Abstract
The opiate system has long been implicated in the rewarding properties of social interactions. In particular, the μ-opioid receptor (MOR) mediates multiple forms of social attachment, including the attachment of offspring to the mother and social bonding between mates. We have previously shown that MOR in the caudate-putamen is involved in partner preference formation in monogamous prairie voles. Here, using in situ hybridization and receptor autoradiography, we mapped in detail the distribution of MOR mRNA and ligand binding in monogamous prairie vole brains and compared MOR binding density with that of promiscuous meadow vole brains. Comparison of MOR binding in these closely related species with distinctly different social behavior revealed that while the distribution of MOR is similar, prairie voles have significantly higher densities of MOR than meadow voles in a majority of regions in the forebrain, including the caudate-putamen, nucleus accumbens shell, lateral septum and several thalamic nuclei, including the anteroventral and anteromedial thalamic nuclei. These differences in MOR expression between prairie and meadow voles could potentially contribute to species differences in behavior, including social attachment.
Keywords: pair bonding, social behavior, social attachment
Introduction
The brain opiate system modulates a number of fundamental processes including pain, analgesia, and the rewarding properties of food, water, sex, and addictive drugs (Turkish and Cooper, 1983, Agmo and Berenfeld, 1990, Yeomans and Gray, 1996, Sora et al., 1997, Gerrits et al., 2003, Fields, 2007). In addition to the effects on analgesia and reward, the opiate system has been proposed to play an important role in modulating social reward, including maternal behavior, social motivation, and social attachments (Nelson and Panksepp, 1998). Social attachment has many parallels with opiate addiction (Panksepp et al., 1978, Insel, 2003, Burkett and Young, 2012). For instance, the distress evoked by separation of the offspring from the parent shares psychological symptoms with opiate withdrawal, can be induced with opioid antagonists, and can be alleviated with opioid agonists (Herman and Panksepp, 1978, Panksepp et al., 1978, Panksepp et al., 1980, Warnick et al., 2005). Furthermore, in Rhesus macaques, acute administration of an opiate antagonist increases maternal and affiliative behavior, while morphine decreases these behaviors (Fabre-Nys et al., 1982, Kalin et al., 1988, Martel et al., 1993).
The opiate system is activated either by exogenous opiate drugs such as morphine and heroin, or by endogenous neuropeptides such as endorphin, enkephalin, and dynorphin (Le Merrer et al., 2009). The targets of these neuropeptides are the opioid receptors, mu (μ), kappa (κ), and delta (δ). The μ-opioid receptor (MOR) seems to be principally involved in modulating the hedonic properties of many addictive drugs as well as endogenous reward and pain (van Ree et al., 1999, Leknes and Tracey, 2008, Loyd et al., 2008). MOR is also the principal receptor implicated in social reward. MOR knockout mice show decreased social exploration toward opposite-sex conspecifics, decreased response to social defeat, and maternal attachment deficits (Moles et al., 2004, Komatsu et al., 2011, Wohr et al., 2011). Rhesus macaques with the C77G polymorphism of the MOR gene show increased infant-mother attachment and increased maternal care (Barr et al., 2008, Higham et al., 2011). This role for MOR seems to be conserved in humans, where an analogous polymorphism in the MOR gene has been correlated with increased social affection, increased responses to social rejection and social rewards, and altered social attachment (Barr et al., 2008, Way et al., 2009, Higham et al., 2011, Troisi et al., 2011).
Recent studies in voles have provided great insights into the neurobiological mechanisms underlying social bonding between mates, or pair bonding (Young and Wang, 2004, McGraw and Young, 2010). Closely related species of voles show strikingly different social attachment behaviors (Thomas and Birney, 1979, Madison, 1980, Getz et al., 1981). Socially monogamous prairie voles (Microtus ochrogaster) are highly affiliative, display biparental care, and form selective pair bonds between mating partners. By contrast, meadow voles (Microtus pennsylvanicus) are asocial and mate promiscuously without forming pair bonds. Pharmacological and genetic manipulation studies have revealed that oxytocin, vasopressin, dopamine and corticotropin-releasing factor (CRF) act in the mesolimbic reward and reinforcement system to facilitate the formation of the social bond between mates (Insel and Young, 2001, Young et al., 2001, Liu and Wang, 2003, Lim et al., 2004, Lim et al., 2007, Ross et al., 2009). Species differences in the expression levels, distribution or regulation of receptors for these neuromodulators have been implicated in the differences in social behavior between prairie and meadow voles (Lim et al., 2004, Lim et al., 2005b, Lim et al., 2007, Ross et al., 2009). More recently, we have shown that MOR in the dorsal caudate putamen (CP) also plays a critical role in pair bond formation (Burkett et al., 2011). Infusion of CTAP, a selective MOR antagonist, into the dorsal CP of female prairie voles just prior to pairing with a male prevents the development of a partner preference, a laboratory proxy for the pair bond (Burkett et al., 2011). Furthermore, peripheral infusion of naltrexone, a nonselective opioid antagonist, results in a partner aversion. While the partner aversion observed after peripheral infusion of opioid antagonist is likely the consequence of the aversive effects of opioid antagonists, the lack of a partner aversion following CP infusion of MOR antagonist suggests that MOR plays a specific role in mating-induced partner preference formation, rather than having non-specific aversive effects.
Recent studies have provided some information on MOR binding, but not mRNA distribution, in prairie vole (Burkett et al., 2011, Resendez et al., 2012). To better understand the MOR system in the prairie vole, we here characterize the distribution of MOR binding and mRNA throughout the prairie vole brain using receptor autoradiography and in situ hybridization. In addition, to explore potential species differences in MOR expression that may be related to species differences in social attachment behaviors, we compare MOR binding density between two vole species with different patterns of social attachment, prairie and meadow voles.
Experimental procedures
Animals
Adult prairie and meadow voles from 10 weeks to 9 months of age were obtained from our breeding colonies at the Yerkes National Primate Research Center. All prairie voles were descended from a wild caught population in Illinois, USA, and meadow voles were descended from a wild caught population from southeastern USA. All cages were maintained on a 14:10 light:dark cycle with the temperature at 20°C. After weaning at 21 days of age, subjects were housed in same sex sibling pairs or trios with water and Purina rabbit chow provided ad libitum. All subjects in this study were sexually naïve.
All experiments were done in accordance with the Institutional Animal Care and Use Committee at Emory University.
MOR Autoradiography
Brains were dissected from male prairie and meadow voles (N=6 each). Brain slices (20 μm) were prepared for MOR autoradiography using 1 nM [Tyr-3,5-3H(N)]-DAMGO ([3H]DAMGO, PerkinElmer, MA) and analyzed as described previously (Loyd et al., 2008). Noncompetitive MOR binding to brain slices was measured using [3H]DAMGO alone. As a control, competitive binding was measured on adjacent sections using [3H]DAMGO in the presence of either a μ-opioid selective antagonist, CTAP (10 μM) or a nonselective opioid antagonist, naltrexone (NTX, 10 μM), to demonstrate that the ligands bound to the vole MOR as described previously in rat. After sixty days exposure to phosphor imaging plates, signals were acquired using a BAS 5000 phosphor imaging scanner (Fujifilm, Tokyo, Japan), and quantified using Fujifilm Multi Gauge software. Signal intensity for each region was calculated by averaging the quantum level/pixel2 from two or three sections bilaterally per animal. Averaged signals in the corpus callosum were used as background and subtracted from each mean value to yield specific binding. Digital images were cropped, transferred to Adobe Photoshop CS (Adobe Systems, San Jose, CA) and brightness and contrast were equally adjusted for all the images from both the prairie and meadow vole brains.
Acetylcholinesterase (AChE) stain
Following MOR autoradiography, slices were counterstained for acetylcholinesterase for accurate identification of the brain regions as described previously (Lim et al., 2005a).
MOR In Situ Hybridization
Sense and antisense 35S-UTP-labeled RNA probes for MOR mRNA were generated as described previously (Inoue et al., 2004, Burkett et al., 2011). The RNA probe was complementary to the prairie vole MOR sequence corresponding to base pairs 341–1409 of mouse MOR cDNA (Genbank accession number U19380). Twenty μm cryosections adjacent to the slices used for MOR autoradiography were hybridized with the probes, and then were exposed to Kodak BioMax MR films for five weeks. The slides were then coated with Kodak NTB emulsion. After four weeks exposure, sections were developed in Kodak D-19 and fixed with Kodak rapid fixer. Sections were then counterstained with thionin. Regional MOR mRNA expressions were graded as very high (++++), high (+++), intermediate (++) and low (+) to give semi-quantitative estimates of signal strength. For film autoradiograms, digital images were obtained using a light box and a SPOT camera (Diagnostic Instruments, Sterling Heights, MI) connected to a computer. Bright-field and dark-field microscope images were taken with Nikon E800 microscope and SPOT camera setup. Brightness and contrast of the images were equally adjusted for all images using Adobe Photoshop CS.
Statistical Analysis
A total of 51 brain regions were analyzed in this study. Optical density was compared between-species using multiple 2-tailed t-tests. Correction for multiple comparisons within the same subjects was performed using the Ryan–Einot–Gabriel–Welsch multiple stepdown procedure (Howell, 2012). Briefly, all 51 comparisons were ordered from highest to lowest based on the difference between the means. Each comparison was then assigned a unique alpha (α), ranging from α=0.05/51 for the largest difference, to α=0.05 for the smallest difference. Alpha values for each comparison appear in Table 1.
Table 1.
Comparison of Prairie and Meadow Voles: MOR Binding and In Situ Hybridization
| Region | Abbreviation | Prairie Voles | Meadow Voles | P-value | α-value | ||
|---|---|---|---|---|---|---|---|
| RNA | Binding | Binding Mean±SE | Binding Mean±SE | ||||
| Telencephalon | |||||||
| Olfactory bulb | |||||||
| mitral cell layer | OB-Mi | ++++ | - | NA | NA | NA | NA |
| external plexiform layer | OB-EPI | - | ++ | 30.1±1.6 | 19.3±0.5 | 0.0002 ** | 0.0017 |
| granule layer | OB-Gr | +++ | + | 27.2±2.1 | 19.3±1.9 | 0.0226 | 0.0022 |
| Cortex | |||||||
| prelimbic cortex | PrL | ++ | ++ | 44.2±1.3 | 27.0±1.7 | 1.11E-05 ** | 0.0014 |
| cingulate cortex | Cg | ++ | ++ | 34.2±1.8 | 22.4±0.7 | 0.0001 ** | 0.0016 |
| insular cortex | Ins | +++ | +++ | 53.8±4.0 | 28.8±2.6 | 0.0004 * | 0.0011 |
| piriform cortex | Pir | ++++ | ++ | 42.6±2.7 | 30.7±1.6 | 0.0038 | 0.0015 |
| Caudate putamen | |||||||
| rostral | CP-ro | +++ | ++++ | 64.9±2.2 | 47.3±2.7 | 0.0005 * | 0.0014 |
| caudal | CP-ca | + | + | 18.3±0.9 | 18.2±0.4 | 0.9465 | 0.0500 |
| Globus pallidus | GP | + | + | 22.6±0.9 | 20.3±1.6 | 0.2267 | 0.0050 |
| Nucleus accumbens | |||||||
| shell | NAcsh | ++ | +++ | 58.9±2.7 | 44.2±1.5 | 0.0008 * | 0.0014 |
| core | NAcc | ++ | +++ | 56.3±2.8 | 51.6±1.6 | 0.1723 | 0.0026 |
| Lateral septal nucleus | LS | ++ | ++ | 43.3±2.7 | 21.6±1.3 | 2.68E-05 ** | 0.0012 |
| Bed nucleus stria terminalis | BNST | +++ | + | 29.8±2.4 | 26.2±1.4 | 0.2505 | 0.0036 |
| Interstitial nucleus, posterior limb of anterior commissure | IPAC | ++++ | ++++ | 72.7±2.2 | 72.1±1.5 | 0.8361 | 0.0167 |
| Amygdaloid nucleus | |||||||
| medial | MeA | + | ++ | 37.1±1.1 | 37.9±3.3 | 0.8108 | 0.0125 |
| posteromedial cortical | PMCo | ++++ | ++++ | 73.9±3.1 | 44.1±2.1 | 1.20E-05 ** | 0.0010 |
| Hippocampus, CA1 field | CA1 | - | - | 5.7±0.9 | 14.2±0.9 | 0.0001 ** | 0.0021 |
| Diencephalon | |||||||
| Thalamic nuclei | |||||||
| anteroventral | AV | ++++ | +++ | 60.9±2.6 | 7.2±1.3 | 3.20E-08 ** | 0.0010 |
| anteromed | AM | ++++ | ++++ | 76.2±2.7 | 25.4±1.8 | 2.79E-07 ** | 0.0010 |
| interanteromedial | IAM | +++ | ++++ | 79.0±3.9 | 31.7±0.9 | 1.89E-06 ** | 0.0010 |
| reuniens | Re | +++ | +++ | 55.3±3.3 | 33.5±0.8 | 0.0003 * | 0.0011 |
| rhomboid | Rh | +++ | ++++ | 83.6±2.8 | 55.7±2.1 | 1.04E-05 ** | 0.0011 |
| paraventricular thalamic nucleus, anterior | PVA | +++ | +++ | 50.1±4.8 | 22.0±1.8 | 0.0018 | 0.0011 |
| mediodorsal, central, caudal | MDC-ca | ++ | + | 24.0±2.5 | 20.4±1.9 | 0.2731 | 0.0038 |
| mediodorsal, central, rostral | MDC-ro | ++ | + | 16.7±3.1 | 28.6±1.1 | 0.0045 | 0.0015 |
| Medial habenular nucleus | MHb | ++++ | +++ | 65.4±4.1 | 61.2±4.9 | 0.5257 | 0.0028 |
| Medial geniculate nucleus | MG | +++ | +++ | 64.6±2.5 | 59.3±0.7 | 0.0686 | 0.0024 |
| Lateral post thalamic nucleus, mediocaudal | LPMC | + | ++ | 38.9±1.9 | 27.6±1.6 | 0.0009 * | 0.0017 |
| Zona incerta | ZI | +++ | +++ | 58.8±2.7 | 41.1±1.6 | 0.0002 ** | 0.0013 |
| fasciculus retroflexus | fr | - | ++ | 47.4±3.2 | 35.6±4.7 | 0.0644 | 0.0016 |
| Nucleus optic tract | OT | + | ++ | 40.9±2.6 | 22.0±2.2 | 0.0003 * | 0.0013 |
| Anterior hypothalamic area | AH | + | + | 20.8±1.8 | 17.0±2.9 | 0.2768 | 0.0031 |
| Lateral hypothalamic area | LH | + | + | 22.6±1.4 | 20.8±2.4 | 0.5259 | 0.0071 |
| Ventromedial hypothalamic nucleus | VMH | + | + | 26.9±3.9 | 21.7±2.0 | 0.2619 | 0.0025 |
| Medial mammillary nucleus, medial | MM | ++ | ++ | 31.1±4.2 | 10.7±1.7 | 0.0012 * | 0.0012 |
| Supramammillary nucleus | SuM | ++ | ++ | 35.8±3.9 | 25.5±2.6 | 0.0556 | 0.0018 |
| Mesencephalon | |||||||
| Superficial gray layer of superior colliculus | SuG | ++ | ++ | 34.6±1.5 | 30.7±3.0 | 0.2671 | 0.0029 |
| Central nucleus of inferior colliculus | CIC | ++ | +++ | 56.8±1.7 | 54.9±2.6 | 0.5629 | 0.0063 |
| Periaqueductal gray | PAG | + | ++ | 31.9±1.1 | 28.2±1.5 | 0.0752 | 0.0033 |
| Paranigral nucleus | PN | +++ | +++ | 63.9±5.9 | 82.0±3.7 | 0.0259 | 0.0013 |
| Interpeduncular nucleus caudal subnucleus | IPC | +++ | ++++ | 87.2±8.6 | 108.9±6.1 | 0.0675 | 0.0012 |
| Substantia nigra, pars reticulata | SNR | - | + | 24.6±1.8 | 15.3±2.1 | 0.0077 | 0.0019 |
| Parabigeminal nucleus | PBG | +++ | + | 24.7±2.1 | 22.2±2.0 | 0.4052 | 0.0042 |
| Rhonbencephalon | |||||||
| Magnocellular nucleus post commissure | MCPC | ++ | ++ | 44.6±4.3 | 43.0±2.0 | 0.7431 | 0.0083 |
| Median raphe nucleus | MnR | ++ | + | 26.0±2.0 | 36.2±1.9 | 0.0049 | 0.0019 |
| Dorsal raphe nucleus | DR | ++ | + | 23.6±1.7 | 24.2±1.7 | 0.8118 | 0.0250 |
| Dorsal tegmental nucleus | DTg | ++ | ++ | 31.8±1.5 | 34.0±2.0 | 0.4039 | 0.0056 |
| Lateral parabrachial nucleus | LPB | +++ | ++ | 44.7±4.8 | 43.5±4.5 | 0.8635 | 0.0100 |
| Central gray of pons | CGPn | + | ++ | 33.0±2.1 | 40.6±2.7 | 0.0509 | 0.0023 |
| Locus coeruleus | LC | +++ | ++ | 37.2±5.3 | 39.5±2.5 | 0.6730 | 0.0045 |
| Parvicellular reticular nucleus, alpha part | PCRtA | +++ | - | NA | NA | NA | NA |
| Pontine reticular nucleus, caudal part | PnC | +++ | - | NA | NA | NA | |
| Cerebellum | |||||||
| molecular layer | Cb-ML | - | ++ | 36.1±3.3 | 44.7±2.5 | 0.0632 | 0.0020 |
| granule cell layer | Cb-GL | +++ | - | NA | NA | NA | NA |
Values represent ligand binding, calculated as mean quantum level of ROI/pixel2 minus background read by the BAS 5000 from n=6 per group after eliminating artificial signals. P-values were calculated by individual t-tests and compared to α values derived from the Ryan step-down method.
p < α species effect,
p < α/5 species effect.
Results
MOR autoradiography and mRNA distribution in the prairie vole brain
Using receptor autoradiography and in situ hybridization, we first investigated MOR ligand binding and mRNA distribution in male prairie vole brains (Fig. 1). The MOR ligand binding was broadly distributed throughout the brain. The expression pattern of the MOR mRNA showed a similar pattern to the protein expression in a majority of brain regions (Fig. 1). Detailed expression analysis is described in Table 1.
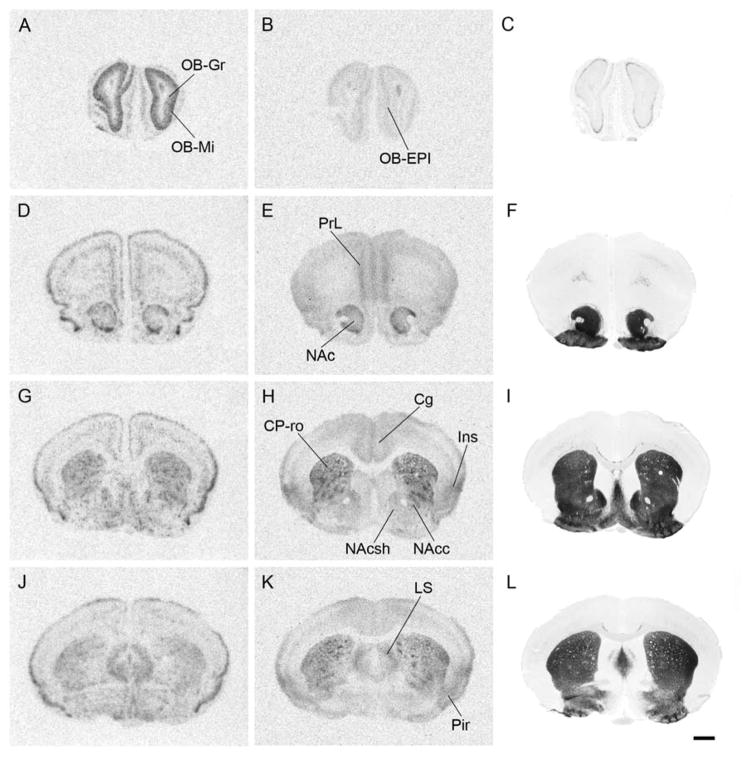
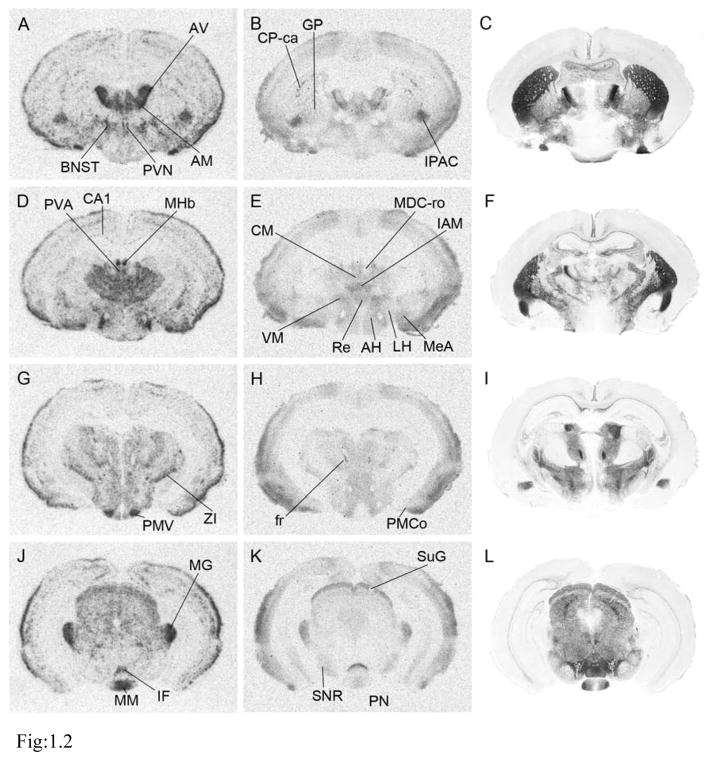
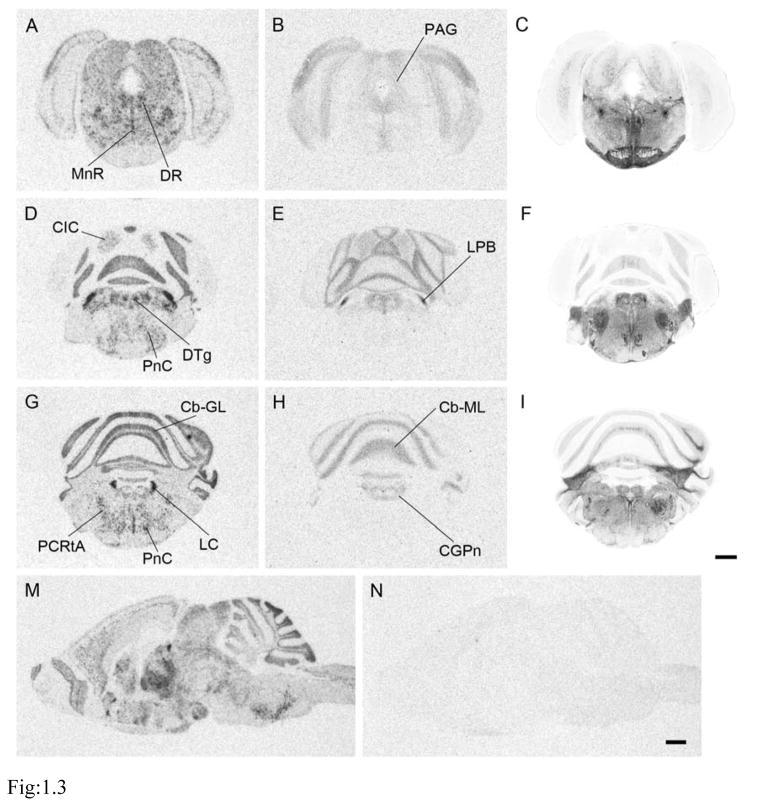
Figure 1.
Representative pictures of prairie vole MOR in situ hybridization (1.1 A, D, G, J; 1.2 A, D, G, J; and 1.3 A, D, G), ligand binding autoradiography (1.1 B, E, H, K; 1.2 B, E, H, K; and 1.3 B, E, H), and acetylcholinesterase staining (1.1 C, F, I, L; 1.2 C, F, I, L; and 1.3 C, F, I) for anatomical reference. Coronal sections in rostral-caudal order: 1.1, from olfactory bulbs to septum; 1.2, from thalamus to substantia nigra; and 1.3, from periaqueductal gray to cerebellum. Fig. 1.3 contains sagittal section pictures of in situ hybridization with antisense- (M) or sense- (N) probe, demonstrating specificity of the antisense probe. See Table 1 for abbreviations. Scale bars in 1.1 L, 1.2 L, 1.3 I, and 1.3 N = 1mm
Specificity of MOR binding and in situ hybridization
To assess the specificity of MOR binding, we examined the binding of [3H]DAMGO with in the presence or absence of either the μ-opioid selective agonist CTAP or nonselective opioid agonist nalotrexone using adjacent prairie vole sections. No signal was detected from the competitive binding distinct from the background signals (data not shown) thus confirmed the specificity of our MOR binding assay as we had previously demonstrated (Burkett et al., 2011). For in situ hybridization, we used sense probes as a control to hybridized to adjacent sections and observed no signal above the background (Fig. 1.3 M and N).
Telencephalon
In the olfactory bulb, very strong MOR mRNA signal was detected in the mitral cell layer and strong signal was detected in the granule layer. Moderate to intermediate MOR binding was observed more widely in the olfactory bulb, including the external plexiform and granule layers (Fig. 1.1A–C, Table 1). MOR mRNA was broadly expressed in the cortex, with several high intensity areas including the medial prefrontal, piriform, and insular cortices (Fig. 1.1 D and G). MOR mRNA was concentrated mainly in layers II and V, whereas binding was mainly apparent in layers I and V (data not shown). Both protein binding and mRNA signal were very high in the rostral part of the dorsal striatum (CP-ro), but both were lower in the caudal part (CP-ca) (Figs. 1.1 G and H, 1.2 A–F). Within the dorsal striatum, both mRNA and MOR binding were clustered in the striosome compartment, which has been reported to be MOR-immunoreactive in the other animals (Miura et al., 2007). mRNA and MOR binding were both strong in the shell and the core of the nucleus accumbens, but were lower than in the dorsal striatum (Fig. 1.1 D–I). In the amygdala, both mRNA and binding signals were strongly observed in the posteromedial cortical amygdaloid nucleus and moderately observed in the medial amygdaloid nucleus (Fig. 1.2 D–I, Table 1). No labeling was observed in the central amygdaloid nucleus, whereas the adjacent interstitial nucleus of the posterior limb of the anterior commissure had both high mRNA and protein binding signals. A very low mRNA signal was observed in the CA1 region of the hippocampus, but no ligand binding signal was detected.
Diencephalon
Dense labeling of both mRNA and receptor binding was detected broadly throughout the thalamus. Prominent signals were detected in subregions such as the anteroventral thalamic nucleus, anteromedial thalamic nucleus, interanteromedial thalamic nucleus and medial habenular nucleus (Fig. 1.2 A–F, Table 1). Strong signals were also observed in the paraventricular thalamic nucleus, anterior part, reuniens thalamic nucleus, zona incerta and medial geniculate nucleus (Fig. 1.2).
Mesencephalon and Rhombencephalon
The paranigral nucleus, interpeduncular nucleus caudal subnucleus and parabigeminal nucleus were prominently labeled with both in situ hybridization and receptor autoradiography (Table 1). Intermediate binding signal was also observed in the periaqueductal gray, superficial gray layer of the superior colliculus, central nucleus of the inferior colliculus, median raphe (MnR) and locus coeruleus (LC) (Figs. 1.2 J–L, 1.3). Intermediate labeling was also detected in the molecular layer of the cerebellum (Fig. 1.3 G–I).
Comparison of MOR mRNA expression and ligand binding in each brain region
Figure 2 illustrates brain regions that show a discrepancy between the ligand binding and mRNA expression of MOR. In the substantia nigra, mRNA was barely detected while MOR binding was high (Fig. 2 A–C). Conversely, a very strong mRNA signal was observed in the LC, but only a moderate ligand binding was detected. mRNA signals were also detected in other nuclei of the brainstem including the parvicellular reticular nucleus, alpha part and the pontine reticular nucleus, caudal part; whereas ligand binding signals were barely detected in these regions (Fig. 1.3G–I). In the cerebellum, mRNA was expressed strongly in the granular layer, whereas protein binding was detected only in the molecular layer (Fig. 2D–F). As described above, control experiments using either competitive cold opioid agonists for MOR binding, or a sense probe for in situ hybridization, did not show any signals throughout the brain, including cerebellum cortex. A dense hybridization pattern throughout the granular layer is a typical characteristic feature of the labeling of granule cells, which project parallel fibers to the molecular layer; suggesting that MOR is expressed in the granule layer and the MOR protein is localized on axon terminals.
Figure 2.
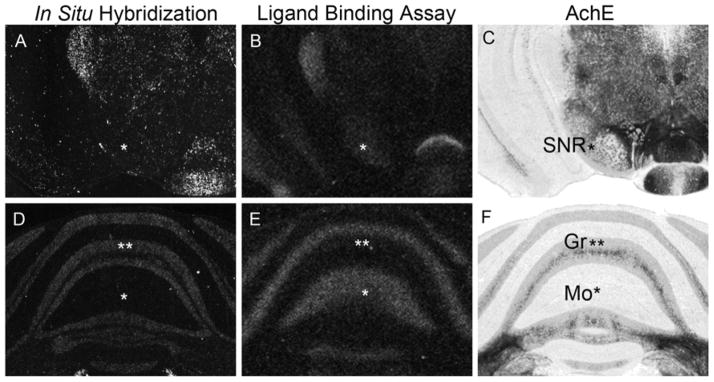
Representative pictures of in situ hybridization (A, D) and ligand binding assay (B, E) of prairie vole MOR in adjacent slices. The regions of interest are indicated by asterisks. Acetylcholinesterase staining (C, F) is presented for anatomical reference. Note the discrepancy between mRNA and protein expressions of MOR in the substantia nigra pars reticulata (SNR, A–C) and in the molecular (Mo) and granule (Gr) layers of the cerebellum (D–F).
Species differences in MOR between prairie and meadow voles
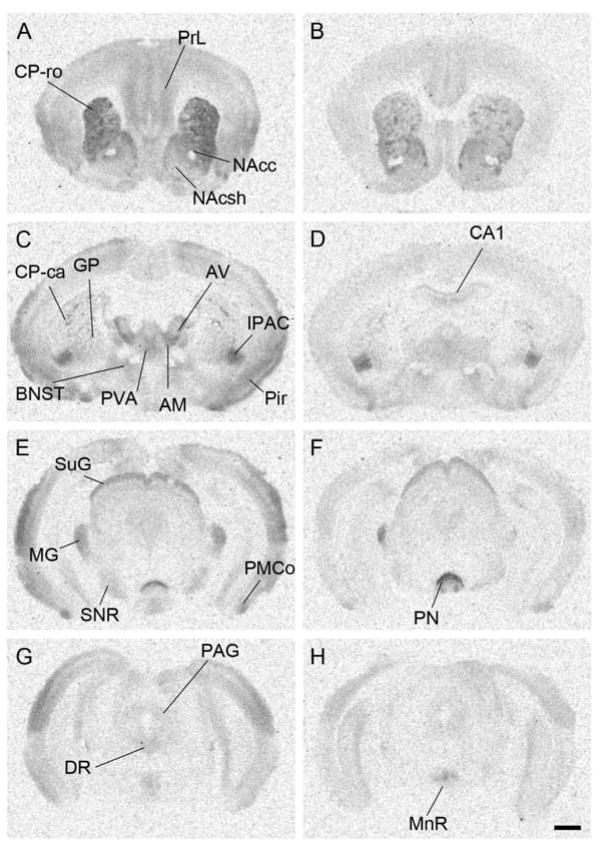
Next, we examined the differences in MOR binding between male prairie and meadow voles. The general pattern of MOR binding was similar between the two species, although quantitative differences were observed (Table 1, Fig. 3). MOR binding density was significantly higher in prairie voles compared to meadow voles in a majority of the forebrain regions, including prelimbic cortex (PrL), caudate putamen, nucleus accumbens shell (NAcsh), lateral septal nucleus (LS) and several thalamic nuclei (Fig. 3). In contrast, the MOR ligand binding in hippocampal CA1 was higher in meadow voles (P = 0.00008). Quantification of the binding profile for both species is described in Table 1.
Figure 3.
Comparison between MOR ligand autoradiography in prairie voles (A, C, E, and G) and meadow voles (B, D, F, and H). Prairie voles showed higher binding signals than meadow voles in many regions tested. Contrarily, binding signals were stronger in meadow voles in CA1, PN, and MnR. See text for more details. Scale bar in H = 1mm
Discussion
Here, we investigated the neuroanatomical distribution of MOR binding and mRNA in the highly social and monogamous prairie vole and the asocial, promiscuously breeding meadow vole using ligand-binding receptor autoradiography and in situ hybridization. Three prior studies have examined MOR ligand binding in prairie voles (Insel and Shapiro, 1992, Resendez et al., 2012, Burkett et al., 2011), and we significantly expand on those findings here to provide the first complete map of MOR mRNA distribution in prairie vole, a substantial increase in knowledge about MOR ligand binding in the meadow vole, and MOR ligand binding from additional hindbrain and cerebellar structures in prairie vole.
Our data demonstrate that MOR mRNA and binding are distributed in a common pattern throughout the brain of prairie and meadow voles. This distribution is also similar to that of rats and mice (Kaufman et al., 1994, Zastawny et al., 1994, Le Merrer et al., 2009). However, in several forebrain regions, including the medial prefrontal cortex, LS, dorsal and ventral striatum, and thalamic nuclei, prairie voles show higher MOR radioligand binding than meadow voles. Conversely, meadow voles had higher binding in hippocampal CA1, and similar or perhaps higher MOR binding in the MnR.
In some areas, there was a clear mismatch between mRNA and ligand binding. For example, in cerebellar cortex, MOR mRNA is localized in the granular layer and the ligand binding in the molecular layer. MOR protein in some brain regions is synthesized in the cell body, transported and incorporated to the membrane of the axon terminals (Aicher et al., 2000a, Aicher et al., 2000b, Jaferi and Pickel, 2009, Pennock and Hentges, 2011). These data are consistent with the hypothesis that MOR mRNA is transcribed in granule cells and the protein is localized in axon terminals in the molecular layer.
It is particularly intriguing that the PrL, LS, CP-ro, and NAcsh show significantly higher density of the MOR protein in prairie voles than in meadow voles, as each of these regions has been implicated in species-specific pair bonding in prairie voles (Liu and Wang, 2003, Lim et al., 2004, Lim et al., 2007, Ross et al., 2009, Burkett et al., 2011). More specifically, our prior study showed that MOR signalling in the CP-ro is necessary for pair bond formation, while MOR in the NAcsh is not. Like MOR in the striatum, expression levels of oxytocin receptors (OTR) in the dorsal and ventral striatum are highly correlated within individual voles. However, OTR in the NAcsh is necessary for pair bonding formation, while OTR in the CP-ro is not (Young et al., 2001). This suggests that MOR and OTR are not directly interacting to influence pairbonding.
Inter-species variation between prairie and meadow voles in neuropeptide receptor density and distribution has played an important role in delineating the brain regions involved in social behavior. Species differences in the density of vasopressin V1aR are known to underlie species differences in male pair bonding between meadow and prairie voles (Young and Wang, 2004), while observed differences in OTR and CRF receptors led to predictions about the brain regions where these receptors act in pair bonding that were subsequently verified experimentally (Lim et al., 2004, Lim et al., 2007, Ross et al., 2009). Our results here, in combination with our prior study showing the necessary role of striatal MOR in pair bond formation (Burkett et al., 2011), suggest that species differences in MOR could also contribute to species differences in pair bonding behavior. In addition, prairie and montane vole pups differ in relation to separation distress, with prairie vole pups showing significantly more distress vocalizations than montane vole pups (Shapiro and Insel, 1990). Given the long literature linking MOR to separation distress in general, and distress vocalizations specifically (Martel et al., 1995), species differences in MOR may contribute to differences in separation distress behavior as well.
Intra-species variation in neuropeptide receptors in the prairie vole also drives differences in social behavior. Individual variation in OTR density in the ventral striatum of prairie voles is a causal mechanism creating individual differences in female pair bonding and maternal behavior (Ross et al., 2009, Keebaugh and Young, 2011), while individual variation in vasopressin V1aR in the ventral pallidum underlies variation in male pair bonding (Barrett et al., 2013). While some individual variation in MOR binding density in the striatum was observed in agreement with Resendez et al. (Resendez et al., 2012), the variation is substantially less than is seen in OTR or V1aR, and it is unknown whether this small variation in MOR is behaviorally relevant.
Although MOR binding was higher in prairie voles in many areas of the brain, meadow voles showed more MOR compared to prairie voles in hippocampal CA1. In the hippocampus, the prairie vole is completely devoid of MOR, whereas MOR is expressed in meadow voles and other promiscuous rodents, including mouse and rat (Mansour et al., 1987, Mansour et al., 1994, Goody et al., 2002). Very low level of MOR in the hippocampus was also reported in the other promiscuous animals such as guinea pigs and rabbits (Robson et al., 1985, Foote and Maurer, 1986). Meadow voles may also have shown higher expression in MnR; though this difference was large (p = 0.005), it was not large enough to survive multiple comparisons correction due to the large number of brain regions analyzed. Although the function in social attachment of opioids in the hippocampus is not known, both of these areas are involved in the serotonergic system, which is implicated in depression and anxiety (Segal, 1975, Baldwin and Rudge, 1995). This suggests the possibility that MOR may modify the serotonergic system to induce behavioral differences other than social attachment. Alternatively, the differences in MOR in these regions may have no influence on social behaviors, and may be unrelated to species differences in social organization in voles.
Insel and Shapiro (1992) performed a cursory examination of MOR in the forebrain of monogamous prairie voles and promiscuous meadow and montane voles, where the only significant difference detected was a lower MOR binding in the LS of montane as compared to prairie. Our more thorough comparison of prairie and meadow voles revealed differences in the majority of forebrain regions, including the LS, with meadow voles showing significantly lower MOR density than prairie voles. Low MOR binding in the LS is also seen in rats and mice (Kitchen et al., 1997) and may be a general characteristic of promiscuous rodents.
These findings may also shed additional light on the roles of the basal ganglia direct and indirect pathways in pair bonding behaviors. In prairie voles, pair bonding is primarily defined by the presence of two observable behaviors: partner preference, a laboratory proxy for pair bond formation; and selective aggression, a behavioral mechanism of bond maintenance (Carter et al., 1995). These behaviors are mutually antagonistic; pair bond formation requires the inhibition of aggressive responses to a potential mate, while selective aggression prevents the formation of new bonds once a partner has been selected. Prior literature on selective aggression has primarily implicated the direct pathway (Le Moine and Bloch, 1995), including κ-opioid receptor (KOR) in the ventral striatum, whose endogenous ligand, dynorphin, is expressed in direct pathway neurons which also express D1 dopamine receptors (Steiner and Gerfen, 1998). Conversely, literature on pair bond formation has implicated the indirect pathway (Young and Wang, 2004; Burkett et al., 2012), including MOR in the dorsal striatum, whose endogenous ligand, enkephalin, is expressed in indirect pathway neurons, which also express D2 dopamine receptors (Steiner and Gerfen, 1998). Our findings and those of Resendez et al. (2012) now suggest some interesting locations where MOR and KOR intersect, which may provide clues as to a mechanism of cross-talk between these behavioral systems. For instance, in the indirect pathway, KOR is expressed in the CP, nucleus accumbens, ventral pallidum, and substantia nigra pars reticulata (SNR); while in the direct pathway, MOR is expressed in the striatum and globus pallidus external segment. Interestingly, one of these nuclei, the SNR, contains KOR and MOR binding but no MOR mRNA, suggesting that MOR is expressed on axon terminals, possibly from indirect pathway neurons originating in the CP. Furthermore, in meadow voles several of these areas contain notably less MOR ligand binding, and thus may contribute to a general increase in aggression and decrease in bonding between conspecifics. Future pharmacological studies could investigate these potential interactions and their roles in pair bond formation.
In conclusion, the distribution of MOR in prairie voles and meadow voles is largely consistent with that reported for other rodents, which is in contrast to the more diverse expression patterns observed across species for other neuropeptide systems such as oxytocin, vasopressin and corticotrophin releasing factor. Furthermore, there is significantly less individual variation in MOR distribution than seen for oxytocin and vasopressin receptors. These observations suggest that the function of the MOR system is more highly conserved and constrained than other peptide systems. Furthermore, there were robust quantitative differences between prairie and meadow voles. Prairie voles showed more MOR protein binding in regions implicated in social attachment and reward, while meadow voles showed more binding in serotonergic regions involved in anxiety related behaviors. These species differences in receptor density may contribute to species differences in social behavior and social attachment.
Highlights.
We compared μ-opioid receptor binding and mRNA in prairie and meadow vole brains.
MOR distribution, but not density, was similar in prairie and meadow vole brains.
Prairie vole showed higher MOR binding in many forebrain regions.
Meadow voles have higher MOR binding in hippocampal CA1.
Mismatches between mRNA and ligand binding revealed regions with axonal expression.
Acknowledgments
We would like to thank members of the Young lab for helpful discussion throughout this project. This work was funded in part by NIH Grant MH64692 to LJY. Additional support was provided by the National Center for Research Resources P51RR165 to YNPRC, which is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132, and the Emory Scholars Program in Interdisciplinary Neuroscience Research to JPB.
Footnotes
Conflict of Interest Statement: None of the authors have any conflicts of interest.
Role of Authors: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: KI, JPB and LJY. Acquisition of data: KI and JPB. Analysis and interpretation of data: KI. Drafting of the manuscript: KI. Critical revision of the manuscript for important intellectual content: JPB and LJY. Statistical analysis: JPB. Obtained funding: LJY. Study supervision: LJY.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agmo A, Berenfeld R. Reinforcing properties of ejaculation in the male rat: role of opioids and dopamine. Behavioral neuroscience. 1990;104:177–182. doi: 10.1037//0735-7044.104.1.177. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Goldberg A, Sharma S, Pickel VM. mu-opioid receptors are present in vagal afferents and their dendritic targets in the medial nucleus tractus solitarius. The Journal of comparative neurology. 2000a;422:181–190. doi: 10.1002/(sici)1096-9861(20000626)422:2<181::aid-cne3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Sharma S, Cheng PY, Liu-Chen LY, Pickel VM. Dual ultrastructural localization of mu-opiate receptors and substance p in the dorsal horn. Synapse. 2000b;36:12–20. doi: 10.1002/(SICI)1098-2396(200004)36:1<12::AID-SYN2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Baldwin D, Rudge S. The role of serotonin in depression and anxiety. International clinical psychopharmacology. 1995;9(Suppl 4):41–45. doi: 10.1097/00004850-199501004-00006. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, Suomi SJ, Heilig M. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, Young LJ. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Hormones and behavior. 2013 doi: 10.1016/j.yhbeh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, Young LJ. Activation of mu-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:2200–2210. doi: 10.1038/npp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl) 2012;224:1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre-Nys C, Meller RE, Keverne EB. Opiate antagonists stimulate affiliative behaviour in monkeys. Pharmacology, biochemistry, and behavior. 1982;16:653–659. doi: 10.1016/0091-3057(82)90432-4. [DOI] [PubMed] [Google Scholar]
- Fields HL. Understanding how opioids contribute to reward and analgesia. Regional anesthesia and pain medicine. 2007;32:242–246. doi: 10.1016/j.rapm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Foote RW, Maurer R. Distribution of opioid binding sites in the guinea pig hippocampus as compared to the rat: a quantitative analysis. Neuroscience. 1986;19:847–856. doi: 10.1016/0306-4522(86)90303-9. [DOI] [PubMed] [Google Scholar]
- Gerrits MA, Lesscher HB, van Ree JM. Drug dependence and the endogenous opioid system. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2003;13:424–434. doi: 10.1016/j.euroneuro.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS, Gavish L. The Mating System of the Prairie Vole, Microtus-Ochrogaster - Field and Laboratory Evidence for Pair-Bonding. Behav Ecol Sociobiol. 1981;8:189–194. [Google Scholar]
- Goody RJ, Oakley SM, Filliol D, Kieffer BL, Kitchen I. Quantitative autoradiographic mapping of opioid receptors in the brain of delta-opioid receptor gene knockout mice. Brain research. 2002;945:9–19. doi: 10.1016/s0006-8993(02)02452-6. [DOI] [PubMed] [Google Scholar]
- Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: evidence for opiate mediation of social affect. Pharmacology, biochemistry, and behavior. 1978;9:213–220. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- Higham JP, Barr CS, Hoffman CL, Mandalaywala TM, Parker KJ, Maestripieri D. Mu-opioid receptor (OPRM1) variation, oxytocin levels and maternal attachment in free-ranging rhesus macaques Macaca mulatta. Behavioral neuroscience. 2011;125:131–136. doi: 10.1037/a0022695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell D. Statistical methods for psychology. 8. Belmont, CA: Wadsworth Publishing; 2012. [Google Scholar]
- Inoue K, Terashima T, Nishikawa T, Takumi T. Fez1 is layer-specifically expressed in the adult mouse neocortex. The European journal of neuroscience. 2004;20:2909–2916. doi: 10.1111/j.1460-9568.2004.03763.x. [DOI] [PubMed] [Google Scholar]
- Insel TR. Is social attachment an addictive disorder? Physiology & behavior. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nature reviews Neuroscience. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Pickel VM. Mu-opioid and corticotropin-releasing-factor receptors show largely postsynaptic co-expression, and separate presynaptic distributions, in the mouse central amygdala and bed nucleus of the stria terminalis. Neuroscience. 2009;159:526–539. doi: 10.1016/j.neuroscience.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain research. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- Kaufman DL, Xia YR, Keith DE, Jr, Newman D, Evans CJ, Lusis AJ. Localization of the delta-opioid receptor gene to mouse chromosome 4 by linkage analysis. Genomics. 1994;19:405–406. doi: 10.1006/geno.1994.1087. [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Young LJ. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Hormones and behavior. 2011;60:498–504. doi: 10.1016/j.yhbeh.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen I, Slowe SJ, Matthes HW, Kieffer B. Quantitative autoradiographic mapping of mu-, delta- and kappa-opioid receptors in knockout mice lacking the mu-opioid receptor gene. Brain research. 1997;778:73–88. doi: 10.1016/s0006-8993(97)00988-8. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Ohara A, Sasaki K, Abe H, Hattori H, Hall FS, Uhl GR, Sora I. Decreased response to social defeat stress in mu-opioid-receptor knockout mice. Pharmacology, biochemistry, and behavior. 2011;99:676–682. doi: 10.1016/j.pbb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiological reviews. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. The Journal of comparative neurology. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nature reviews Neuroscience. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- Lim MM, Bielsky IF, Young LJ. Neuropeptides and the social brain: potential rodent models of autism. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2005a;23:235–243. doi: 10.1016/j.ijdevneu.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z, Young LJ. CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Hormones and behavior. 2007;51:508–515. doi: 10.1016/j.yhbeh.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Nair HP, Young LJ. Species and sex differences in brain distribution of corticotropin-releasing factor receptor subtypes 1 and 2 in monogamous and promiscuous vole species. The Journal of comparative neurology. 2005b;487:75–92. doi: 10.1002/cne.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Wang X, Murphy AZ. Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:14007–14017. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DM. Space Use and Social Structure in Meadow Voles, Microtus Pennsylvanicus. Behav Ecol Sociobiol. 1980;7:65–71. [Google Scholar]
- Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ. mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain research. 1994;643:245–265. doi: 10.1016/0006-8993(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Martel FL, Nevison CM, Rayment FD, Simpson MJ, Keverne EB. Opioid receptor blockade reduces maternal affect and social grooming in rhesus monkeys. Psychoneuroendocrinology. 1993;18:307–321. doi: 10.1016/0306-4530(93)90027-i. [DOI] [PubMed] [Google Scholar]
- Martel FL, Nevison CM, Simpson MJ, Keverne EB. Effects of opioid receptor blockade on the social behavior of rhesus monkeys living in large family groups. Developmental psychobiology. 1995;28:71–84. doi: 10.1002/dev.420280202. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Young LJ. The prairie vole: an emerging model organism for understanding the social brain. Trends in neurosciences. 2010;33:103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Saino-Saito S, Masuda M, Kobayashi K, Aosaki T. Compartment-specific modulation of GABAergic synaptic transmission by mu-opioid receptor in the mouse striatum with green fluorescent protein-expressing dopamine islands. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:9721–9728. doi: 10.1523/JNEUROSCI.2993-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: opiates alleviate separation distress. Biological psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neuroscience and biobehavioral reviews. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Pennock RL, Hentges ST. Differential expression and sensitivity of presynaptic and postsynaptic opioid receptors regulating hypothalamic proopiomelanocortin neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:281–288. doi: 10.1523/JNEUROSCI.4654-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. kappa-Opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. J Neurosci. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson LE, Gillan MG, Kosterlitz HW. Species differences in the concentrations and distributions of opioid binding sites. European journal of pharmacology. 1985;112:65–71. doi: 10.1016/0014-2999(85)90239-0. [DOI] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Physiological and pharmacological evidence for a serotonergic projection to the hippocampus. Brain research. 1975;94:115–131. doi: 10.1016/0006-8993(75)90881-1. [DOI] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Birney EC. Parental Care and Mating System of the Prairie Vole, Microtus-Ochrogaster. Behav Ecol Sociobiol. 1979;5:171–186. [Google Scholar]
- Troisi A, Frazzetto G, Carola V, Di Lorenzo G, Coviello M, Siracusano A, Gross C. Variation in the {micro}-opioid receptor gene (OPRM1) moderates the influence of early maternal care on fearful attachment. Social cognitive and affective neuroscience. 2011 doi: 10.1093/scan/nsr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkish S, Cooper SJ. Fluid consumption in water-deprived rats after administration of naloxone or quaternary naloxone. Progress in neuro-psychopharmacology & biological psychiatry. 1983;7:835–839. doi: 10.1016/0278-5846(83)90078-7. [DOI] [PubMed] [Google Scholar]
- van Ree JM, Gerrits MA, Vanderschuren LJ. Opioids, reward and addiction: An encounter of biology, psychology, and medicine. Pharmacological reviews. 1999;51:341–396. [PubMed] [Google Scholar]
- Warnick JE, McCurdy CR, Sufka KJ. Opioid receptor function in social attachment in young domestic fowl. Behavioural brain research. 2005;160:277–285. doi: 10.1016/j.bbr.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Moles A, Schwarting RK, D’Amato FR. Lack of social exploratory activation in male mu-opioid receptor KO mice in response to playback of female ultrasonic vocalizations. Social neuroscience. 2011;6:76–87. doi: 10.1080/17470911003765560. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW. Selective effects of naltrexone on food pleasantness and intake. Physiology & behavior. 1996;60:439–446. doi: 10.1016/s0031-9384(96)80017-5. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Hormones and behavior. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nature neuroscience. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zastawny RL, George SR, Nguyen T, Cheng R, Tsatsos J, Briones-Urbina R, O’Dowd BF. Cloning, characterization, and distribution of a mu-opioid receptor in rat brain. Journal of neurochemistry. 1994;62:2099–2105. doi: 10.1046/j.1471-4159.1994.62062099.x. [DOI] [PubMed] [Google Scholar]