Abstract
Tumor necrosis factor-α (TNF) is an inflammatory cytokine that induces context-dependent proliferation, survival, and apoptosis responses in hepatocytes. TNF stimulates and enhances growth factor-mediated hepatocyte proliferation and survival following partial hepatectomy, but also acts in concert with other inflammatory cytokines of the innate immune response during viral infection to induce apoptosis in hepatocytes. In other epithelial cell types, TNF has recently been shown to stimulate autocrine release of transforming growth factor-α (TGF-α) and interleukin-1 (IL-1) family ligands. Here, we examine the role of these autocrine ligands in modulating TNF-induced proliferation and apoptosis in primary hepatocytes. We show that TNF-induced hepatocyte proliferation is regulated by an inducible, coupled, and self-antagonizing autocrine cascade involving the pro-proliferative TGF-α and IL-1 receptor antagonist (IL-1ra) ligands and antiproliferative IL-1α/β ligands. Moreover, cooperative stimulation of hepatocyte proliferation by combined TNF and TGF-α; treatment is self-limited through antiproliferative autocrine IL-1α/β feedback We show that TNF potently induces apoptosis of adenovirus-infected hepatocytes in a manner similarly determined through the integrated activity of a coupled TGF-α–IL-1α/β–IL-1ra autocrine cascade. Exogenous TGF-α can either enhance or diminish apoptosis in adenoviral vector-treated and TNF-treated hepatocytes, in a biphasic relationship also mediated by autocrine IL-1α/β feedback.
Conclusion
We demonstrate that TNF-induced hepatocyte proliferation and apoptosis are both governed by a self-antagonizing TGF-α–IL-1α/β–IL-1ra autocrine cascade in vitro, and thus identify multiple molecular targets for control of TNF-regulated hepatocyte phenotypic responses related to liver regeneration and adenoviral gene therapy.
Tumor necrosis factor-α (TNF) can stimulate multiple disparate hepatocyte responses—proliferation, survival, or apoptosis—depending on the cellular context. TNF binds and activates the receptor TNFR, leading to downstream activation of the c-Jun N-terminal kinase (JNK), inhibitor of nuclear factor-κB (IκB) kinase (IKK)–nuclear factor (NF)-κB, and p38 signaling pathways and caspase cascade.1 The modulation of these and other intracellular signaling pathways by concomitant synergistic and antagonistic cytokine stimuli, viruses, and/or pharmacological treatments determine specific cell responses to TNF.2–4
In the partial hepatectomy (PHx) model of liver regeneration, normally quiescent hepatocytes are stimulated to proliferate in a process regulated by multiple redundant signaling pathways and molecules.5 Following PHx, Kupffer cells are activated and secrete TNF and interleukin (IL)-6. These cytokines stimulate the transcription of a set of “immediate early” genes and a G0–G1 cell cycle progression in hepatocytes.6,7 TNF signaling primes hepatocytes for DNA replication through subsequent stimulation by hepatocyte growth factor and epidermal growth factor receptor (EGFR) ligands.5,7–9 In primary hepatocyte cultures, TNF not only potentiates growth factor-stimulated proliferation, but acts as a mitogen itself6,10 through the induced release of autocrine transforming growth factor-α (TGF-α) and its activation of serine-threonine protein kinase B (PKB)/Akt and extracellular signal-regulated kinase (ERK).11,12 The IL-1 receptor (IL-1R) agonists IL-1α and IL-1β antagonize hepatocyte proliferation when produced by nonparenchymal cells in vivo during liver regeneration and when added exogenously to mitogenic factors in vitro13,14
Hepatocytes are resistant to apoptosis stimulated by TNF alone as it activates both proapoptotic and anti-apoptotic signaling pathways.1,4 Consequently, pharmacologic or genetic interference with antiapoptotic signaling is commonly used to examine TNF-induced hepatocyte apoptosis.4 In diseased and/or virus-infected hepatocytes, TNF signaling contributes to apoptotic and necrotic cell death.4,15 Recently, we have shown that infection with a replication-deficient adenoviral vector (Adv) potently sensitizes human epithelial cell lines, including the C3A hepatocarcinoma cell line, to TNF-induced apoptosis via both proapoptotic and antiapoptotic signaling pathways.3 Adenoviral gene therapy vectors targeting the liver and other organs are often compromised due to hepatocyte death induced by both the viral vector itself and cytokines of the innate immune response such as TNF and IL-1β.16 Therefore, Adv infection might provide a physiologically relevant environment to potentiate TNF-induced apoptosis in primary hepatocytes and could lead to insights in liver adenoviral gene therapy.
Hepatocyte death responses to TNF and other inflammatory cytokines can be antagonized by many of the same growth factors that stimulate hepatocyte proliferation8,17 or by naturally occurring inhibitors of cytokine signaling such as IL-1 receptor antagonist (IL-1ra).18 Whereas many of the factors, such as TNF, that affect hepatocytes during injury or stress arise primarily from exogenous sources, hepatocytes themselves secrete growth factors and cytokines that act in autocrine fashion to enhance or oppose exogenous stimuli.8,9,11,12,17,19 Recently, we have demonstrated that the response of human epithelial cell lines to TNF involves release of TGF-α, IL-1α, and IL-1ra, which provide conflicting and interlinked autocrine feedback signals governing apoptotic responses to TNF.2,20 Hepatocytes express TGF-α, IL-1α, IL-1β, and IL-1ra and their receptors but it is unknown whether these ligands operate via interlinked autocrine circuits to modulate hepatocyte proliferation and apoptosis responses to TNF.9,12,21,22
Here we show that rat hepatocyte proliferation and apoptosis responses to TNF are both mediated by an inducible, coupled, and self-antagonizing TGF-α–IL-1α/β–IL-1ra autocrine cascade. The net effect of this coupled autocrine cascade is pro-proliferative as induced by TNF alone but proapoptotic when induced by TNF in Adv-infected hepatocytes. Moreover, elucidation of this self-antagonizing autocrine cascade is a useful paradigm that helps rationalize the diverse landscape of hepatocyte phenotypic responses to TNF and TGF-α costimulation and their induction of autocrine IL-1α/β signaling.
Materials and Methods
Primary hepatocytes were isolated from male Fisher rats using collagenase perfusion.23 Rat hepatocytes were seeded and cultured on collagen type I gels in growth factor–free hepatocyte growth medium.24 For proliferation studies, hepatocytes were stimulated with recombinant TNF and/or TGF-α 24 hours after seeding. For apoptosis studies, hepatocytes were infected with a replication-deficient Adv (Adv.β-gal) 24 hours after seeding and, 24 hours later, were stimulated with TNF and/or TGF-α. Autocrine ligand perturbations were added 1 hour before cytokine stimulation. Hepatocyte proliferation was analyzed by 5-bromo-2’-deoxyuridine (BrdU) incorporation 24–48 hours after cytokine stimulation and flow cytometric quantification of BrdU+-albumin+ cells. Hepatocyte apoptosis was analyzed by flow cytometric quantification of cleaved caspase 3+ cells at 24 hours after cytokine stimulation. Conditioned media samples were analyzed by enzyme-linked immunoabsorbent assay and lactate dehydrogenase (LDH) assays to quantify cytokine release and cell death, respectively. Cell lysates were analyzed using multiplexed bead-based assays to quantify phosphoprotein signaling. Additional and more detailed experimental procedures are included in the Supplementary Material.
Results
TNF-Induced Hepatocyte Proliferation Is Regulated by a Set of Coupled, Self Antagonizing Autocrine Circuits Involving TGF-α, IL-1α/β, and IL-1ra
Rat hepatocytes were cultured on a collagen gel monolayer for 24 hours then stimulated with TNF and other cotreatments for 48 hours, with BrdU added from 24–48 hours to capture maximal cytokine-induced hepatocyte DNA synthesis.6,10,11 Cells were harvested and analyzed by flow cytometry for intracellular albumin and BrdU to quantify the fraction of proliferating hepatocytes (Fig. 1A). Proliferation of nonparenchymal cells was negligible across all treatments as measured by BrdU+-albu-min− cells (Fig. 1A; data not shown) and by assaying changes in abundance of nonparenchymal cells by flow cytometry (Supplementary Table 1).
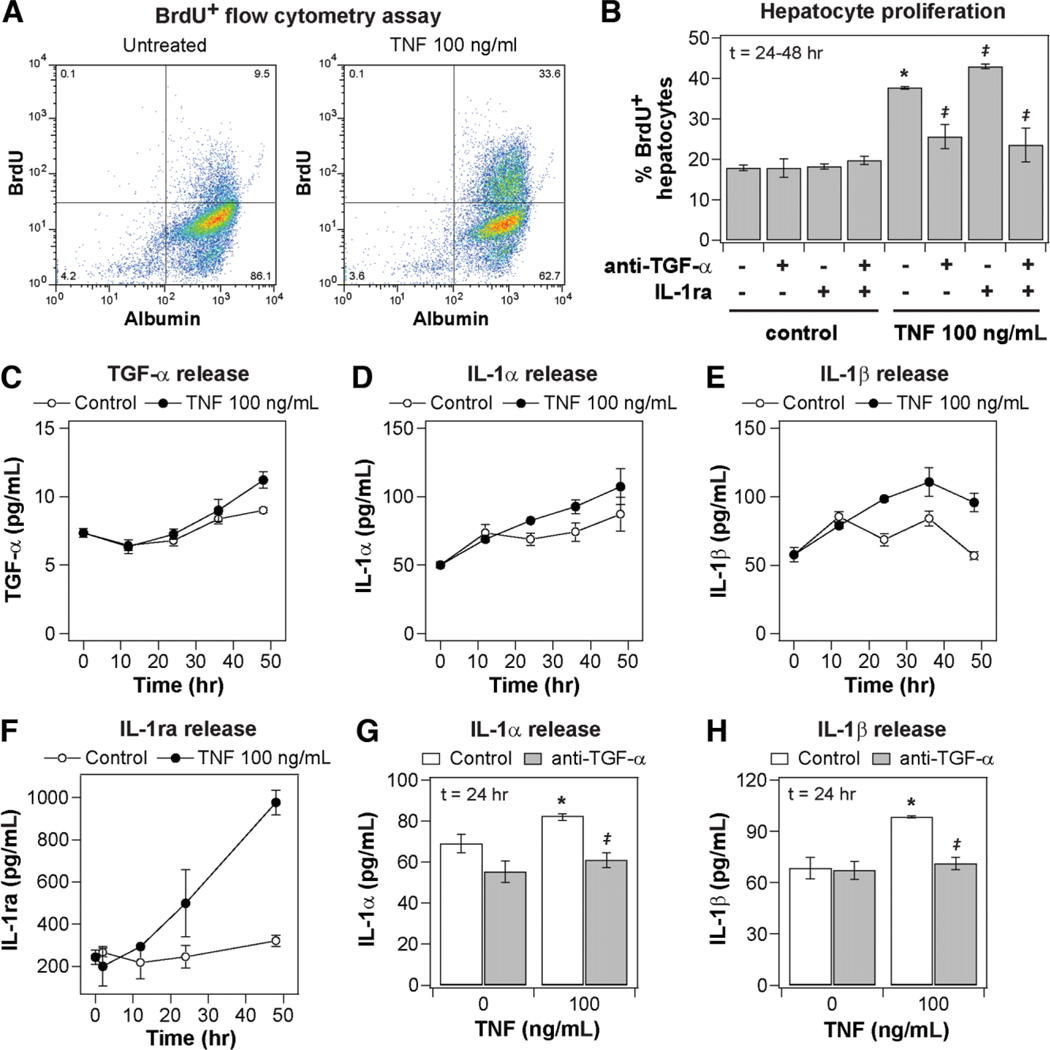
Fig. 1.
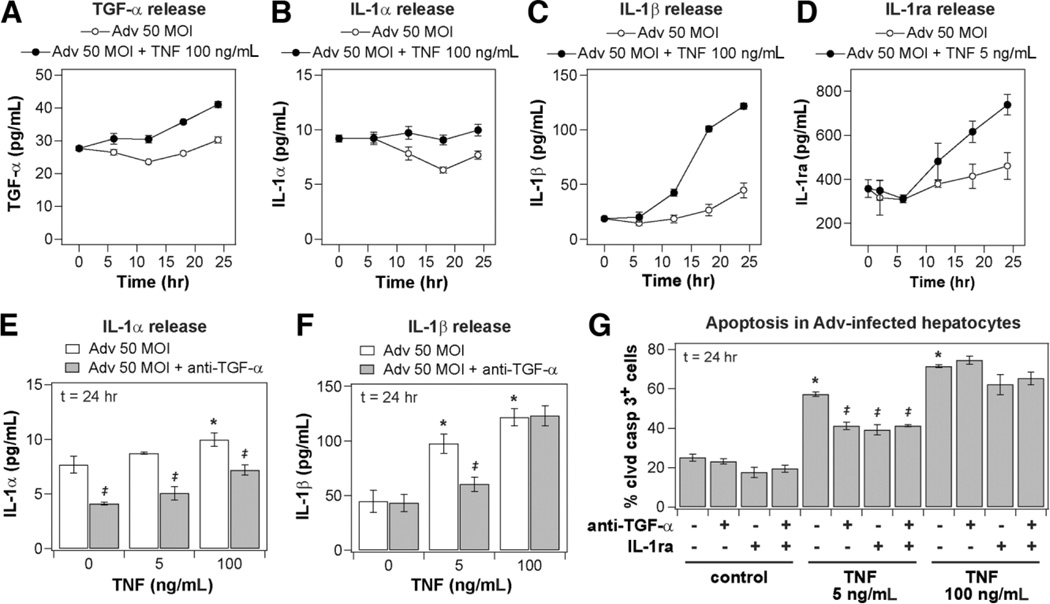
Coupled and self-antagonizing autocrine TGF-α, IL-1α/β, and IL-1ra circuits regulate TNF-induced hepatocyte proliferation. Primary rat hepatocytes were isolated, treated, and assayed as described in the Supplementary Experimental Procedures for proliferation studies. (A) Representative scatter plots of BrdU+ flow cytometry assays for cells stimulated with carrier only (left) or 100 ng/mL TNF (right). The proliferative fraction of cultured hepatocytes is reported as percentage of BrdU+ cells within the albumin+ (hepatocyte) population. (B) Regulation of TNF-induced hepatocyte proliferation by autocrine TGF-α and IL-1α/β. Autocrine TGF-α and IL-1α/β ligand activity was inhibited by pretreatment with 10 µg/mL anti-TGF-α and/or 10 µg/mL IL-1ra 1 hour before mock or 100 ng/mL TNF treatment. (C–F) Conditioned media samples were collected from 0 to 48 hours following mock or 100 ng/mL TNF treatment and were assayed for (C) autocrine TGF-α, (D) IL-1α, (E) IL-1β, and (F) IL-1ra release by quantitative enzyme-linked immunoabsorbent assay (ELISA). Differences between each pair of ligand release time courses were assessed using two-way ANOVA (TGF-α: P< 0.022; IL-1α: P< 0.032; IL-1β: P< 10−4; IL-1ra: P< 0.005). (G–H) Perturbation of TNF-induced IL-1α and IL-1β release at 24 hours after stimulus in the presence of 10 µg/mL anti-TGF-α. In (B), (G), and (H), differences between mock and TNF treatments are labeled as significant (*) if P< 0.05. Differences between pairs of uninhibited and inhibitor treatments are labeled as significant (‡) if P< 0.05. Data are presented as the mean ± SEM of three biological samples (B,F–H) and in six biological samples (C–E).
TNF modestly stimulated hepatocyte proliferation compared to basal media alone (Fig. 1B). Pretreatment with an antibody neutralizing TGF-α activity (Fig. 1B) or the EGFR kinase inhibitor CI1033 (Supplementary Fig. 2) reduced hepatocyte proliferation stimulated by TNF to basal levels, demonstrating that autocrine TGF-α is necessary for TNF-induced hepatocyte proliferation in accordance with previous reports.11,12 Pretreatment with IL-1ra, an inhibitor of IL-1α/β binding to IL-1R, slightly increased hepatocyte proliferation stimulated by TNF (Fig. 1B), showing that autocrine IL-1α/β inhibits TNF-induced hepatocyte proliferation. Pretreatment with iso-form-specific neutralizing antibodies for IL-1α and IL-1β showed that IL-1β contributes a larger antiproliferative autocrine effect (Supplementary Fig. 2). Moreover, the increased proliferation observed under IL-1α/β inhibition was not evident when TGF-α was also inhibited (Fig. 1B), indicating that the antiproliferative effects of autocrine IL-1α/β are contingent on autocrine TGF-α activity. TNF stimulated a slight increase in TGF-α release (Fig. 1C) and more substantial increases in IL-1α (Fig. 1D), IL-1β (Fig. 1E), and IL-1ra (Fig. 1F) release over 48 hours of treatment compared to untreated controls. TNF-induced autocrine IL-1α and IL-1β release, at 24 hours after stimulus, were both completely inhibited by neutralizing autocrine TGF-α activity (Fig. 1G,H), in accordance with reports in mammary and colonic epithelial cells.2 TNF-induced IL-1ra release was not dependent on autocrine TGF-α or IL-1α/β activity (data not shown). Thus, TNF-induced hepatocyte proliferation is regulated by a set of coupled and self-antagonizing autocrine circuits involving pro-proliferative TGF-α, antiproliferative IL-1α/β, and IL-1ra, with the release and antiproliferative effects of autocrine IL-1α/β contingent on TNF-induced autocrine TGF-α signaling (Fig. 3F).
Fig. 3.
Exogenous TGF-α has limited effect in synergizing with TNF-induced hepatocyte proliferation due to an antiproliferative IL-1α/β autocrine circuit. Primary rat hepatocytes were isolated, treated, and assayed as described in the Supplementary Experimental Procedures for proliferation studies. (A) Hepatocyte proliferation induced by 0, 1, 10, or 100 ng/mL exogenous TGF-α with either mock or 100 ng/mL TNF cotreatment. (B,C) IL-1α (B) and IL-1β (C) release at 24 hours after stimulus induced by 0, 1, 10, or 100 ng/mL exogenous TGF-α with either mock or 100 ng/mL TNF cotreatment. Dashed red lines indicate ligand release level in the absence of exogenous TGF-α stimulus for clarity. (D) “Cue-response landscape” plot of hepatocyte proliferation and autocrine IL-1α/β release induced by multiple combinations of TNF and TGF-α stimuli. Mean values of three biological samples are plotted for BrdU+ hepatocytes (lines and vertices; z-axis) and total autocrine IL-1α/β concentration at 24 hours after stimulus (interpolated surface color map) for multiple concentrations of exogenous TNF and either inhibited, autocrine (uninhibited), or exogenous TGF-α. See Supplementary Table 2 for details of treatment conditions and measured values plotted. (E) Hepatocyte proliferation induced by 100 ng/mL TNF, 1 ng/mL TGF-α, or TNF + TGF-α in the absence or presence of 10 µg/mL IL-1ra. (F) A molecular “logic” model of the effects of autocrine TGF-α, IL-1α/β, and IL-1ra ligands in regulating TNF-induced hepatocyte proliferation. Exogenous TNF stimulates the release of antiproliferative autocrine IL-1α and IL-1β ligands contingent of the activity of autocrine TGF-α, a pro-proliferative ligand itself, and the release of IL-1ra independent of activity of other autocrine ligands. Thus, this coupled autocrine circuit is self-antagonizing in its control of hepatocyte proliferation. (A–C,E) Differences between pairs of treatments connected by brackets are labeled as significant (*) if P< 0.05; and data are presented as the mean ± SEM of three biological samples.
Autocrine TGF-α and IL-1α/β Contribute to Multiple Signaling Pathways Related to TNF-Induced Hepatocyte Proliferation
To investigate how autocrine TGF-α and IL-1α/β contribute to TNF-induced hepatocyte proliferation, we quantified phosphoprotein levels in signaling pathways associated with TNF and hepatocyte proliferation. TNF-induced hepatocyte proliferation in vitro is dependent on autocrine TGF-α and its activation of EGFR and downstream signaling through the pro-proliferative Akt and ERK pathways.11,12 Here, TNF-induced activation of the Akt and ERK signaling, on both transient and sustained time scales, was dependent on autocrine TGF-α (Fig. 2A,B). IL-1 ligands activate multiple pathways downstream of IL-1R that are shared by TNF signaling, including JNK, IKK–NF-κB, and p38, but it is uncertain how these pathways contribute to IL-1’s antagonism of hepatocyte proliferation.14,25 TNF-induced activation of JNK and IKK– NF-κB signaling (as measured by p-IκB-α) at 2–12 hours after stimulus were partially dependent on autocrine IL-1α/β signaling (Fig. 2C,D). These signaling pathways were similarly perturbed upon inhibition of autocrine TGF-α, again demonstrating that autocrine IL-1α/β signaling is contingent on autocrine TGF-α activity. Although both JNK and IKK–NF-κB are activated by TNF immediately following PHx and are associated with pro-proliferative functions, these pathways are not absolutely necessary for hepatocyte proliferation. 1,4–6,26 Sustained signaling via autocrine stimulation can govern cellular behaviors in counterintuitive manners, as has been observed in mammary epithelial cells in which TNF-induced autocrine IL-1α signaling contributes to sustained activation of IKK–NF-κB signaling, which is associated with a proapoptotic function rather than its canonical antiapoptotic role.2,20 Similarly, the JNK and IKK–NF-κB pathways could function, through their sustained activation, to antagonize hepatocyte proliferation through mechanisms such as accumulation of reactive oxygen species, which is associated with JNK signaling1 and inhibits hepatocyte proliferation.27
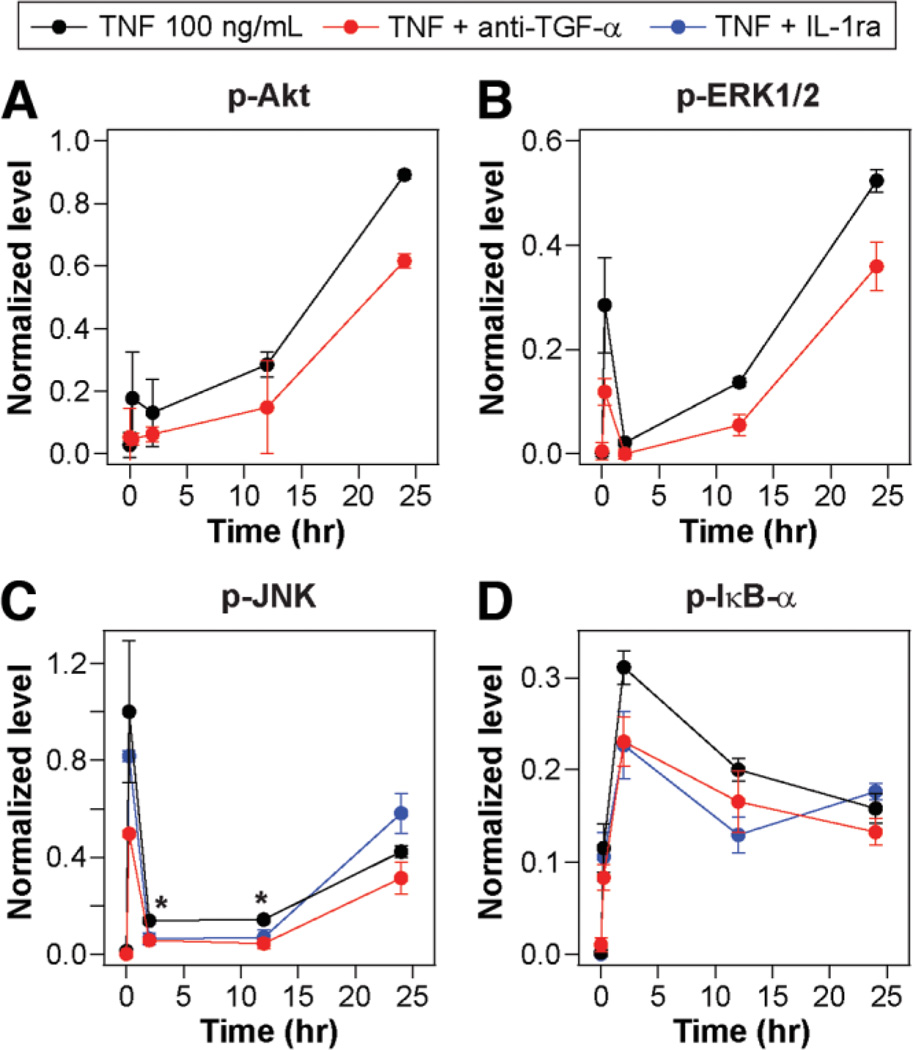
Fig. 2.
Autocrine TGF-α and IL-1α/β contribute to TNF-induced phosphoprotein signaling regulating hepatocyte proliferation. Primary rat hepatocytes were isolated, treated, and assayed as described in the Supplementary Experimental Procedures for proliferation studies. Lysates were collected from 0, 0.25, 2, 12, and 24 hours following 100 ng/mL TNF treatment with mock, 10 µg/mL anti-TGF-α, or 10 µg/mL IL-1ra pretreatments to perturb autocrine ligand activity. Lysates were analyzed using multiplexed phosphoprotein assays for (A) p-Akt, (B) p-ERK1/2, (C) p-JNK, and (D) p-lκB-α. (A,B) IL-1ra inhibition treatments were unchanged from uninhibited treatments and thus not shown. Differences between uninhibited and inhibited phosphoprotein signaling time courses were assessed using two-way ANOVA (p-Akt, anti-TGF-α: P< 0.02; p-ERK1/2, anti-TGF-α: P< 0.01; p-JNK, anti-TGF-α: P< 0.02; p-JNK, IL-1ra: P= 0.58; p-lκB-α, anti-TGF-α: P< 0.01; p-lκB-α, IL-1ra: P< 0.03). (C) Individual time points that demonstrated significant differences in both comparisons are labeled (*) if P< 0.05. Data are presented as the mean ± SEM of three biological samples.
Intracellular phosphoprotein levels can provide evidence of autocrine ligand activities with greater temporal resolution than can be inferred from their accumulation in culture media (Fig. 1C–F). The transient (15 minutes after stimulus) activation of the MAPK–ERK pathway (Fig. 2B; Supplementary Fig. 3B) depends on autocrine TGF-α, indicating that TGF-α release and activity immediately follows TNF stimulation. Similarly, the prolonged (2 hours after stimulus) activation of the JNK–c-Jun (Fig. 2C; Supplementary Fig. 3C) and IKK–NF-κB (Fig. 2D) pathways depends on autocrine IL-1α/β, indicating the early release and activity of IL-1α/β. Last, the inability of exogenously added IL-1ra to perturb late-phase JNK and IKK–NF-κB signaling (at ∼24 hours) indicates the onset of detectable autocrine IL-1ra activity. Together, these data suggest that TGF-α, IL-1α/β, and IL-1ra operate in a coupled and time-varying autocrine “cascade”; TNF stimulates the immediate release of TGF-α and its activation of Akt and ERK signaling, the slightly delayed release of IL-1α/β and its activation of JNK and IKK–NF-κB signaling, and the late-phase release of IL-1ra, which antagonizes IL-1 signaling (Fig. 8).
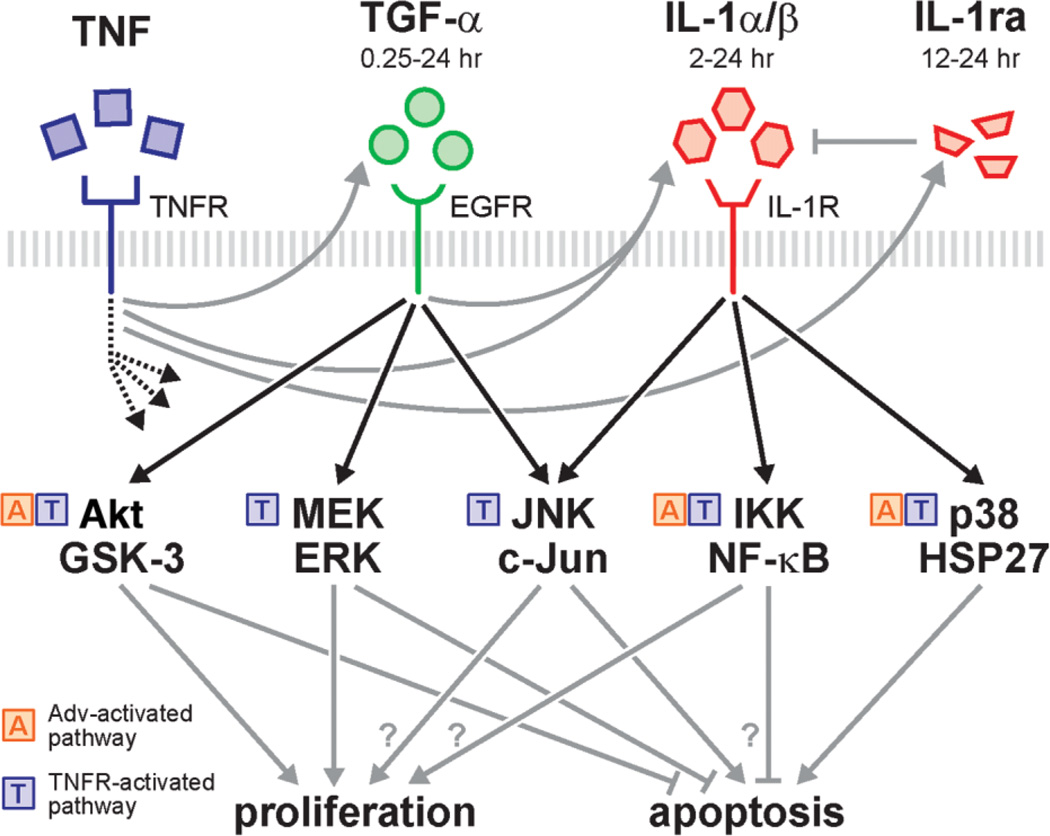
Fig. 8.
A TGF-α-IL-1α/β-IL-1ra autocrine cascade contributes to TNF-induced hepatocyte proliferation and Adv-infection sensitized apoptosis through the regulation of multiple shared signaling pathways. TNF activates the Akt, ERK, JNK, IKK-NF-κB, and p38 signaling pathways, both directly downstream of the TNF receptor1,4 and indirectly through this autocrine cascade. Replication-deficient Adv infection can activate Akt, NF-κB, and p38 pathway signaling.3,30 TNF induces TGF-α release, which activates Akt, ERK, and JNK signaling over a time scale of 0.25-24 hours. Induced IL-1α/β release is contingent on both TNF and autocrine TGF-α. Autocrine IL-1α/β activates JNK, IKK-NF-κB, and p38 signaling over a time scale of 2-24 hours. Independent of autocrine TGF-α or IL-1α/β, TNF also induces the release of IL-1ra, which antagonizes IL-1α/β ligand activity and accumulates over a time scale of 12–24 hours. These signaling pathways have diverse function in regulating hepatocyte proliferation and apoptosis.1,4,5,11
TNF-TGF-α Cooperation in Inducing Hepatocyte Proliferation Is Self-Limited by Release of Autocrine IL-1α/β
Exogenous TGF-α stimulated a dose-dependent increase in hepatocyte proliferation in the absence of TNF and cotreatment with TNF did not elicit additional hepatocyte proliferation (Fig. 3A), in discordance with previous reports demonstrating cooperation between TNF and EGFR ligands in stimulating hepatocyte proliferation in vitro under some media formulations.6,10,28 We note that exogenous TNF and TGF-α exhibited slightly cooperative stimulation of hepatocyte proliferation when insulin was removed from the culture media (Supplementary Fig. 4). We were motivated to ask whether cooperation between exogenous TNF and TGF-α in the presence of insulin could be limited by antiproliferative autocrine IL-1α/β.
To examine whether autocrine IL-1α/β could affect TNF-TGF-α cooperativity in inducing hepatocyte proliferation, hepatocyte IL-1α/β release and proliferation were assayed under multiple combinations of exogenous TNF and autocrine/exogenous TGF-α stimuli and were plotted in a multivariate “cue-response landscape” (Fig. 3D). In this perspective, the two hepatocyte responses (IL-1α/β release and proliferation) show disparate dependencies on the two stimulatory cues (TNF and TGF-α). That is, the conditions that lead to maximal IL-1α/β release (100 ng/mL TNF + 1 ng/mL TGF-α; Fig. 3B – D) are different than those associated with maximal proliferation (100 ng/mL TNF +100 ng/mL TGF-α; Fig. 3A,D). This suggested that autocrine IL-1α/β could serve as a negative regulator of hepatocyte proliferation under certain exogenous TNF-TGF-α costimuli conditions—in particular those with intermediate exogenous TGF-α concentrations. Following this, IL-1ra pretreatment elicited a slight increase in proliferation stimulated by 100 ng/mL TNF and a more substantial increase upon 100 ng/mL TNF + 1 ng/mL TGF-α costimulation, but not a significant increase in proliferation stimulated by 1 ng/mL TGF-α only (Fig. 3E). Moreover, TNF + TGF-α + IL-1ra treatment significantly increased hepatocyte proliferation compared to both TNF + IL-1ra and TGF-α + IL-1ra treatments (Fig. 3E), demonstrating TNF and TGF-α can stimulate additive induction of hepatocyte proliferation, even in the presence of insulin, but this cooperation is inhibited by their induction of antiproliferative autocrine IL-1α/β release. Thus, induced autocrine IL-1α/β release not only antagonizes hepatocyte proliferation stimulated by exogenous TNF, contingent on its induced autocrine release of TGF-α, but also potently self-limits hepatocyte proliferation induced by exogenous TNF-TGF-α co-stimulation (Fig. 3F).
Adv Infection Synergistically Sensitizes Hepatocytes to TNF-Induced Apoptosis
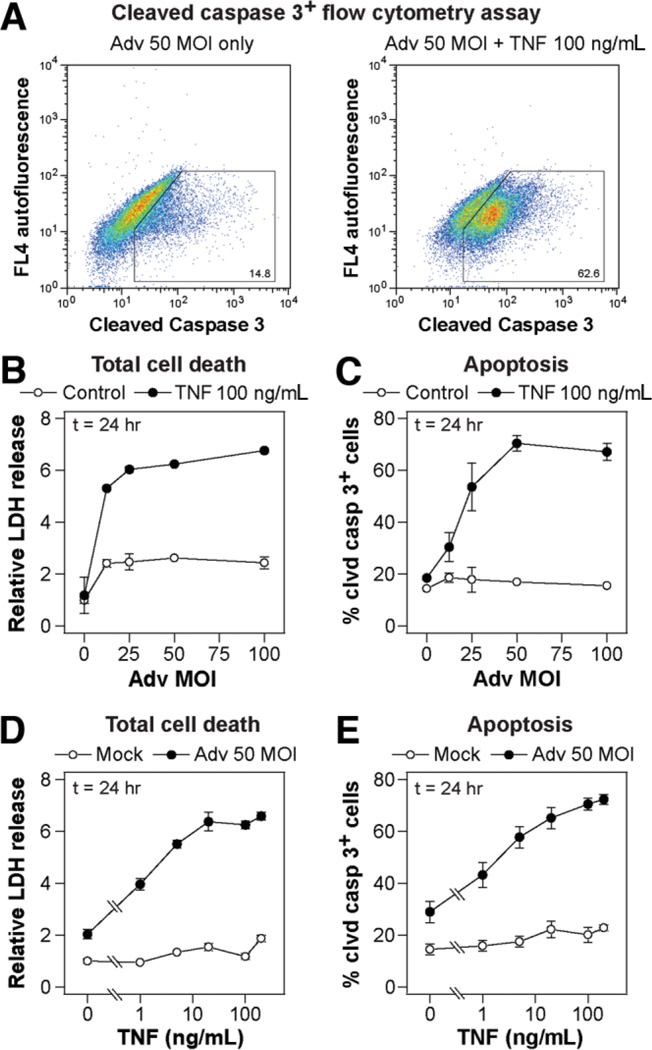
Common models of TNF-induced hepatocyte apoptosis require nonphysiological interference with antiapoptotic signaling pathways.1,4 Here, we examined TNF-induced hepatocyte apoptosis in the context of adenoviral infection because of the relevance of this condition to physiological and therapeutic applications and because we have found it to be an important modulator of TNF-induced apoptosis in other epithelial cell types.3,29 Rat hepatocytes were cultured on collagen gel for 24 hours, then infected with a replication-deficient adenovirus expressing a β-gal transgene at a multiplicity of infection (MOI) of 0 to 100. A total of 24 hours after Adv infection, hepatocytes were stimulated with TNF and other cotreatments for 24 hours. Cells were harvested and analyzed by flow cytometry for the cleaved form of the effector caspase 3 (Fig. 4A), and LDH release (an indicator of loss of plasma membrane integrity in necrotic and apoptotic cell death) was measured in culture supernatants. Over a range of infection levels, Adv potently sensitized TNF-induced apoptosis as measured by both LDH release (Fig. 4B) and cleaved caspase 3 + cells (Fig. 4C) with the sensitization effect plateauing at ∼50 MOI Adv, which was used for all subsequent Adv infections. At points in time later than 24 hours after cytokine stimulation, hepatocyte death induced by Adv infection alone increased significantly, reducing the level of observed synergy between Adv and TNF in inducing hepatocyte death (data not shown). In hepatocytes in-fected with 50 MOI Adv, TNF induced apoptosis as measured by both LDH release (Fig. 4D) and cleaved caspase 3+ cells (Fig. 4E), with half-maximal and maximal responses induced at ∼5 ng/mL and ∼100 ng/mL TNF, respectively. Thus, Adv infection potently sensitizes hepatocytes to TNF-induced apoptosis and provides a physiologically relevant model to examine the role of autocrine ligands in regulating TNF-induced apoptosis.
Fig. 4.
Replication-deficient Adv infection synergistically sensitizes hepatocytes to TNF-induced apoptosis. Primary rat hepatocytes were isolated, treated, and assayed as described in the Supplementary Experimental Procedures for apoptosis studies. (A) Representative scatter plots of a cleaved caspase 3+ flow cytometry assay for cells infected with 50 MOI Adv and stimulated with carrier only (left) or 100 ng/mL TNF (right). (B,C) Effect of Adv infection level on (B) TNF-induced total cell death as assayed by (C) relative LDH release and apoptosis as assayed by the cleaved caspase 3+ cells. Cells were infected with storage buffer only or 12.5, 25, 50, or 100 MOI Adv and then stimulated with mock or 100 ng/mL TNF for 24 hours. (B,D) LDH release values were normalized to mock treatment condition at 24 hours after stimulus. (D,E) Effect of TNF concentration on Adv infection-sensitized (D) total cell death and (E) apoptosis. Cells were infected with storage buffer only or 50 MOI Adv and then stimulated with 0, 1, 5, 20, 100, or 200 ng/mL TNF for 24 hours. (B–E) Differences between all pairs of cell death and apoptosis dose-response curves were assessed using two-way ANOVA and were all statistically significant (P < 10−4); and data are presented as the mean ± SEM of three biological samples.
TNF-Induced Apoptosis in Adv-Infected Hepatocytes Is Regulated by a Coupled, Proapoptotic TGF-α–IL-1α/β–IL-1ra Autocrine Cascade
Because we found that a coupled TGF-α–IL-1α/β–IL-1ra autocrine cascade regulates hepatocyte proliferation induced by TNF, and a similar autocrine mechanism operates in determining apoptotic responses of colonic epithelial cells,2,20 we investigated whether this autocrine cascade regulates TNF-induced apoptosis in Adv-infected hepatocytes. In hepatocytes infected with 50 MOI Adv, TNF treatment significantly up-regulated the release of autocrine TGF-α, IL-1α, IL-1β, and IL-1raas measured over 24 hours (Fig. 5A – D). Adv infection alone induced an increased release of TGF-α but not IL-1α, IL-1β, or IL-1ra compared to uninfected control cells (Supplementary Fig. 6 and data not shown). Inhibition of autocrine TGF-α reduced the release of IL-1α induced by both Adv infection alone and Adv infection followed by 5 or 100 ng/mL TNF treatment (Fig. 5E) but only reduced the release of IL-1β upon Adv + 5 ng/mL TNF treatment (Fig. 5F) and did not perturb the release of autocrine IL-1ra (data not shown). Pretreatment with anti-TGF-α, IL-1ra, or both inhibitors significantly reduced TNF-induced apoptosis in Adv-infected hepatocytes treated with 5 ng/mL TNF, but did not perturb apoptosis in a statistically significant manner under 0 or 100 ng/mL TNF treatments (Fig. 5G). In Adv-infected hepatocytes, pretreatment with CI1033 and anti-IL-1β also reduced apoptosis induced by 5 ng/mL TNF (Supplementary Fig. 7).
Fig. 5.
Autocrine TGF-α, IL-1α/β, and IL-1ra regulate TNF-induced apoptosis in Adv-infected hepatocytes in a coupled autocrine circuit. Primary rat hepatocytes were isolated, treated, and assayed as described in the Supplementary Experimental Procedures for apoptosis studies. (A–D) Conditioned media samples were collected from 0-24 hours following mock, (D) 5 ng/mL or (A–C) 100 ng/mL TNF treatment in hepatocytes infected with 50 MOI Adv and were assayed for autocrine (A) TGF-α, (B) IL-1α, (C) IL-1β, and (D) IL-1ra release by quantitative enzyme-linked immunoabsorbent assay (ELISA). Differences between each pair of ligand release time courses were assessed using two-way ANOVA and were all statistically significant (P< 10∼3). Perturbation of (E) IL-1α and (F) IL-1β release induced by 0, 5, or 100 ng/mL TNF treatment at 24 hours after stimulus in 50 MOI Adv-infected hepatocytes in the presence of 10 µg/mL anti-TGF-α. (G) Regulation of TNF-induced apoptosis in Adv-infected hepatocytes by autocrine TGF-α and IL-1α/β. Autocrine TGF-α and IL-1α/β ligand activity was inhibited by pretreatment with 10 µg/mL anti-TGF-α and/or 10 µg/mL IL-1ra 1 hour before 0, 5, or 100 ng/mL TNF treatment in 50 MOI Adv-infected hepatocytes. (E–G) Differences between mock and TNF treatments are labeled as significant (*) if P< 0.05 and differences between pairs of uninhibited and inhibitor treatments are labeled as significant (‡) if P< 0.05. Data are presented as the mean ± SEM of (A–C) six biological samples and (D–G) three biological samples.
Thus, in Adv-infected hepatocytes, TNF-induced apoptosis is regulated by the coupled activity of autocrine TGF-α and IL-1α/β. At subsaturating TNF concentrations (5 ng/mL), the induced release of IL-1α and IL-1β is contingent on autocrine TGF-α, the induced release of IL-1ra is independent of autocrine TGF-α or IL-1α/β, and the net effect of the coupled TGF-α–IL-1α/β autocrine circuit in Adv-infected hepatocytes is proapoptotic. At saturating concentrations (100 ng/mL), TNF induces TGF-α and IL-1α/β release in Adv-infected hepatocytes, but these mediators only provide a negligible contribution to apoptosis and the requirement of autocrine TGF-α activity for IL-1β release is not apparent. Taken together, the observations across all treatment conditions imply that autocrine TGF-α can act paradoxically as a proapoptotic signal in Adv-infected hepatocytes by coupling TNF treatment to release of proapoptotic autocrine IL-1α/β (Fig. 7F).
Fig. 7.
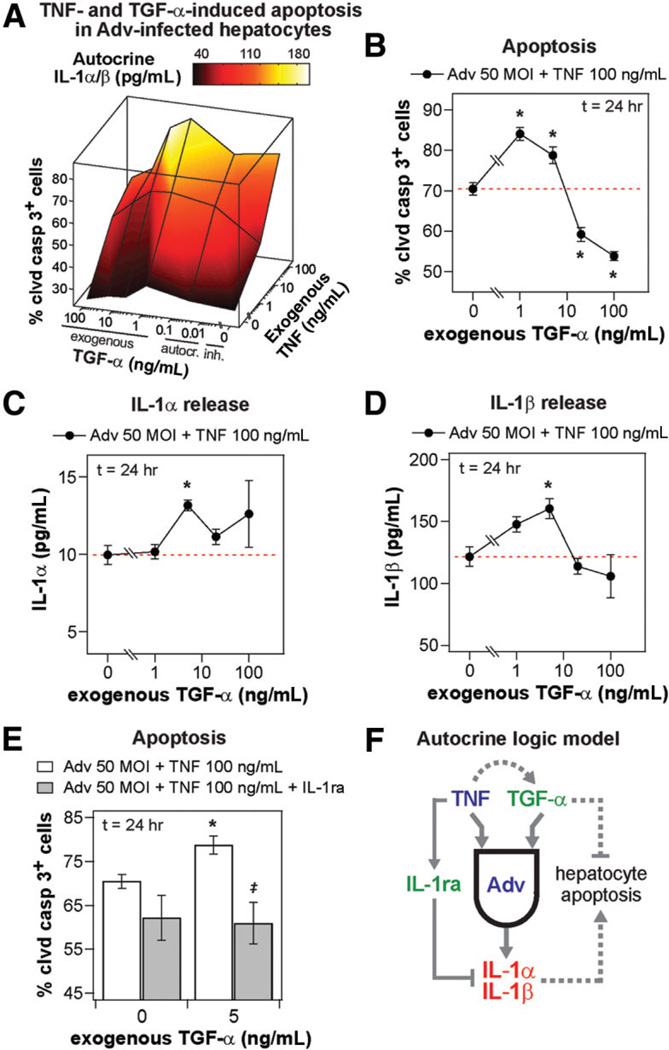
Exogenous TGF-α biphasically regulates apoptosis/survival in Adv-infected, TNF-treated hepatocytes through an autocrine IL-1α/β circuit. Primary rat hepatocytes were isolated, treated, and assayed as described in the Supplementary Experimental Procedures for apoptosis studies. (A) “Cue-response landscape” plot of apoptosis and autocrine IL-1α/β release induced by multiple combinations of TNF and TGF-α stimuli in 50 MOI Adv-infected hepatocytes. Mean values of three biological samples are plotted for cleaved caspase 3+ cells (lines and vertices; z-axis) and total autocrine IL-1β/β concentration (interpolated surface color map) at 24 hours after stimulus for multiple concentrations of exogenous TNF and either inhibited, autocrine (uninhibited), or exogenous TGF-α. See Supplementary Table 3 for details of treatment conditions and measured values plotted. (B) Apoptosis induced by 0, 1, 5, 20, or 100 ng/mL exogenous TGF-α with 100 ng/mL TNF cotreatment in 50 MOI Adv-infected hepatocytes. Dashed red lines indicate apoptosis level in the absence of exogenous TGF-α stimulus for clarity. (C) IL-1α and (D) IL-1β release at 24 hours after stimulus induced by 0, 1, 5, 20, or 100 ng/mL exogenous TGF-α with 100 ng/mL TNF cotreatment in 50 MOI Adv-infected hepatocytes. Dashed red lines indicate ligand release level in the absence of exogenous TGF-α stimulus for clarity. (E) Apoptosis induced by 100 ng/mL TNF only treatment or 100 ng/mL TNF + 5 ng/mL TGF-α cotreatment in the absence or presence of 10 µg/mL IL-1ra in 50 MOI Adv-infected hepatocytes. (F) A molecular “logic” model of the effects of autocrine TGF-α, IL-1α/β, and IL-1ra ligands in regulating TNF-induced apoptosis in Adv-infected hepatocytes. Exogenous TNF stimulates the release of proapoptotic autocrine IL-1α and IL-1β contingent of the activity of autocrine TGF-α, which is an antiapoptotic ligand at saturating exogenous concentrations but not at autocrine concentrations, and the release of IL-1ra independent of activity of other autocrine ligands. (B–E) Differences between control treatments and treatments with exogenous TGF-α are labeled as significant (*) if P< 0.05. (E) Differences between pairs of control treatments and IL-1ra treatments are labeled as significant (‡) if P< 0.05. (B–E) Data are presented as the mean ± SEM of three biological samples.
Autocrine TGF-α andIL-1α/β Contribute to Multiple Signaling Pathways Related to TNF-Induced Apoptosis in Adv-Infected Hepatocytes
To investigate how autocrine TGF-α and IL-1α/β signaling contributes to TNF-induced hepatocyte apoptosis, we quantified phosphoprotein signaling in hepatocytes infected with 50 MOI Adv and stimulated with TNF at 5 ng/mL, a sub-saturating concentration that showed dependence on autocrine signaling in its induction of apoptosis. In Adv-infected hepatocytes, TNF activated antiapoptotic ERK signaling, dependent on autocrine TGF-α, but did not significantly activate Akt (Fig. 6A,B). Adv infection itself strongly activates Akt signaling,3,30 which can be observed by comparing basal Akt activation in uninfected (Fig. 2A) and Adv-infected (Fig. 6A) cells. TNF-induced activation of proapoptotic JNK and p38 signaling (at ∼6 to 12 hours after stimulus) were both dependent on autocrine IL-1α/β signaling, as observed by direct inhibition with IL-1ra or through the inhibition of autocrine TGF-α (Fig. 6C,D). In contrast to uninfected hepatocytes (Fig. 2D), TNF-induced IKK–NF-κB activation in Adv-infected hepatocytes was only marginally dependent on autocrine signaling (Supplementary Fig. 8E). As observed in uninfected hepatocytes, TGF-α, IL-1α/β, and IL-1ra operate in an autocrine cascade that contributes to multiple signaling pathways related to TNF-induced apoptosis signaling in Adv-infected hepatocytes (Fig. 8).
Fig. 6.
Autocrine TGF-α and IL-1α/β contribute to TNF-induced phosphoprotein signaling regulating hepatocyte apoptosis. Primary rat hepatocytes were isolated, treated, and assayed as described in the Supplementary Experimental Procedures for apoptosis studies. Lysates were collected 0, 0.25, 2, 6, 12, and 24 hours following 5 ng/mL TNF treatment of 50 MOI Adv-infected hepatocytes with mock, 10 µg/mL anti-TGF-α, or 10 µg/mL IL-1ra pretreatments to perturb autocrine ligand activity. Lysates were analyzed using multiplexed phosphoprotein assays for (A) p-Akt, (B) p-ERK1/2, (C) p-JNK, and (D) p-p38. (A,B) IL-1ra inhibition treatments were unchanged from uninhibited treatments and thus not shown. Differences between uninhibited and inhibited phosphoprotein signaling time courses were assessed using two-way ANOVA (p-Akt, anti-TGF-α: P= 0.22; p-ERK1/2, anti-TGF-α: P< 10−4; p-JNK, anti-TGF-α: P< 10−4; p-JNK, IL-1ra: p < 0.01; p-p38, anti-TGF-α: P< 0.0003; p-p38, IL-1ra: P= 0.27). (D) Individual time points that did demonstrate significant differences in both comparisons are labeled (*) if P< 0.05. Data are presented as the mean ± SEM of three biological samples.
TGF-α Biphasically Regulates Apoptosis/Survival in Adv-Infected, TNF-Treated Hepatocytes Mediated by Autocrine IL-α/β
To further investigate the paradoxical roles of TGF-α in regulating TNF-induced apoptosis in Adv-infected hepatocytes, we treated Adv-infected hepatocytes with multiple combinations of exogenous TNF and autocrine/exogenous TGF-α stimuli and assayed IL-1α/β release and apoptosis. In examining the “cue-response landscape” of autocrine IL-1α/β release and apoptosis induced by these TNF-TGF-α costimuli in Adv-infected hepatocytes, we observed that both IL-1α/β release and apoptosis responses were maximally stimulated at intermediate concentrations of exogenous TGF-α for both 5 and 100 ng/mL TNF treatments (Fig. 7A). This indicated that exogenous TGF-α could induce increased apoptosis in Adv-infected, TNF-treated hepatocytes through the correlated release of additional autocrine IL-1α/β. In Adv-infected hepatocytes treated with 100 ng/mL TNF, exogenous TGF-α cotreatment increased apoptosis at 1 and 5 ng/mL TGF-α and a decreased apoptosis at 20 and 100 ng/mL TGF-α (Fig. 7B). In comparison, release of autocrine IL-1α (Fig. 7C) and IL-1β (Fig. 7D) was up-regulated for TNF cotreated with TGF-α at 5 ng/mL but not other concentrations. Therefore, at high concentrations, exogenous TGF-α rescued TNF-induced apoptosis, in agreement with the recognized antiapoptotic role of EGFR ligands,8,17 but, at intermediate concentrations, exogenous TGF-α contributed to TNF-induced apoptosis, mirroring its role as a proapoptotic autocrine mediator in Adv-infected, TNF-treated hepatocytes. A similar biphasic TGF-α synergism and antagonism in regulating apoptosis has been observed in interferon-γ-sensitized, TNF-treated human colonic epithelial cells.2 Furthermore, the up-regulation of apoptosis in Adv-infected, TNF-treated hepatocytes by 5 ng/mL exogenous TGF-α, which coincided with increased IL-1α/β release, was completely attenuated in the presence of IL-1α/β inhibition, confirming that autocrine IL-1α/β act as mediators of the increased apoptosis stimulated by exogenous TGF-α (Fig. 7E).
Given that exogenous TGF-α at saturating concentrations acted as an antiapoptotic stimulus, we asked if autocrine or subsaturating exogenous TGF-α could serve as an antiapoptotic stimulus when decoupled from its induction of proapoptotic IL-1α/β release. In Adv-infected hepatocytes pretreated with IL-1ra, then stimulated with 100 ng/mL TNF, TGF-α at autocrine or subsaturating exogenous (5 ng/mL) concentrations induced a slight, but not statistically significant, reduction in apoptosis compared to treatment with anti-TGF-α (Supplementary Fig. 9). The negligible antiapoptotic effect of autocrine and subsaturating exogenous TGF-α are likely due to its limited ability to further supplement antiapoptotic Akt signaling in the presence of Adv infection and insulin (Fig. 6A);3 and, instead, saturating concentrations of TGF-α are required to exert a substantial antiapoptotic effect. Thus, an integrated balance of signaling by exogenous TNF, autocrine and exogenous TGF-α, and autocrine IL-1α/β determines hepatocyte apoptosis responses in the presence of Adv infection. At autocrine and subsaturating exogenous concentrations, TGF-α exerts a negligible antiapoptotic stimulus that is overwhelmed by proapoptotic signaling from exogenous TNF and induced autocrine IL-1α/β, which are antagonized by released IL-1ra. But, at saturating exogenous concentrations, TGF-α effectively antagonizes proapoptotic signaling by these factors (Fig. 7F).
Discussion
We have demonstrated that hepatocyte responses to TNF are regulated by an inducible, coupled, and self-antagonizing TGF-α–IL-1α/β–IL-1ra autocrine cascade. This autocrine cascade promotes both TNF-induced apoptosis in hepatocytes infected with Adv—a therapeutically and physiologically relevant sensitization for hepatocyte apoptosis induced by TNF developed herein—and TNF-induced proliferation in the absence of viral infection. Although not investigated here, the expression and/or posttranslational processing and shedding of TGF-α, IL-1α/β, and IL-1raligands are regulated by a number of signaling pathways activated by TNF and Adv infection, including JNK, IKK–NF-κB, and p38.11,19,31,32 TNF-induced autocrine TGF-α and IL-1α/β contribute to multiple intracellular signaling pathways that govern both hepatocyte proliferation and apoptosis (Fig. 8). Autocrine TGF-α regulates pro-proliferative/antiapoptotic signaling through the ERK and, in the absence of Adv infection, Akt pathways.11,12 Autocrine IL-1α/β regulates proapoptotic signaling through JNK and p38 pathways. From our results, it is unclear how autocrine IL-1α/β signaling antagonizes hepatocyte proliferation, but one possibility is through the sustained activation of JNK signaling and its association with anti-proliferative reactive oxygen species accumulation.27 When added exogenously, IL-1 antagonizes hepatocyte proliferation and induces production of nitric oxide.14 Nitric oxide has been shown to impair DNA synthesis through its activation of ribonucleotide reductase,14 but could also inhibit DNA synthesis, and effector caspase activation, through its S-nitrosylation and deactivation of initiator caspases,33 whose cleavage and activation is necessary for both DNA synthesis34 and apoptosis. Thus, autocrine-dependent signaling modulates diverse signaling pathways and further investigation is necessary to identify how the pathways assayed herein and other signaling mechanisms interact to govern hepatocyte responses to TNF.
Our results also underscore the challenges in unraveling the complexities of context-dependent cues such as TNF, and we note several factors that should be considered in interpretation of our results. In this study, we inferred the activity of autocrine factors by assaying their accumulation in culture medium and their control of multiple phosphoprotein signaling pathways and resultant cellular responses. The rate of ligand accumulation in culture medium does not, however, completely reflect activity of autocrine factors, especially when receptor-mediated ligand consumption is significant compared to production.35 The outcome of autocrine effects can be influenced by cell density, which differed in the proliferation and apoptosis studies here, through the modulation of local ligand concentrations achieved by the net effects of ligand production and uptake.36 Cell density could similarly influence hepatocyte autocrine signaling in physiological processes such as liver regeneration, in which hepatocyte cell density varies in different microenvi-ronments and time points following PHx.5 Further, ligand-dependent receptor degradation, prolonged culture duration, and viral infection can all modulate receptor expression levels in hepatocytes,15,37 which in turn could lead to amplification and/or attenuation of the exogenous and autocrine ligand activities observed here. Finally, autocrine factors not examined in this study might also be involved in hepatocyte responses to TNF. These could include other ligands that are processed by ADAM17/TACE (a protease that regulates TGF-α release) in hepatocytes such as the EGFR li-gands heparin-binding EGF-like growth factor and amphiregulin and even TNF itself.8,38
Results herein, and those previously reported, collectively illustrate the integral role of autocrine factors in hepatocyte proliferation, apoptosis and survival,39 and transformation17 responses to exogenous cytokine stimuli and implicate diverse autocrine signaling connections between cytotoxic, inflammatory, and mitogenic ligands in hepatocytes. Integration of opposing positive and negative feedback mechanisms, such as those observed here, has previously been proposed to confer robustness in the control of cell phenotypic responses.2,40 The disruption of self-limiting control mechanisms present in the TNF-induced TGF-α–IL-1α/β–IL-1ra autocrine cascade could provide the means by which hepatocyte pathophysiological responses to inflammatory cytokine stimuli arise and lead to oncogenic transformation and hepatocellular carcinoma.25,41 Our results also suggest ways that manipulation of autocrine loops may influence therapeutic interventions in liver disease. For example, targeted interference with IL-1 signaling has been shown to reduce hepatotoxicity and improve efficacy of Adv gene therapy in vivo,16 positive effects that may arise in part by disruption of autocrine IL-1 signaling. Our findings that TGF-α is proapoptotic at, and slightly above, concentrations associated with autocrine secretion imply that adenoviral gene therapies might be especially hepatotoxic under conditions in which both TNF and TGF-α (or other EGFR ligands) are mildly up-regulated, as is observed following PHx.5 Clearly, the delicate balance between opposing signals requires careful examination using quantitative experimental models to achieve the desired outcomes. Moreover, further development of animal models will be critical to parse the complex autocrine and paracrine signaling mechanisms regulating TNF signaling in hepatocytes and other liver cell types in vivo and to validate any therapeutic interventions directed toward the autocrine control mechanisms identified in this study.
Supplementary Material
Acknowledgment
We thank Kevin Janes and Kath-ryn Miller-Jensen for helpful discussions, Laura Vineyard, Romie Littrell, and Ta-Chun Hang for technical assistance, and Glenn Paradis of the MIT Center for Cancer Research for flow cytometry advice.
Supported in part by National Institutes of Health (NIH) grant P50-GM68762 to D.A.L.; DoD Institute for Collaborative Biotechnologies grant to D.A.L.; Massachusetts Institute of Technology (MIT) Biotechnology Process Engineering Center grant to L.G G; MIT Center for Environmental Health Sciences grant NIH U19ES011399; NIH grant CA76541 to D.B.S.; and a Whitaker Foundation Graduate Fellowship to B.D.C.
Abbreviations
- Adv
adenoviral vector
- Akt
also known as protein kinase B (PKB)
- ANOVA
analysis of variance
- β-gal
β-galactosidase
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- GSK3α/β
glycogen synthase kinase-3α/β
- HGF
hepatocyte growth factor
- HGM
hepatocyte growth medium
- HSP27
heat shock protein 27
- IκB
inhibitor of nuclear factor-κB
- IKK
IκB kinase
- IL
interleukin
- IL-1ra
IL-1 receptor antagonist
- IL-1R
IL-1 receptor
- JNK
c-Jun N-terminal kinase
- LDH
lactate dehydrogenase
- MEK
MAPK–ERKkinase
- MOI
multiplicity of infection
- NF-κB
nuclear factor-κB
- PHx
partial hepatectomy
- SEM
standard error of the mean
- TGF-α
transforming growth factor-α
- TNF
tumor necrosis factor-α
- TNFR
tumor necrosis factor receptor
Footnotes
Animal Experimentation Statement: All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the NIH (NIH publication 86-23).
Potential conflict of interest: Nothing to report.
Supplementary material for this article can be found on the Hepatology Web site (http://interscience.wiley.com/jpages/0270-9139/suppmat/index.html).
References
- 1.Schwabe RF, Brenner DA. Mechanisms of liver injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 2.Janes KA, Gaudet S, Albeck JG, Nielsen UB. Lauffenburger DA, Sorger PK. The response of human epithelial cells to TNF involves an inducible autocrine cascade. Cell. 2006;124:1225–1239. doi: 10.1016/j.cell.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Miller-Jensen K, Janes KA, Wong YL, Griffith LG, Lauffenburger DA. Adenoviral vector saturates Akt pro-survival signaling and blocks insulin-mediated rescue of tumor necrosis-factor-induced apoptosis. J Cell Sci. 2006;119:3788–3798. doi: 10.1242/jcs.03102. [DOI] [PubMed] [Google Scholar]
- 4.Plumpe J, Streetz K, Manns MP, Trautwein C. Tumour necrosis factor alpha—mediator of apoptosis and cell proliferation of hepatocytes. Ital J Gastroenterol Hepatol. 1999;31:235–243. [PubMed] [Google Scholar]
- 5.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 6.Diehl AM, Yin M, Fleckenstein J, Yang SQ, Lin HZ, Brenner DA, et al. Tumor necrosis factor-alpha induces c-jun during the regenerative response to liver injury. Am J Physiol. 1994;267:G552–G561. doi: 10.1152/ajpgi.1994.267.4.G552. [DOI] [PubMed] [Google Scholar]
- 7.Webber EM, Bruix J, Pierce RH, Fausto N. Tumor necrosis factor primes hepa-tocytes for DNA replication in the rat. Hepatology. 1998;28:1226–1234. doi: 10.1002/hep.510280509. [DOI] [PubMed] [Google Scholar]
- 8.Berasain C, Garcia-Trevijano ER, Castillo J, Erroba E, Santamaria M, Lee DC, et al. Novel role for amphiregulin in protection from liver injury. J Biol Chem. 2005;280:19012–19020. doi: 10.1074/jbc.M413344200. [DOI] [PubMed] [Google Scholar]
- 9.Mead JE, Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of anautocrine mechanism. Proc Natl Acad Sci U S A. 1989;86:1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iocca HA, Isom HC. Tumor necrosis factor-alpha acts as a complete mi-togen for primary rat hepatocytes. Am J Pathol. 2003;163:465–476. doi: 10.1016/s0002-9440(10)63676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Argast GM, Campbell JS, Brooling JT, Fausto N. Epidermal growth factor receptor transactivation mediates tumor necrosis factor-induced hepato-cyte replication. J Biol Chem. 2004;279:34530–34536. doi: 10.1074/jbc.M405703200. [DOI] [PubMed] [Google Scholar]
- 12.Gallucci RM, Simeonova PP, Toriumi W, Luster MI. TNF-alpha regulates transforming growth factor-alpha expression in regenerating murine liver and isolated hepatocytes. J Immunol. 2000;164:872–878. doi: 10.4049/jimmunol.164.2.872. [DOI] [PubMed] [Google Scholar]
- 13.Boulton R, Woodman A, Calnan D, Selden C, Tam F, Hodgson H. Nonparenchymal cells from regenerating rat liver generate interleukin-1alpha and −1beta: a mechanism of negative regulation of hepatocyte proliferation. Hepatology. 1997;26:49–58. doi: 10.1053/jhep.1997.v26.pm0009214451. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Wang M, Carr BI. The inhibitory effect of interleukin 1beta on rat hepatocyte DNA synthesis is mediated by nitric oxide. Hepatology. 1998;28:430–435. doi: 10.1002/hep.510280221. [DOI] [PubMed] [Google Scholar]
- 15.Wang WH, Gregori G, Hullinger RL, Andrisani OM. Sustained activation of p38 mitogen-activated protein kinase and c-Jun N-terminal kinase pathways by hepatitis B virus X protein mediates apoptosis via induction of Fas/FasL and tumor necrosis factor (TNF) receptor 1/TNF-alpha expression. Mol Cell Biol. 2004;24:10352–10365. doi: 10.1128/MCB.24.23.10352-10365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shayakhmetov DM, Li ZY, Ni S, Lieber A. Interference with the IL-1-signaling pathway improves the toxicity profile of systemically applied adenovirus vectors. J Immunol. 2005;174:7310–7319. doi: 10.4049/jimmunol.174.11.7310. [DOI] [PubMed] [Google Scholar]
- 17.Del Castillo G, Murillo MM, Alvarez-Barrientos A, Bertran E, Fernandez M, Sanchez A, et al. Autocrine production of TGF-beta confers resistance to apoptosis after an epithelial-mesenchymal transition process in hepato-cytes: Role of EGF receptor ligands. Exp Cell Res. 2006;312:2860–2871. doi: 10.1016/j.yexcr.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Fujioka N, Mukaida N, Harada A, Akiyama M, Kasahara T, Kuno K, et al. Preparation of specific antibodies against murine IL-1ra and the establishment of IL-1ra as an endogenous regulator of bacteria-induced fulminant hepatitis in mice. J Leukoc Biol. 1995;58:90–98. doi: 10.1002/jlb.58.1.90. [DOI] [PubMed] [Google Scholar]
- 19.Russell WE, Kaufmann WK, Sitaric S, Luetteke NC, Lee DC. Liver regeneration and hepatocarcinogenesis in transforming growth factor-alpha-targeted mice. Mol Carcinog. 1996;15:183–189. doi: 10.1002/(SICI)1098-2744(199603)15:3<183::AID-MC4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 20.Janes KA, Albeck JG, Gaudet S, Sorger PK, Lauffenburger DA, Yaffe MB. A systems model of signaling identifies a molecular basis set for cytokine-induced apoptosis. Science. 2005;310:1646–1653. doi: 10.1126/science.1116598. [DOI] [PubMed] [Google Scholar]
- 21.Gabay C, Porter B, Guenette D, Billir B, Arend WP. Interleukin-4 (IL-4) and IL-13 enhance the effect of IL-1beta on production of IL-1 receptor antagonist by human primary hepatocytes and hepatoma HepG2 cells: differential effect on C-reactive protein production. Blood. 1999;93:1299–1307. [PubMed] [Google Scholar]
- 22.Tsukui T, Kikuchi K, Mabuchi A, Sudo T, Sakamoto T, Asano G, et al. Production of interleukin-1 by primary cultured parenchymal liver cells (hepatocytes) Exp Cell Res. 1994;210:172–176. doi: 10.1006/excr.1994.1026. [DOI] [PubMed] [Google Scholar]
- 23.Hwa AJ, Fry RC, Sivaraman A, So PT, Samson LD, Stolz DB, Griffith LG. Rat liver sinusoidal endothelial cells survive without exogenous VEGF in 3D perfused co-cultures with hepatocytes. FASEB J. 2007;21:2564–2579. doi: 10.1096/fj.06-7473com. [DOI] [PubMed] [Google Scholar]
- 24.Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, et al. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 26.Chaisson ML, Brooling JT, Ladiges W, Tsai S, Fausto N. Hepatocyte-specific inhibition of NF-kappaB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J Clin Invest. 2002;110:193–202. doi: 10.1172/JCI15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beyer TA, Xu W, Teupser D, auf dem Keller U, Bugnon P, Hildt E, et al. Impaired liver regeneration in Nrf2 knockout mice: role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008;27:212–223. doi: 10.1038/sj.emboj.7601950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serandour AL, Loyer P, Garnier D, Courselaud B, Theret N, Glaise D, et al. TNFalpha-mediated extracellular matrix remodeling is required for multiple division cycles in rat hepatocytes. Hepatology. 2005;41:478–486. doi: 10.1002/hep.20602. [DOI] [PubMed] [Google Scholar]
- 29.Miller-Jensen K, Janes KA, Brugge JS, Lauffenburger DA. Common effector processing mediates cell-specific responses to stimuli. Nature. 2007;448:604–608. doi: 10.1038/nature06001. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F, Cheng J, Hackett NR, Lam G, Shido K, Pergolizzi R, et al. Adenovirus E4 gene promotes selective endothelial cell survival and angio-genesis via activation of the vascular endothelial-cadherin/Akt signaling pathway. J Biol Chem. 2004;279:11760–11766. doi: 10.1074/jbc.M312221200. [DOI] [PubMed] [Google Scholar]
- 31.Burns K, Martinon F, Tschopp J. New insights into the mechanism of IL-1beta maturation. Curr Opin Immunol. 2003;15:26–30. doi: 10.1016/s0952-7915(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 33.Kim JE, Tannenbaum SR. S-Nitrosation regulates the activation of endogenous procaspase-9 in HT-29 human colon carcinoma cells. J Biol Chem. 2004;279:9758–9764. doi: 10.1074/jbc.M312722200. [DOI] [PubMed] [Google Scholar]
- 34.Gilot D, Serandour AL, Ilyin GP, Lagadic-Gossmann D, Loyer P, Corlu A, et al. A role for caspase-8 and c-FLIPL in proliferation and cell-cycle progression of primary hepatocytes. Carcinogenesis. 2005;26:2086–2094. doi: 10.1093/carcin/bgi187. [DOI] [PubMed] [Google Scholar]
- 35.Monine MI, Berezhkovskii AM, Joslin EJ, Wiley HS, Lauffenburger DA, Shvartsman SY. Ligand accumulation in autocrine cell cultures. Biophys J. 2005;88:2384–2390. doi: 10.1529/biophysj.104.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tschumperlin DJ, Dai G, Maly IV, Kikuchi T, Laiho LH, McVittie AK, et al. Mechanotransduction through growth-factor shedding into the extracellular space. Nature. 2004;429:83–86. doi: 10.1038/nature02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheving LA, Zhang L, Stevenson MC, Kwak ES, Russell WE. The emergence of ErbB2 expression in cultured rat hepatocytes correlates with enhanced and diversified EGF-mediated signaling. Am J Physiol Gastrointest Liver Physiol. 2006;291:G16–G25. doi: 10.1152/ajpgi.00328.2005. [DOI] [PubMed] [Google Scholar]
- 38.Kiso S, Kawata S, Tamura S, Miyagawa J, Ito N, Tsushima H, et al. Expression of heparin-binding epidermal growth factor-like growth factor in the hepatocytes of fibrotic rat liver during hepatocarcinogenesis. J Gas-troenterol Hepatol. 1999;14:1203–1209. doi: 10.1046/j.1440-1746.1999.02007.x. [DOI] [PubMed] [Google Scholar]
- 39.Castillo J, Erroba E, Perugorria MJ, Santamaria M, Lee DC, Prieto J, et al. Amphiregulin contributes to the transformed phenotype of human hepatocellular carcinoma cells. Cancer Res. 2006;66:6129–6138. doi: 10.1158/0008-5472.CAN-06-0404. [DOI] [PubMed] [Google Scholar]
- 40.Stelling J, Sauer U, Szallasi Z, Doyle FJ3rd, Doyle J. Robustness of cellular functions. Cell. 2004;118:675–685. doi: 10.1016/j.cell.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Webber EM, Wu JC, Wang L, Merlino G, Fausto N. Overexpression of transforming growth factor-alpha causes liver enlargement and increased hepatocyte proliferation in transgenic mice. Am J Pathol. 1994;145:398–408. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.