The mechanism responsible for pancreatitis, the inflammatory diseases of the pancreas, was attributed to autodigestion more than 115 years ago by Dr Chiari.1 This idea has remained widely accepted because it seems obvious. After all, the exocrine pancreas is specialized in the synthesis of digestive enzymes, and activated digestive enzymes capable of injuring the gland have been detected within the pancreas, pancreatic juice, and plasma in clinical as well as experimental forms of pancreatitis.2 Thus, it is not surprising that pancreatitis research was focused for many years on determining the mechanisms to explain the initiation of pancreatic autodigestion.
In 1904, Pavlov was awarded the Nobel Prize for his work, which included the observation that enterokinase was responsible for activation of “protease activity” in the intestine. The digestive enzymes were later crystallized by Kunitz and Northrop from bovine pancreas in 1936 and John Northrop was awarded the Nobel Prize for Chemistry in 1946 for showing the activation of proenzymes, pepsinogen, trypsinogen, and chymotrypsinogen and that trypsinogen, the inactive precursor of the protease trypsin, was its key target. Once activated, trypsin became the activator of the other proenzymes. Thus, it was logical to consider trypsin as the initiator of the autodigestive process observed in pancreatitis. However, for many years it was not clear where or how trypsinogen might be activated. It was the development of new animal models of the disease in the mid 20th century, along with improvements in technology, that led to the first real progress in understanding the molecular mechanisms involved in the initiation of pancreatitis. One of the key observations was that trypsinogen was activated intracellularly early in the course of experimental pancreatitis.3,4 This led to the hypothesis that intracellular activation of trypsinogen was the key mechanism responsible for the initiation of acute pancreatitis. This hypothesis was perhaps best described by Steer.3 In this model, trypsinogen activation was due to perturbations in intracellular trafficking of proteins, such that lysosomal hydrolases such as cathepsins and digestive enzyme zymogens become co-localized with lysosomes leading to trypsinogen activation by cathepsin B and subsequent cellular damage.
This hypothesis was supported indirectly by a large number of studies. For example, inhibition of trypsinogen activation with pharmacologic inhibitors of cathepsins seemed to ameliorate the disease.5 However, the strongest evidence supporting the trypsin paradigm came from the observation originally made by Whitcomb et al,6 that mutations of the cationic trypsinogen gene are associated with hereditary pancreatitis. Furthermore, mutations of the potent pancreatic serine protease inhibitor Kazal type 1 (SPINK1) also predispose patients to pancreatitis.7 Correspondingly, transgenic expression of SPINK1 ameliorates secretagogue-induced pancreatitis in mice.8 Other support for the paradigm includes the observations that genetic deletion of cathepsin B reduced the severity of experimental pancreatitis.9 With so much support, the trypsin hypothesis became the central paradigm of the field.
However, objective evaluation of the major supports of a central role of trypsinogen activation as the most important initiator of pancreatitis indicates that they are indirect and inconclusive. The greatest obstacle is that the standard experimental animal models of acute pancreatitis cause generalized, nonspecific damage, rather than specific activation of trypsinogen. Pharmacologic protease inhibitors have tended to lack specificity and interfere with multiple pathways that are important in pancreatitis.10 Recently, newer inhibitors have actually suggested that trypsin is not involved in the activation of its zymogen or other digestive enzymes in the injured acinar cell.11 Even the genetic support from hereditary pancreatitis studies is inconclusive. Some mutations associated with hereditary pancreatitis seem not to alter enzymatic properties of the protease at all, but rather lead to misfolding and consequently to endoplasmic reticular stress.12 Most important, there is no clear-cut proof that protease inhibitors alter the clinical course of acute pancreatitis in >70 clinical trials performed over the past 40 years.13 The most plausible explanation for the inconsistency between the popular paradigm and clinical reality is that the animal models utilized to develop the paradigm are not suitable to dissect the specific role of intracellular trypsinogen activation in pancreatitis.
In this issue of Gastroenterology, Dawra et al14 described a novel genetic mouse model that will be extremely valuable for studies on the role of trypsin in pancreatitis. In this model, the authors genetically deleted the most prominent form of trypsinogen in the mice, trypsinogen-7, an equivalent gene of human cationic trypsinogen. They showed that deletion of trypsinogen-7 reduced the total trypsinogen content by 60%, but did not affect physiologic function. After caerulein treatment, these mice lacked pathologic activation of trypsinogen, which occurs during early stages of caerulein-induced pancreatitis in control mice. Absence of trypsinogen activation in mice lacking trypsinogene-7 led to near complete inhibition of acinar cell death in vitro and a 50% reduction in acinar necrosis in vivo. However, these mice showed similar degrees of local and systemic inflammation.
In their model, Dawra et al14 found that trypsinogen-7 gene deletion only decreased acinar cell trypsinogen content by about 60%, and yet was able to completely block caerulein induced intracellular trypsin activity. It remains unclear whether this specific isoform is the only one normally activated in this model of experimental pancreatitis, or whether simply reducing the total level of trypsinogen would have the same effect. Lack of an obvious phenotype in trypsinogen-7–deficient mice under control conditions suggests that trypsinogen expression levels are normally in excess of what is actually needed physiologically. Another possibility is that the mice compensate for the reduced trypsin by increasing other mechanisms, which are as yet unknown. Further studies with these animals will clearly provide more interesting insights on pancreatic physiology.
The observation that intracellular trypsin is important for acinar cell death is consistent with previous results. Our laboratory developed a mutant trypsinogen that is activated by a mammalian propeptide processing endoprotease located in the trans-Golgi network, PACE (also called furin). When this construct was expressed in pancreatic acinar cells, spontaneous intracellular trypsinogen activation led to acinar cell apoptosis through caspase-dependent and -independent pathways.15 Similar observations were made by Sahin-Toth et al 16 using a mutant human trypsinogen with increased autoactivation properties.16 Taken together, these data firmly demonstrated that intracellular trypsin is harmful and can directly induce cell death.
The Dawra et al14 study confirms that trypsin activity is not necessary for caerulein-induced pancreatitis. The issue of whether trypsin activity is sufficient to cause pancreatitis was recently answered using an in vivo model in which PACE-trypsinogen was conditionally expressed in transgenic mice.17 Surprisingly, the animals were able to compensate for considerable levels of active trypsin without obvious injury. However, when the level of active trypsin was increased by gene dosage, the compensatory mechanisms were unable to cope and acute pancreatitis developed. Thus, this model indicated for the first time that active intracellular trypsin is sufficient to cause acute pancreatitis. Interestingly, the animals that survived high levels of active trypsin expression in their acinar cells did not develop chronic pancreatitis, but rather developed fatty replacement of acinar cells. Therefore, although trypsinogen activation is sufficient to induce acute pancreatitis under these experimental conditions, it seems unlikely that trypsinogen activation alone is responsible for chronic pancreatitis as it occurs in patients.
Another important early event during the development of pancreatitis is acinar cell expression of inflammatory regulators including chemokines and cytokines.18 Acinar cell–derived regulators lead to the infiltration and activation of inflammatory cells and the initiation of an inflammatory cascade. Their expression is regulated by inflammation related transcription factors including nuclear factor (NF)-κB18 and EGR-119. Dawra et al elegantly demonstrated that caerulein caused comparable levels of intra-acinar NF-κB activation in mice lacking trypsinogen-7. Other studies in which cathepsin B was either genetically depleted or inhibited also had significant reductions in trypsin activity, but found that systemic inflammation was little affected.9 In concordance with these observations, we found that intracellular trypsin did not activate NF-κB.15 Therefore, a separation exists between intra-acinar trypsin activation and the initiation of other critical pathways of pancreatitis, as we have previously suggested.10 However, trypsin could activate NF-κB during the progression of pancreatitis, because extracellular trypsin is able to activate NF-κB.15
Perhaps the most important message from the recent studies with genetic mouse models is that multiple pathways are activated simultaneously by various etiologies that cause pancreatitis. Trypsinogen activation is definitely not the only factor that determines the severity of pancreatitis. Indeed, it has been demonstrated that acute pancreatitis can be initiated by genetically activating the NF-κB pathway.20 Elevated Ras signaling is also able to induce pancreatitis.21 The notion that trypsin is sufficient but not required for the development of pancreatitis might be able to explain the failed clinical trials with trypsin inhibitors.
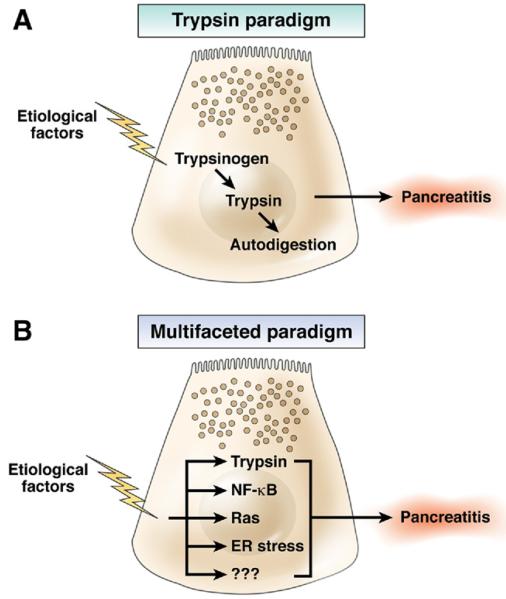
Taken, together the accumulating evidence suggests that active trypsin is but 1 component of the multifaceted response of the acinar cell to injury. Acute pancreatitis is not only associated with pancreatic damage, but also with a large number of sequelae including edema, coagulation, and vascular complications, infiltration of immune cells, local and systemic inflammation, and except in the most severe cases, resolution and repair. This leads to a new paradigm of multifaceted mechanisms involved in pancreatitis (Figure 1). Certainly necrosis is what Chiari was looking at 115 years ago and he was correct that autodigestion occurred in severe acute pancreatitis. However, it is clearly time to broaden the focus of pancreatitis research from its strong focus on trypsinogen activation (Figure 1A) to other mechanisms that are also critical to the initiation of the disease (Figure 1B). These studies will be facilitated by the emergence of new genetic models such as that developed by Dawra et al.14
Figure 1.
A comparison of models depicting the role of trypsin in acute pancreatitis. In the original “trypsin paradigm,” the activation of trypsin was the 1 key mechanism upon which all of the aspects of pancreatitis followed. In the newer “multifaceted paradigm,” trypsin activity is 1 of several interrelated mechanisms that is simultaneously activated when acinar cells are injured. With the multifaceted model, different mechanisms can be more affected by specific causal factors and, therefore, can explain observed differences in pancreatitis initiated by different etiologies.
See “Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis,” by Dawra R, Sah RP, Dudeja V, et al, on page 2210.
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Chiari H. Über die Selbstverdauung des menschlichen Pankreas. Zeitschrift für Heilkunde. 1896;17:69–96. [Google Scholar]
- 2.Geokas MC, Rinderknecht H. Free proteolytic enzymes in pancreatic juice of patients with acute pancreatitis. Am J Dig Dis. 1974;19:591–598. doi: 10.1007/BF01073012. [DOI] [PubMed] [Google Scholar]
- 3.Steer ML. Search for the trigger mechanism of pancreatitis. Gastroenterology. 1984;86:764–766. [PubMed] [Google Scholar]
- 4.Gaisano HY, Gorelick FS. New insights into the mechanisms of pancreatitis. Gastroenterology. 2009;136:2040–2044. doi: 10.1053/j.gastro.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Van Acker GJ, Saluja AK, Bhagat L, et al. Cathepsin B inhibition prevents trypsinogen activation and reduces pancreatitis severity. Am J physiol Gastrointest Liver Physiol. 2002;283:G794–G800. doi: 10.1152/ajpgi.00363.2001. [DOI] [PubMed] [Google Scholar]
- 6.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 7.Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 8.Nathan JD, Romac J, Peng RY, et al. Transgenic expression of pancreatic secretory trypsin inhibitor-I ameliorates secretagogue-induced pancreatitis in mice. Gastroenterology. 2005;128:717–727. doi: 10.1053/j.gastro.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 9.Halangk W, Lerch MM, Brandt-Nedelev B, et al. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han B, Ji B, Logsdon CD. CCK independently activates intracellular trypsinogen and NF-kappaB in rat pancreatic acinar cells. Am J Physiol Cell Physiol. 2001;280:C465–C472. doi: 10.1152/ajpcell.2001.280.3.C465. [DOI] [PubMed] [Google Scholar]
- 11.Halangk W, Kruger B, Ruthenburger M, et al. Trypsin activity is not involved in premature, intrapancreatic trypsinogen activation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G367–G374. doi: 10.1152/ajpgi.00315.2001. [DOI] [PubMed] [Google Scholar]
- 12.Kereszturi E, Szmola R, Kukor Z, et al. Hereditary pancreatitis caused by mutation-induced misfolding of human cationic trypsinogen: a novel disease mechanism. Hum Mutat. 2009;30:575–582. doi: 10.1002/humu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh VP, Chari ST. Protease inhibitors in acute pancreatitis: lessons from the bench and failed clinical trials. Gastroenterology. 2005;128:2172–2174. doi: 10.1053/j.gastro.2005.03.087. [DOI] [PubMed] [Google Scholar]
- 14.Dawra R, Sah RP, Dudeja V, et al. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology. 2011;141:2210–2217. doi: 10.1053/j.gastro.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji B, Gaiser S, Chen X, et al. Intracellular trypsin induces pancreatic acinar cell death but not NF-kappaB activation. J Biol Chem. 2009;284:17488–17498. doi: 10.1074/jbc.M109.005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kereszturi E, Sahin-Toth M. Intracellular autoactivation of human cationic trypsinogen mutants causes reduced trypsinogen secretion and acinar cell death. J Biol Chem. 2009;284:33392–33399. doi: 10.1074/jbc.M109.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaiser S, Daniluk J, Liu Y, et al. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut. 2011;60:1379–1388. doi: 10.1136/gut.2010.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han B, Logsdon CD. Cholecystokinin induction of mob-1 chemokine expression in pancreatic acinar cells requires NF-kappaB activation. Am J Physiol. 1999;277:C74–C82. doi: 10.1152/ajpcell.1999.277.1.C74. [DOI] [PubMed] [Google Scholar]
- 19.Ji B, Chen XQ, Misek DE, et al. Pancreatic gene expression during the initiation of acute pancreatitis: identification of EGR-1 as a key regulator. Physiol Genomics. 2003;14:59–72. doi: 10.1152/physiolgenomics.00174.2002. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Ji B, Han B, et al. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122:448–457. doi: 10.1053/gast.2002.31060. [DOI] [PubMed] [Google Scholar]
- 21.Ji B, Tsou L, Wang H, et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–1082. doi: 10.1053/j.gastro.2009.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]