Abstract
The current study investigates locomotor activity in a novel environment and correlates these activity levels with cocaine self-administration in rats that were either trained or untrained on a lever-pressing task prior to cocaine self-administration. The authors report that it is the rate of learning the lever-pressing task, not cocaine self-administration, that correlates with locomotor activity. The results suggest that a correlation between locomotor activity and cocaine self-administration is secondary to a link between locomotor activity and rate of learning to lever press for a reward. The authors conclude that locomotor activity is not necessarily an indicator of propensity to self-administer cocaine and demonstrate that environmental novelty and rate of learning an operant task are important considerations when designing experiments on drug-seeking behaviors.
Keywords: addiction, learning, self-administration, cocaine, locomotion
Behavioral models of drug abuse have demonstrated that a correlation exists between locomotor activity and vulnerability to psychostimulant self-administration in rats. Typically, rats with greater locomotor activity in a novel environment acquire higher levels of psychostimulant self-administration (Deroche, Le Moal, & Piazza, 1999; Piazza, Deminiere, Le Moal, & Simon, 1989; Piazza, Deroche-Gamonent, Rouge-Pont, & Le Moal, 2000). However, increased locomotor activity has also been characterized as an index of novelty seeking (exploration) and stress (Robbins, 1977; Van den Buuse, Van Acker, Fluttert, & De Kloet, 2001), complicating its link with drug abuse. Locomotor activity might also reflect individual differences in learning, which may themselves be important contributors to performance in behavioral paradigms of drug seeking. It is currently unclear whether rats with high locomotor activity self-administer more cocaine because they are especially susceptible to cocaine reinforcement or whether they are instead more adept at learning a particular operant task than those with low locomotor activity.
The current experiments were conducted to determine the contribution of operant training to subsequent cocaine self-administration. We hypothesized that rats with high locomotor activity would acquire food self-administration more rapidly than those with low locomotor activity and that, once all rats were proficient at food self-administration, there would be no correlation between locomotor activity and cocaine self-administration. We also hypothesized that rats that had already learned an operant lever-pressing task prior to cocaine exposure would display more stable responding for cocaine, as such responding would not be obscured by individual differences in learning. Last, we hypothesized that if differences in cocaine self-administration were due to differences in learning, any correlation between locomotion and cocaine self-administration in rats with no previous experience on an operant task would be lost once all rats gained proficiency at this task.
Method
Subjects and Surgical Procedures
Seventy-two male Sprague–Dawley rats (Charles River, MA) weighing 275 g at the beginning of behavioral testing were housed 3 to a cage during food training and were individually housed following surgery. The colony was kept at constant temperature and followed a standard 12-hr light–dark cycle, with food and water available ad libitum. Rats were tested at the same time during their light cycle each day. All experimental protocols were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Prior to cocaine self-administration, rats were anesthetized with a ketamine–xylazine–acepromazine mixture and implanted with a chronic jugular catheter using methods previously described (Caine & Koob, 1993). In brief, catheters were constructed by connecting microrenethane tubing (i.d. = .012 in [.305 mm], o.d. = .025 in [.635 mm]) to a 26-gauge guide cannula (Plastics One, Roanoke, VA) and then securing the guide cannula to polypropylene mesh (Small Parts, Miami, FL) with cranioplastic cement. Following surgery, all rats received a daily administration of the antibiotic ticarcillin (20 mg/day at 100 mg/ml; GlaxoSmithKline, Research Triangle Park, NC). Catheter patency was tested by infusion of the fast-acting barbiturate Brevital (methohexital sodium; 1 mg/0.1 ml; Lilly, Indianapolis, IN). Catheters were deemed patent in rats showing profound loss of muscle tone within 5 s of infusion.
Food and Cocaine Self-Administration
Self-administration training was conducted in operant-conditioning chambers enclosed within sound-attenuating cabinets (MED Associates, St. Albans, VT). Each chamber contained an active and an inactive lever as well as a food dispenser and two stimulus lights. Presses on the active lever resulted in either a 120-μl infusion of cocaine over a 4-s period via a Razel pump (Razel Scientific Instruments, Fairfax, VT) or delivery of a 45-mg food pellet (Bio-Serv, Frenchtown, NJ). Presses on the inactive lever were recorded but were without consequence. Presses on the active lever resulted in a 20-s time-out, which was signaled by illumination of the stimulus light and retraction of the active lever. Once stable responding for food reinforcement was achieved at a fixed ratio (FR1: one barpress = one food pellet; 8 days, 60-min sessions, n = 32), rats were shifted to a higher fixed ratio (FR5: five barpresses = one food pellet, 60-min sessions) for a period of 7 days. Following surgical recovery (5 days), food-trained rats were again allowed to lever press for food (FR5; 3 days). Rats then lever pressed for cocaine (0.08 mg/infusion; approximately 0.25 mg/kg/infusion) on an FR5 schedule of reinforcement for three daily sessions of 3 hr each (n = 25). The 7 remaining food-trained rats served as yoked controls (data not shown). To replicate previous findings (Piazza et al., 1989), rats untrained with food on the operant task were given the opportunity to lever press for cocaine for a period of 7 days (n = 15). A low dose of cocaine was used for self-administration because previous research indicates that such doses are best for detecting differences in proclivity to self-administer cocaine (Marinelli & White, 2000; Piazza et al., 2000). No priming infusions were given at any time. Rats were never food deprived. Cocaine hydrochloride was supplied by the Research Triangle Institute (Research Triangle Park, NC) under the National Institute on Drug Abuse Drug Supply Program and was dissolved in physiological saline (0.9%).
Locomotor Testing
Rats were tested for basal locomotor activity for a period of 15 min using a MED Associates ENV-515 open-field test environment coupled to an ENV-520 48-channel infrared controller. Locomotor testing was conducted 24 hr prior to the first day of behavioral training or testing. Number of horizontal and vertical beam breaks as well as stereotypic behaviors were recorded. Locomotor testing was conducted at the same time each day (1:00–2:30 p.m.).
Data Analysis
Values are given as arithmetic means ± SEM. A median split based on locomotor activity was conducted as previously described (Piazza et al., 1989) to compare rats with high and low cocaine self-administration. Learning of lever pressing behavior was characterized by an initial period in which rate of lever pressing was low and a second phase in which performance rapidly improved. This learning was reflected in a bimodal distribution of frequency of active lever pressing over sessions. Therefore, the transition between these two phases represents a point of change in lever-pressing behavior. The lever-pressing rate marking the transition between these two phases was, therefore, used as a threshold of learning. Plotting frequency of active lever presses over days (see Figure 1) and then fitting this histogram with a polynomial curve demonstrated a bimodal distribution of the data at about the 30th active lever press. This suggests that, on average, rats pressing fewer than 30 times/hr are not yet aware of the relationship between lever pressing and reward. Learning threshold was then set as the day on which active lever presses exceeded 30/hr. Learning threshold, cumulative responses, self-administration, and locomotor activity were analyzed using linear regression analysis. Comparisons between groups were conducted using a two-way analysis of variance followed by the Holman-Sidak post hoc test for multiple comparisons. Paired comparisons were made using a Student's t test (two-tailed). Pearson rs were analyzed using a single-sample Student's t test to compare residuals. All statistical tests were parametric and were conducted using commercially available software (Excel and SigmaStat).
Figure 1.
Bimodal distribution of frequency fo active lever pressing over sessions. Learning threshold was defined as the day on which active lever presses exceeded 30 per hour.
Results
Locomotor Activity and Cocaine Self-Administration
Although locomotor activity has been suggested to predict cocaine self-administration (Piazza et al., 1989), in the current experiment locomotor activity and cocaine self-administration did not correlate in rats that were trained to self-administer food prior to subsequent testing for cocaine self-administration in the same behavioral context (see Figure 2; n = 25).
Figure 2.
Locomotor activity (beam breaks) and self-administration on Days 1–3 of cocaine self-administration in rats (n = 25) trained with food on the lever-pressing task prior to cocaine access.
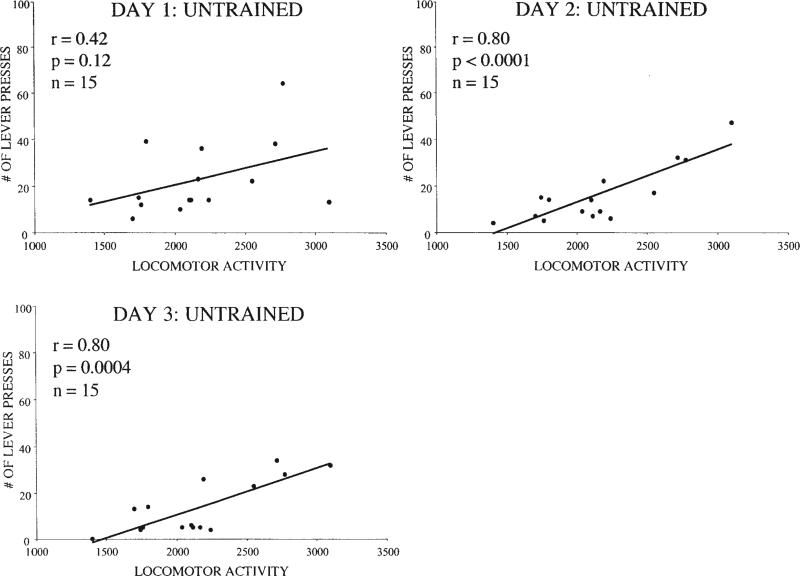
The relationship between cocaine self-administration and locomotor activity was also examined in rats that were untrained on the operant task prior to cocaine self-administration. Consistent with previous reports (e.g., Piazza et al., 1989), locomotor activity was found to correlate with cocaine self-administration in untrained rats, such that those with greater locomotor activity self-administered more cocaine (see Figure 3; n = 15). This correlation was significant on Day 2 of cocaine self-administration (r = .80, p = .0001) and remained significant through Day 4 (r = .57, p = .027; data not shown).
Figure 3.
Locomotor activity (beam breaks) and self-administration on Days 1–3 of cocaine self-administration in rats (n = 15) untrained on the lever pressing task prior to cocaine access.
Locomotor Activity and Food Self-Administration
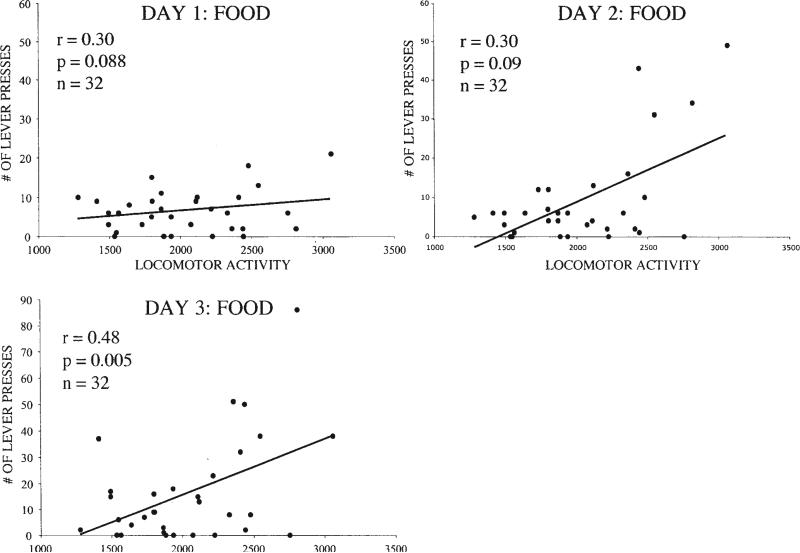
Acquisition of food self-administration was examined to determine whether the difference seen in cocaine self-administration between rats that were either trained or untrained on the operant task was due to differences in exposure to the operant task or testing environment. Locomotor activity was found to correlate with the acquisition of food self-administration (see Figure 4; n = 32), suggesting that locomotor activity does not specifically predict a propensity to self-administer psychostimulants. This correlation was at trend level on Day 1 of training (r = .30, p = .088), became significant on Day 3 of training (r = .48, p = .005), and remained significant until Day 8 (r = .33, p = .067; data not shown), as rats with low locomotor activity gradually improved on the operant task.
Figure 4.
Locomotor activity (beam breaks) and self-administration on Days 1–3 of training (with food) on the lever-pressing task (n = 32).
Responses on the Inactive Lever
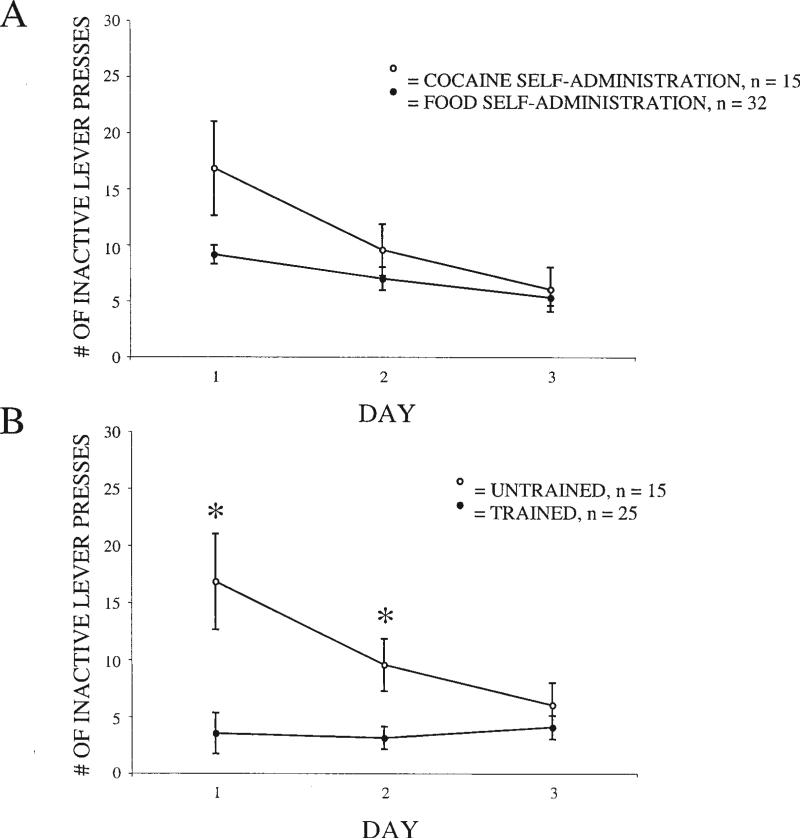
Although the number of responses on the inactive lever was identical for the food self-administration and cocaine self-administration groups during the first 3 days of exposure to the operant task (see Figure 5A), inactive lever responses differed significantly between the trained and untrained groups during the first 3 days of cocaine exposure (see Figure 5B, p < .001). Specifically, rats that were trained with food on the operant task prior to cocaine exposure had significantly fewer responses on the inactive lever during the first 2 days of cocaine exposure than those that were untrained on the operant task. This effect was significant on the first day of cocaine exposure, t(38) = 4.58, p < .001, remained significant through the second day of cocaine exposure, t(38) = 2.20, p = .03, and lost significance on Day 3, t(38) = 0.68, p = .50. This finding suggests that locomotor behavior in a novel environment leads to increased lever pressing irrespective of reward, as animals familiar with the environment press the inactive lever less than those exposed to the environment for the first time.
Figure 5.
A: Responses on the inactive lever during the first 3 days of exposure to the operant task in rats receiving either food (n = 32) or cocaine (n = 15) access. B: Responses on the inactive lever during the first 3 days of cocaine self-administration in rats that were either trained (with food, n = 32) or untrained (n = 15) on the operant task prior to cocaine access. Note that untrained rats in Figure 4B are the same group as cocaine self-administration rats in Figure 4A. Analysis of variance demonstrated a significant main effect for group ( p < .001), and post hoc tests revealed a significant difference between groups on Day 1 and Day 2. *p < .05. Error bars represent standard error.
Food and Cocaine Self-Administration
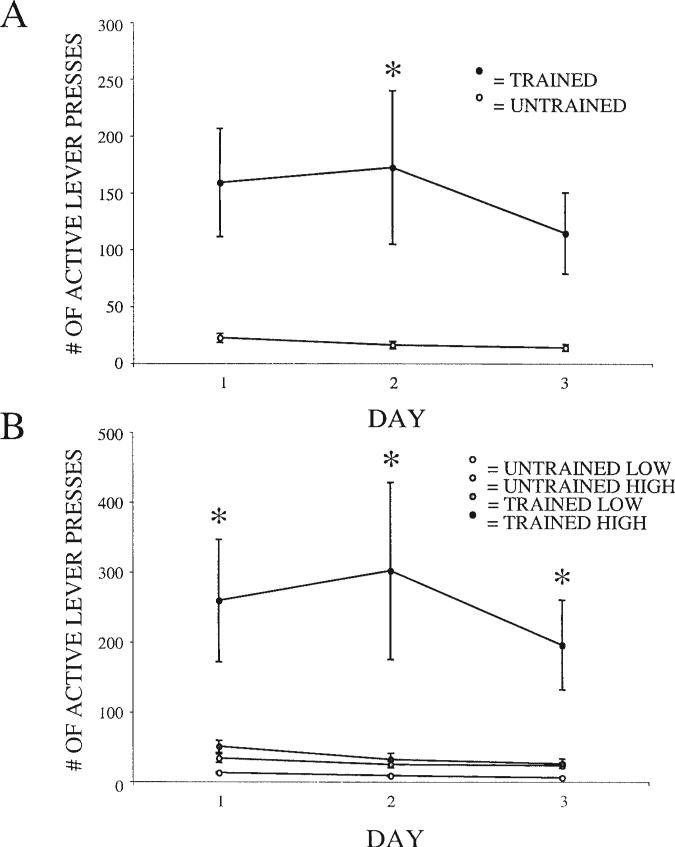
The magnitude of cocaine self-administration differed significantly between rats that were trained with food prior to cocaine access and those that were not ( p = .002). Trained rats self-administered significantly more cocaine during the second day of cocaine exposure than untrained rats (see Figure 6A), t(38) = –2.23, p = .028. To examine this effect in more detail, we conducted a median split based on cocaine intake to divide rats into high- and low-responder groups as previously described (Piazza et al., 1989). This analysis revealed a significant main effect of group ( p < .001). Post hoc tests indicated that this effect was due to differences in high-responding rats, as pretrained high-responders self-administered significantly more cocaine than all other groups (see Figure 6B), t(18) = –4.68, p < .001, t(18) = –4.66, p < .001, and t(18) = –4.15, p < .001, for trained high-responders versus untrained low-responders, trained lowresponders, and untrained high-responders, respectively. Even when comparing their 4th and 5th days of cocaine self-administration with trained rats on their 1st day of cocaine self-administration, untrained rats self-administered significantly less cocaine than trained rats, t(38) = 2.76, p = .01, and t(38) = 2.79, p = .01, respectively. This effect lost significance on Day 6 (data not shown), as untrained rats began to self-administer more cocaine. This outcome suggests that trained rats self-administer more cocaine than untrained rats because they are already familiar with the operant task, not because cocaine is more reinforcing.
Figure 6.
A: Active lever presses in trained (n = 25) and untrained (n = 15) rats during the first 3 days of cocaine access. Analysis of variance demonstrated a significant main effect for group ( p = .002), and post hoc tests revealed a significant difference between groups on Day 2. *p < .05. B: Cocaine self-administration median split from trained (n = 25) and untrained (n = 15) rats. High = high cocaine self-administration; low = low cocaine self-administration. Analysis of variance demonstrated a significant main effect for group ( p < .001), and post hoc tests showed the trained high group to be significantly different from all other groups. *p < .01. Error bars represent standard error.
Learning Thresholds
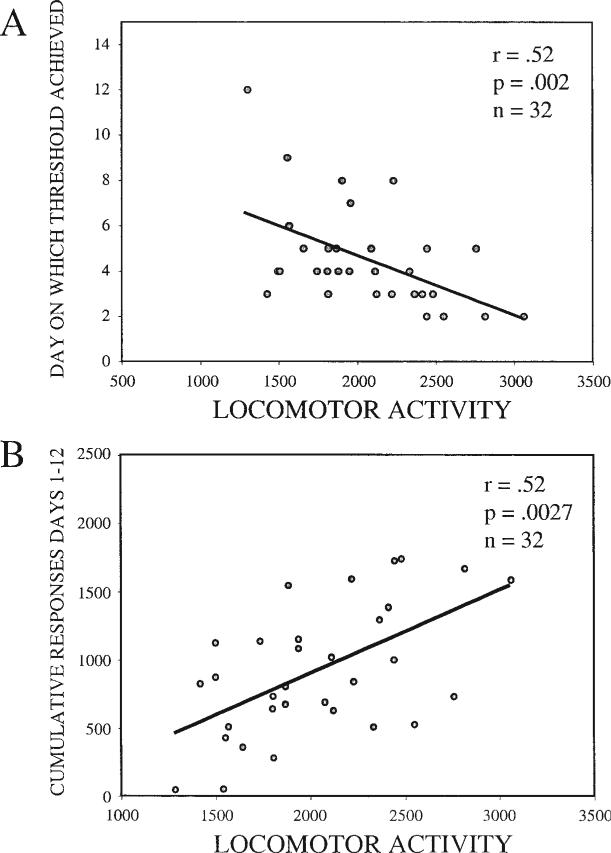
Learning threshold for the operant lever-pressing task (≥ 30 presses/hr on the active lever; see Method section) was found to be significantly correlated with locomotor activity (see Figure 7A; n = 32, r = –.52, p = .002). Additionally, cumulative number of responses on the active lever was found to correlate with locomotor activity during food self-administration (see Figure 7B; n = 32, r = .52, p = .0027). Both results again suggest that locomotor activity does not specifically predict a propensity to self-administer psychostimulants but instead may determine the amount of time required to learn an operant task.
Figure 7.
A: Day on which learning threshold (≥ 30 presses on the active lever) was achieved during food training (n = 32, r = .52, p = .002). B: Cumulative responses on Days 1–12 of food training (n = 32, r = .52, p = .0027).
Locomotor Activity Between Groups
Average locomotor activity was not different between groups of trained (n = 25, M = 2,018.40 ± 84.76) and untrained (n = 15, M = 2,160.09 ± 119.23) rats, suggesting that the differences in cocaine self-administration in trained versus untrained rats are not due to differences in baseline locomotor activity.
Discussion
The current results suggest that locomotor activity per se does not predict levels of cocaine self-administration; rather self-administration is correlated with facility in learning an operant task, which is in turn correlated with locomotor activity. Performance on the operant task is correlated with locomotor activity regardless of the reinforcer. A correlation has previously been reported between locomotor activity in a novel environment and psychostimulant self-administration (Deroche et al., 1999; Piazza et al., 1989, 2000). However, such correlations were observed in rats that were neither habituated to the testing apparatus nor trained on the operant task prior to drug self-administration. Our results demonstrate that this correlation is largely due to individual differences in rates of learning. Indeed, when training was omitted in the current study, a significant positive correlation was found between locomotor activity and cocaine self-administration. In keeping with these results, other researchers have noted a lack of correlation between locomotor activity and drug self-administration in food-trained rats (Kosten, Miserendino, & Kehoe, 2000; Phillips et al., 1994). It is also of interest that locomotor activity is predictive of neither cocaine- nor amphetamine-conditioned place preference (Erb & Parker, 1994; Gong, Neill, & Justice, 1996; Kosten & Miserendino, 1998). If locomotor activity is indeed an indicator of psychostimulant reward, one might expect a positive correlation between locomotor activity and conditioned place preference.
Correlations between locomotor activity and operant performance were not confined to cocaine self-administration. A significant correlation was also found in the current study between locomotor activity and food self-administration. Additionally, a significant correlation was also found between locomotor activity and the rate at which the operant task was learned. Both results suggest that locomotor activity contributes to learning in a novel environment but not to learning cocaine self-administration per se. It follows that differences in learning will contribute to cocaine self-administration in instances in which rats have not been trained on an operant task prior to drug exposure. High rates of cocaine self-administration in rats unfamiliar with an operant task may, therefore, reflect more rapid acquisition of the operant behavior, not greater reinforcing or rewarding effects of cocaine.
The correlation between locomotor activity and cocaine self-administration in untrained rats was no longer significant by Day 5, suggesting that the correlation is dependent on the various learning curves of the rats, perhaps as a direct result of lower locomotor activity or exploratory behavior. Because these rats with low rates of learning are given time to practice and improve, the correlation between self-administration and locomotor activity disappears. Consistent with this finding, Pierre and Vezina (1997) have found that the correlation between locomotor activity and psychostimulant self-administration is lost after the first week of testing in untrained rats.
The operant response used in the current task was a lever-press response rather than a nose poke, as has been used previously in most studies. Although these behaviors are substantially different, several investigators have suggested that a nose poke is a more automatic or natural behavior for a rat and, therefore, an easier operant to perform. However, use of a natural or automatic response could be even more likely to amplify differences in rates of learning. It may be that with a more complex operant, such as lever pressing, the rats more readily learn the association between the behavior and the administration of the reward. Additionally, nose pokes are tightly linked with novelty and exploration. Piazza et al. noted that “the acquisition of amphetamine self-administration is initially based on novelty induced nose pokes, which constitute one of the main behavioral patterns of the rat” (1990, p. 340), making it difficult to interpret such behavior with respect to drug seeking.
Although it may be suggested that, because of prior experience with food self-administration, trained rats are actually food seeking when pressing the active lever during cocaine self-administration sessions, this possibility is unlikely. Rats on a similar schedule of food reinforcement have been shown to significantly increase their responding on Day 1 of food extinction and decrease their responding on Day 2 of food extinction (Ahmed & Koob, 1997), suggesting that the increase in cocaine self-administration reported here in trained rats is not due to extinction behavior. Additionally, rats in the current study were never food deprived, as food deprivation has been shown to increase both stress levels and psychostimulant self-administration (Lu, Shepard, Hall, & Shaham, 2003), and this should have also attenuated motivation for food seeking.
Previous research has shown that corticosterone levels increase as environmental novelty increases (Araujo, DeLucia, Scavone, & Planeta, 2003) and that rats with higher corticosterone levels self-administer more cocaine than those with lower corticosterone levels (Deroche, Marinelli, Le Moal, & Piazza, 1997; Piazza et al., 1991). Additionally, the locomotor effects of psychostimulants have been found to be greater when administered in a novel environment (Badiani et al., 1998). Taken together with the current findings, these results indicate that novelty and stress act to increase locomotor activity, thereby increasing self-administration in rats that might, under other conditions, manifest very different patterns of drug consumption, because when stress and novelty are minimized with habituation and training a different group of high-responders emerges. Even though training a rat to self-administer a psychostimulant is far less time consuming when one does not train it first on the operant task, our results suggest that facilitating the acquisition of drug self-administration with novelty and stress should be approached with caution.
We conclude that basal locomotor activity is not a predictor of cocaine self-administration in rats that have been trained on the operant task prior to cocaine access and suggest that individual differences in learning and environmental novelty are responsible for the correlation previously reported between locomotor activity and psychostimulant self-administration.
Acknowledgments
This work was supported by National Institutes of Health Grants DA14639 and DA14735.
Contributor Information
Jennifer M. Mitchell, Department of Neurology, University of California, San Francisco
Chris L. Cunningham, Department of Behavioral Neuroscience, Oregon Health & Science University.
Gregory P. Mark, Department of Behavioral Neuroscience, Oregon Health & Science University.
References
- Ahmed SH, Koob GF. Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berlin) 1997;132:289–295. doi: 10.1007/s002130050347. [DOI] [PubMed] [Google Scholar]
- Araujo AP, DeLucia R, Scavone C, Planeta CS. Repeated predictable or unpredictable stress: Effects of cocaine-induced locomotion and cyclic AMP-dependent protein kinase activity. Behavioural Brain Research. 2003;139:75–81. doi: 10.1016/s0166-4328(02)00088-8. [DOI] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: Modulation by environmental novelty. Journal of Neuroscience. 1998;18:10579–10593. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993 Jun 18;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Deroche V, Le Moal M, Piazza PV. Cocaine self-administration increases the incentive motivational properties of the drug in rats. European Journal of Neuroscience. 1999;11:2731–2736. doi: 10.1046/j.1460-9568.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Le Moal M, Piazza PV. Glucocorticoids and behavioral effects of psychostimulants: II. Cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. Journal of Pharmacology and Experimental Therapeutics. 1997;281:1401–1407. [PubMed] [Google Scholar]
- Erb SM, Parker LA. Individual differences in novelty-induced activity do not predict amphetamine-induced place conditioning. Pharmacology Biochemistry and Behavior. 1994;48:581–586. doi: 10.1016/0091-3057(94)90317-4. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Justice JB., Jr. Locomotor response to novelty does not predict cocaine place preference conditioning in rats. Pharmacology Biochemistry and Behavior. 1996;53:191–196. [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ. Dissociation of novelty- and cocaine-conditioned locomotor activity from cocaine place conditioning. Pharmacology Biochemistry and Behavior. 1998;60:785–791. doi: 10.1016/s0091-3057(97)00388-2. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Research. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: A review. Neuroscience & Biobehavioral Reviews. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of mid-brain dopamine neurons. Journal of Neuroscience. 2000;20:8876–8875. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GD, Howes SR, Whitelaw RB, Wilkinson LS, Robbins TW, Everitt BJ. Isolation rearing enhances the locomotor response to cocaine and a novel environment, but impairs the intravenous self-administration of cocaine. Psychopharmacology (Berlin) 1994;115:407–418. doi: 10.1007/BF02245084. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere J-M, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989 Sep 29;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere J-M, Maccari S, Mormede P, LeMoal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behavioural Pharmacology. 1990;1:339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. Journal of Neuroscience. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere J-M, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proceedings of the National Academy of Sciences, USA. 1991;88:2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: The contribution of response to novelty and prior exposure to the drug. Psychopharmacology (Berlin) 1997;129:277–284. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- Robbins TW. A critique of the methods available for the measurement of spontaneous motor activity. In: Iversen LL, Iversen DS, Snyder SH, editors. Handbook of psychopharmacology. Vol. 7. Plenum Press; New York: 1977. pp. 37–80. [Google Scholar]
- Van den Buuse M, Van Acker SA, Fluttert M, De Kloet ER. Blood pressure, heart rate, and behavioral responses to psychological “novelty” stress in freely moving rats. Psychophysiology. 2001;38:490–499. doi: 10.1017/s0048577201990687. [DOI] [PubMed] [Google Scholar]