertices (depicted by white arrows). Myosin IIA (C, green) and myosin IIB (D, red), while both shared a similar basal distribution where they were highly localized to actin-rich areas of the cell’s cryptic lamellipodia (white arrow depicts a region where stress fibers stretch between neighboring cells with diminished myosin IIA staining). Myosin activity (pMyosin) was greatest in the region of N-cadherin junctions, both at the migrating cells’ apical domains (I), and strikingly at the atypical N-cadherin junction we have identified in the cryptic lamellipodia (K,depicted by the white arrow). This region of myosin activity was also enriched in F-actin (Fig. L-N, arrowhead). Horizontal long white arrows indicate the direction of migration. Mag. Bar =20 μm. Studies are representative of at least three independent experiments.
Discrete sites of myosin activation revealed at the apical and basal surfaces of epithelial cells moving in response to injury
To reveal potential sites of actomyosin force within an epithelium migrating in response to injury, we examined the subcellular localization of active myosin (pMyosin). For these studies we labeled with an antibody that recognizes the dually phosphorylated (Thr18/Ser19) regulatory light chain (RLC) of myosin II at D1 post-injury. Phosphorylation of myosin II regulates its activity and ability to interact with actin and generate contractile force (Vicente-Manzanares et al., 2009). Active myosin had a punctate distribution along apical cell-cell interfaces that are rich in N-cadherin junctions, with some concentration at the cell vertices (Fig. 6H,I). This pattern suggests differences in myosin-generated forces along apical regions of cell-cell contact of an epithelial sheet responding to wounding. Most exciting; however, was the finding that active myosin was concentrated in the cryptic lamellipodia rich in atypical N-cadherin junctions that had formed in the direction of migration along the basal regions of these moving epithelial cells (Fig. 6J,K). This myosin activity was also a region enriched in F-actin (Fig. 6L-N, arrowhead). These results suggest that myosin activation is tightly regulated in a spatiotemporal manner in both apical and basal microdomains to generate localized areas of force both to maintain cohesive cell-cell interactions and to promote migration for injury repair.
Myosin II function regulates the normal wound response
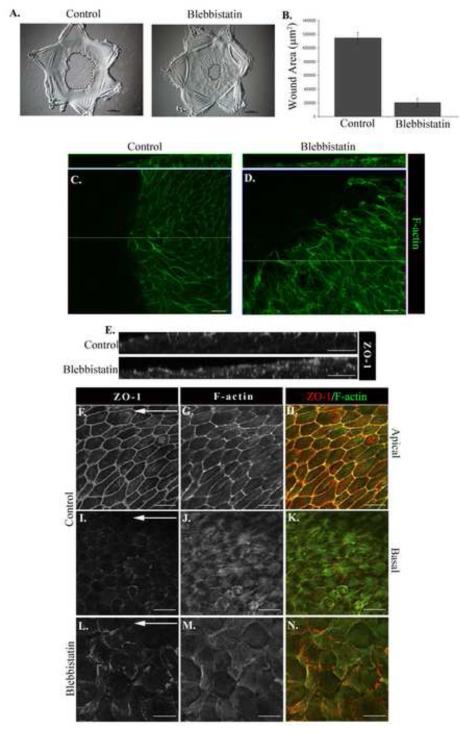
To investigate the functional role of myosin II in the response of an epithelium to wounding, ex vivo mock cataract surgery wounded cultures were exposed to the non-muscle myosin II ATPase inhibitor blebbistatin beginning at the time of injury and examined through D1 in culture. While the open wound area in the control wounded explants was 1,146,500±1,147μm2 at D1 in culture, the rate of lens cell migration increased 5-fold when myosin II activity was inhibited, with a remaining open wound area of only 204,322±204μm2 (Fig. 7A, B). Clearly without the mechanical constrains of myosin activity the epithelial cells were able to fill the wound area with incredible speed. However, did this result reflect an unregulated wound response that would result in ineffective wound repair? In order to investigate the mechanism behind myosin II function in the regulation of wound repair, and whether faster wound closure is necessarily a measure of improved wound repair, we examined various properties of the migrating epithelium when myosin II activity was inhibited. Cultures grown in the presence or absence of blebbistatin were labeled at D1 post-injury with F-actin and examined by confocal microscopy to assess the morphology of cells in the migrating epithelium. Analysis focused on the cells near the leading edge of the migrating epithelium revealed that the migrating lens cells responding to injury in the absence of myosin II activity had lost their sheet-like organization and the individual cells failed to maintain a characteristic epithelial morphology (Fig. 7C,D). The cells were elongated, a morphology more typical of mesenchymal cells, and flattened (height in blebbistatin 11.58±1.0μm compared to 19.13±1.6μm in the control cultures). The collapse of these cells along their z-axis is paralleled by a dramatic increase in size along their xy axis (105.82±5.51μm2 (control) compared to 416.57±24.15μm2 (blebbistatin)). These morphological changes were accompanied by disruption of both the organization and integrity of the epithelial monolayer (Fig. 7D). Cells became intermingled, often acquiring the ability to move over one another, properties unusual for epithelial cells, best illustrated in an orthogonal view (Fig. 7D, F-actin; Fig. 7E, ZO-1). These results show that the loss of myosin function induced the lens epithelial cells to move quicker, in a highly disorganized manner and revealed an essential role for myosin II in maintaining the integrity of an epithelium for collective movement and effective wound repair.
Figure 7. Myosin function is necessary for effective wound repair.
Wounded lens epithelia were cultured for one day in the presence or absence of the myosin II inhibitor blebbistatin. (A) Phase contrast imaging showed faster migration onto the wounded area in the presence of blebbistatin (Mag. Bar = 500μm), data quantified in (B). Wounded cultures were examined by confocal imaging following labeling for F-actin (C,D,G,H,J,K,M, N), or ZO-1 (E,F,H,I,K, L,N) to analyze cell shape and cell-cell connectivity. Control study in F-K was imaged at the cells’ apical (F-H) and basal (I-K) domains. Images shown are either a representative single optical plane from a z-stack (C-D, F-N) or an orthogonal cut of a collected z-stack (E). Loss of myosin function resulted in extensive disorganization of the monolayer, discontinuity of cell-cell junctions, loss of cortical actin, and disorganization of basal actin structures. Mag. Bar = 20μm. Studies are representative of at least three independent experiments.
We also examined the fate of cell-cell junctions in wounded epithelium migrating in the absence of myosin activity. As shown above, in control, wounded cultures the junctional protein ZO-1 remained concentrated at apical cell-cell junctions (Fig. 7E,F), similar to unwounded cultures, with little localization along lateral cell-cell interfaces. In the presence of blebbistatin ZO-1 junctions were no longer restricted to the cells’ apical domains and failed to retain characteristics of a continuous, interconnecting junctional network (Fig. 7L). ZO-1 junctions appeared randomly distributed amidst this disorganized group of lens cells migrating into the wound area, often as if these junctions had collapsed and redistributed as the cells developed their altered morphology, no longer clearly defining an apical-specific cell interface of neighboring epithelial cells (Fig. 7E orthogonal view). Individual focal planes of the z-stack from which this orthogonal cut was created reveal the disorganization of both ZO-1 junctions and the epithelial monolayer (Supplemental Figure 2). In cells in direct contact with the lens basement membrane capsule, ZO-1 was present in both cell-cell junctions and in puncta at their basal surfaces (Fig. 7L) that may be what remains of the specialized ZO-1 junction of the cryptic lamellipodia present in a normal lens epithelium responding to wounding. In the absence of myosin activity the cells also failed to organize the interconnecting F-actin stress fiber network along their basal surfaces that we have shown in lens epithelial cells undergoing wound repair. Instead, basal F-actin was now organized as thin F-actin filaments, with some concentration at the edge of the cells (Fig. 7M). In blebbistatin-treated cultures, myosin IIB distribution followed changes in F-actin, becoming localized along the newly organized actin thin filaments (Supplemental Fig. 5B). These results suggest that blebbistatin altered the wound response by releasing an essential regulation of the normal wound repair process, leading to ineffective wound healing.
Discussion
An ex vivo mock cataract lens surgery model provided a unique opportunity to study the functional properties of cohesively moving cells in a physiologically relevant setting, as these epithelial cells migrated in response to injury on their endogenous matrix capsule. Our studies reveal functional features of the wounded epithelium critical to coordinating their collective movement in response to injury. We found regional-specific organization of the actin cytoskeleton and cell-cell junctions. This provides a mechanism by which the cells’ apical and basal domains can be regulated independently to impact the coordinate behavior and function of the jointly moving epithelial cells (Fig. 8, model). There is also a central role for myosin II mediated mechanical cues, which was found necessary to maintain the integrity, organization and controlled movement of the monolayer in response to injury (Fig. 8, model). In the absence of myosin function, epithelial cells responding to wounding rapidly covered the wound area. While in some context this could be considered a more rapid wound-repair, in actuality this wound-repair was ineffective as judged by the fact that the cells failed to maintain a classical epithelial morphology and their organization as an epithelial monolayer. While still connected during their movement, this was no longer the collective movement of an intact epithelium. Therefore, the rapid filling of a cell-denuded area of basement membrane is not equivalent to effective wound-repair. These results imply that the myosin forces that regulate the collective movement of an epithelium in response to wounding also regulate the rate of migration. Loss of myosin function has been shown to lead to an increased rate of migration in other cell types both in single cell migration (Even-Ram et al., 2007; Liu et al., 2010) and collective migration (Matsubayashi et al., 2011), but it has not been examined before in terms of its effect on the integrity of an epithelial monolayer. The increased rate of migration of lens epithelial cells when myosin activity is blocked provides the cells with properties often associated with cancer cells, where cells become intermingled and move over one another. Maintaining the normal cuboidal apical to basal arrangement of moving epithelial cells during collective migration is essential to maintaining tissue integrity. These results emphasize the importance of myosin restrictive cues for orchestrating the collective migration of an epithelium for effective wound repair.
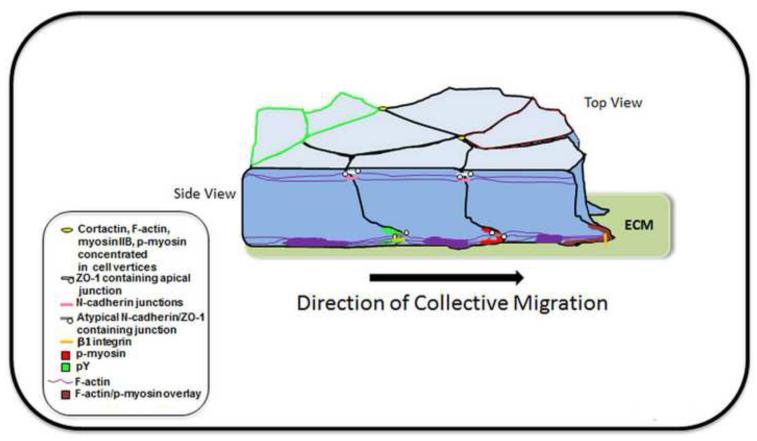
Figure 8. Model of the functional features of a wounded epithelium collectively moving in response to injury.
This model shows how cell behavior and function can be independently regulated at cells apical and basal domains through distinct junctional complexes and cytoskeletal organization to coordinate the collectivity of movement of an epithelium in response to injury. An apical N-cadherin/ZO-1 junctional complex linked at specific domains to pMyosin activity provides the mechanical forces that maintain integrity of the epithelial monolayer as it moves to repair the adjacent wounded area. The myosin IIB isoform has a role in this process at cell vertices, regions of contact between three or more cells, which together with pMyosin has the potential to create tension forces that provide stable reference points for cells to remodel and undergo rearrangements within the moving monolayer. The atypical N-cadherin/ZO-1 junctions we have discovered that are unique to the cryptic lamellipodia and the action of pMyosin with these crucial junctional structures are poised to drive the protrusive forces required for the forward, collective movement of a wounded epithelium. This mechanism is reflected in the coordinated pointing of all the cryptic lamellipodia in the direction of migration, and the basal domains of these cells preceding their apical domains into the wounded area. While β1 integrin is also localized to the cells’ basal surfaces of these migrating cells, its presence is much less prominent than the atypical N-cadherin/ZO-1 junctions, suggesting the importance of N-cadherin/ZO-1/pMyosin-linked protrusive forces for driving the forward, collective movement of an epithelium. Tyrosine phosphorylation, found discretely localized to both apical and basal junctional regions, provides a mechanism by which to spatially regulate junctional remodeling during injury repair. This model shows how distinct structure and myosin-linked function of cell-cell junctions at the apical and basal domains of injured epithelia coordinate effective wound repair.
Within a cohesively moving monolayer cells require the ability to alter their cell-cell interactions, and undergo cellular rearrangements while maintaining their connectivity and the collective integrity of the group, a defining feature of collective migration. Tyrosine phoshorylation of molecular components of cell-cell junctions, like β-catenin, provides one way that collectively moving cells can locally remodel their cell-cell interactions without compromising the integrity of the monolayer. We found that the cell-cell junctions located in both apical and basal domains of the migrating epithelium have the potential to be remodeled through pY, and that their phosphorylation occurs simultaneously in a coordinated manner. A similar mechanism is utilized during drosophila elongation, a dynamic process in which cells alter their interactions through polarized junctional remodeling dependent on β-catenin tyrosine phosphorylation (Tamada et al., 2012).
Local regulation of myosin-II mediated forces provides a mechanism by which cells can preserve their cell-cell interactions through tensional force during cellular rearrangements. In the wounded lens epithelium, active myosin was distributed at discrete regions along the cells’ apical cell-cell borders including a concentration at some cell vertices known to be critical regulatory points for epithelial remodeling (Cavey and Lecuit, 2009). This type of distribution can provide the cells with the ability to carefully balance myosin tensional forces along their apicolateral domains and alter their interactions as they change position within the moving monolayer. In part, the specificity of phospho-myosin localization during wound repair may reflect differences that were observed in the distribution of myosin II isoforms at the apical junctional complex. Here, myosin IIA had a similar distribution to N-cadherin/ZO-1 adhesion junctions and cortical actin cytoskeleton. In the absence of myosin activity ZO-1 was no longer present in a continuous, interconnecting junctional network that is believed to be critical to maintaining the integrity of an epithelium for effective wound repair. The concentration of myosin IIB at apical cell vertices, and its coincidence with high levels of F-actin, cortactin (an actin-polymerizing molecule), and phosphomyosin suggested that these vertices are important sites of force generation in collectively migrating cells. The link between actin polymerization and tension was demonstrated in a study showing that the WAVE2-Arp2/3 actin nucleator complex supports junctional tension at epithelial adherens junctions by recruiting myosin II (Verma et al., 2012). It is tempting to speculate that in a similar manner, enhanced actin polymerization at cell vertices acts locally to recruit increased levels of myosin IIB to create larger amounts of tension at these sites. Consistent with this hypothesis myosin IIB has a greater potential to generate more tension then myosin IIA due to its higher duty ratio (greater amount of time it remains associated with actin) (De La Cruz and Ostap, 2004; Rosenfeld et al., 2003; Wang et al., 2003). Cell vertices may act as stable points of interaction under increased myosin IIB-mediated tension providing reference points of attachment among interacting cells during collective movement.
The atypical N-cadherin junctions discovered in the cryptic lamellipodia of lens epithelial cells migrating as a sheet in response to injury constitute a new category of junction. These atypical junctions resemble nascent cadherin junctions (Kobielak et al., 2004; Vaezi et al., 2002; Vasioukhin et al., 2000) but in that they also contain ZO-1 and link to stress fibers. The association with F-actin stress fibers is similar to the discontinuous adherens junction described in endothelial cells (Millan et al., 2010). The atypical junctions connect the protrusive end of one forward moving epithelial cell with the back end of the cell preceding it, moving in the direction of wound closure. These junctions are enriched for active myosin which is an ideal candidate for mediation of protrusive forces through the atypical N-cadherin junction to promote the forward movement of the basal aspects of collectively moving cells. The prominence of N-cadherin/ZO-1 junctions in the cryptic lamellipodia as compared to β1 integrins implies the importance of these N-cadherin cell-cell junctions in regulating the protrusive forces that guide collective migration. The organization state of the atypical junction is perturbed in the absence of myosin function, providing further evidence that these junctions are tension-regulated and involved in mechanosensing. Cadherin junctions have been shown to undergo flow-like movement in a basal-apical direction which could allow cells the ability to slide along one another during movement (Kametani and Takeichi, 2007). This mechanism is expected to be important to the function of atypical cadherin junctions so that cells can glide along one another as they move within a monolayer for collective movement.
Conclusion
Findings from our studies show spatial differences in cell structure and function at the apical and basal domains of collectively moving cells in response to injury. Regional-specific organization and integrity of the jointly moving epithelium is regulated in part through myosin-mediated mechanical cues which are essential to regulating the behavior of interacting cells to maintain collectivity of migration and for an effective wound healing response to injury.
Supplementary Material
Highlights.
○ We studied junctional remodeling within a moving epithelium during wound repair
○ The apical junctional complex maintains the collectivity of a migrating epithelium
○ Atypical junctions in cryptic lamellipodia at cells’ basal domains drive migration
○ Active myosin concentrates to atypical junctions in basal lamellipodia
○ Myosin activity crucial to maintain epithelial integrity and effective wound repair
Acknowledgements
We thank Dr. Fulton, CMII 23 (Myosin IIB) developed by Drs. Conrad and Conrad, JG22 (β1 developed by Dr. Gottlieb and AA4. 3 (αtubulin) developed by Dr. Walsh were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. This work was supported by National Institutes of Health Grants to A.S.M. (EY021784, EY014258, EY014798) and J.L.W. (EY019571)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–95. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- Betapudi V, Licate LS, Egelhoff TT. Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 2006;66:4725–33. doi: 10.1158/0008-5472.CAN-05-4236. [DOI] [PubMed] [Google Scholar]
- Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, Dunn AR. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc Natl Acad Sci U S A. 2012;109:12568–73. doi: 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey M, Lecuit T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb Perspect Biol. 2009;1:a002998. doi: 10.1101/cshperspect.a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. Tyrosine phosphorylation and cadherin/catenin function. Bioessays. 1997;19:883–91. doi: 10.1002/bies.950191008. [DOI] [PubMed] [Google Scholar]
- De La Cruz EM, Ostap EM. Relating biochemistry and function in the myosin superfamily. Curr Opin Cell Biol. 2004;16:61–7. doi: 10.1016/j.ceb.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- Farooqui R, Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci. 2005;118:51–63. doi: 10.1242/jcs.01577. [DOI] [PubMed] [Google Scholar]
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–57. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–9. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci. 2009;122:3203–8. doi: 10.1242/jcs.036525. [DOI] [PubMed] [Google Scholar]
- Kametani Y, Takeichi M. Basal-to-apical cadherin flow at cell junctions. Nat Cell Biol. 2007;9:92–8. doi: 10.1038/ncb1520. [DOI] [PubMed] [Google Scholar]
- Katsube T, Takahisa M, Ueda R, Hashimoto N, Kobayashi M, Togashi S. Cortactin associates with the cell-cell junction protein ZO-1 in both Drosophila and mouse. J Biol Chem. 1998;273:29672–7. doi: 10.1074/jbc.273.45.29672. [DOI] [PubMed] [Google Scholar]
- Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolega J. Cytoplasmic dynamics of myosin IIA and IIB: spatial ‘sorting’ of isoforms in locomoting cells. J Cell Sci. 1998;111(Pt 15):2085–95. doi: 10.1242/jcs.111.15.2085. [DOI] [PubMed] [Google Scholar]
- le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–15. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckband DE, le Duc Q, Wang N, de Rooij J. Mechanotransduction at cadherin-mediated adhesions. Curr Opin Cell Biol. 2011;23:523–30. doi: 10.1016/j.ceb.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Leonard M, Zhang L, Zhai N, Cader A, Chan Y, Nowak RB, Fowler VM, Menko AS. Modulation of N-cadherin junctions and their role as epicenters of differentiation-specific actin regulation in the developing lens. Dev Biol. 2011;349:363–77. doi: 10.1016/j.ydbio.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong L, Menko AS, Grunwald GB. Differential expression of N- and B-cadherin during lens development. Invest Ophthalmol Vis Sci. 2000;41:3503–10. [PubMed] [Google Scholar]
- Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–65. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Liu CS, Wormstone IM, Duncan G, Marcantonio JM, Webb SF, Davies PD. A study of human lens cell growth in vitro. A model for posterior capsule opacification. Invest Ophthalmol Vis Sci. 1996;37:906–14. [PubMed] [Google Scholar]
- Liu Z, van Grunsven LA, Van Rossen E, Schroyen B, Timmermans JP, Geerts A, Reynaert H. Blebbistatin inhibits contraction and accelerates migration in mouse hepatic stellate cells. Br J Pharmacol. 2010;159:304–15. doi: 10.1111/j.1476-5381.2009.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi Y, Razzell W, Martin P. ‘White wave’ analysis of epithelial scratch wound healing reveals how cells mobilise back from the leading edge in a myosin-II-dependent fashion. J Cell Sci. 2011;124:1017–21. doi: 10.1242/jcs.080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M. Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 1992;118:703–14. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin P, Phillips CL, Adelstein RS, Pollard TD. Differential localization of myosin-II isozymes in human cultured cells and blood cells. J Cell Sci. 1994;107(Pt 11):3077–90. doi: 10.1242/jcs.107.11.3077. [DOI] [PubMed] [Google Scholar]
- Millan J, Cain RJ, Reglero-Real N, Bigarella C, Marcos-Ramiro B, Fernandez-Martin L, Correas I, Ridley AJ. Adherens junctions connect stress fibres between adjacent endothelial cells. BMC Biol. 2010;8:11. doi: 10.1186/1741-7007-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322:1502–5. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- Ng MR, Besser A, Danuser G, Brugge JS. Substrate stiffness regulates cadherin-dependent collective migration through myosin-II contractility. J Cell Biol. 2012;199:545–63. doi: 10.1083/jcb.201207148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin MW, Itoh K, Adelstein RS, Bridgman PC. Localization of myosin II A and B isoforms in cultured neurons. J Cell Sci. 1995;108(Pt 12):3661–70. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- Rorth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009;25:407–29. doi: 10.1146/annurev.cellbio.042308.113231. [DOI] [PubMed] [Google Scholar]
- Rosenfeld SS, Xing J, Chen LQ, Sweeney HL. Myosin IIb is unconventionally conventional. J Biol Chem. 2003;278:27449–55. doi: 10.1074/jbc.M302555200. [DOI] [PubMed] [Google Scholar]
- Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12:696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabdanov E, Borghi N, Brochard-Wyart F, Dufour S, Thiery JP. Role of E-cadherin in membrane-cortex interaction probed by nanotube extrusion. Biophys J. 2009;96:2457–65. doi: 10.1016/j.bpj.2008.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada M, Farrell DL, Zallen JA. Abl regulates planar polarized junctional dynamics through beta-catenin tyrosine phosphorylation. Dev Cell. 2012;22:309–19. doi: 10.1016/j.devcel.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell. 2002;3:367–81. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–19. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Verma S, Han SP, Michael M, Gomez GA, Yang Z, Teasdale RD, Ratheesh A, Kovacs EM, Ali RG, Yap AS. A WAVE2-Arp2/3 actin nucleator apparatus supports junctional tension at the epithelial zonula adherens. Mol Biol Cell. 2012;23:4601–10. doi: 10.1091/mbc.E12-08-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Horwitz AR. Cell migration: an overview. Methods Mol Biol. 2011;769:1–24. doi: 10.1007/978-1-61779-207-6_1. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–90. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol. 2007;176:573–80. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitorino P, Hammer M, Kim J, Meyer T. A steering model of endothelial sheet migration recapitulates monolayer integrity and directed collective migration. Mol Cell Biol. 2011;31:342–50. doi: 10.1128/MCB.00800-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JL, Wolff IM, Zhang L, Menko AS. Activation of SRC kinases signals induction of posterior capsule opacification. Invest Ophthalmol Vis Sci. 2007;48:2214–23. doi: 10.1167/iovs.06-1059. [DOI] [PubMed] [Google Scholar]
- Walker JL, Zhai N, Zhang L, Bleaken BM, Wolff I, Gerhart J, George-Weinstein M, Menko AS. Unique precursors for the mesenchymal cells involved in injury response and fibrosis. Proc Natl Acad Sci U S A. 2010;107:13730–5. doi: 10.1073/pnas.0910382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kovacs M, Hu A, Limouze J, Harvey EV, Sellers JR. Kinetic mechanism of non-muscle myosin IIB: functional adaptations for tension generation and maintenance. J Biol Chem. 2003;278:27439–48. doi: 10.1074/jbc.M302510200. [DOI] [PubMed] [Google Scholar]
- Weber GF, Bjerke MA, DeSimone DW. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell. 2012;22:104–15. doi: 10.1016/j.devcel.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber GF, Menko AS. Actin filament organization regulates the induction of lens cell differentiation and survival. Dev Biol. 2006;295:714–29. doi: 10.1016/j.ydbio.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Weed SA, Karginov AV, Schafer DA, Weaver AM, Kinley AW, Cooper JA, Parsons JT. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J Cell Biol. 2000;151:29–40. doi: 10.1083/jcb.151.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–34. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- Weijer CJ. Collective cell migration in development. J Cell Sci. 2009;122:3215–23. doi: 10.1242/jcs.036517. [DOI] [PubMed] [Google Scholar]
- Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–26. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S. Cadherin-actin interactions at adherens junctions. Curr Opin Cell Biol. 2011;23:515–22. doi: 10.1016/j.ceb.2011.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.