Abstract
The burden of diabetes is increasing globally. Identifying novel preventable risk factors is an urgent need. In 2011, the U.S. National Toxicological Program (NTP) conducted a workshop to evaluate the epidemiologic and experimental evidence on the relationship of environmental chemicals with obesity, diabetes and metabolic syndrome. Although the evidence was insufficient to establish causality, the NTP workshop review identified an overall positive association between some environmental chemicals and diabetes. In this systematic review, our objective was to summarize the epidemiological research published since the NTP workshop. We identified a total of 29 articles (7 on arsenic, 3 on cadmium, 2 on mercury, 11 on persistent organic pollutants, 3 on phthalates and 4 on bisphenol A) including 7 prospective studies. Considering consistency, temporality, strength, dose-response, and biological plausibility (confounding), we concluded that the evidence is suggestive but not sufficient for a relationship between arsenic and persistent organic pollutants, and insufficient for mercury, phthalates and bisphenol A. For cadmium the epidemiologic evidence does not seem to suggest an association with diabetes. Important research questions include the need of additional prospective studies and the evaluation of the dose-response relationship, the role of joint exposures, and effect modification with other comorbidities and genetic variants.
Keywords: Systematic review, environmental chemicals, diabetes, arsenic, cadmium, mercury, persistent organic pollutants, bisphenol A, phthalates, type 2 diabetes, epidemiology
Introduction
The burden of diabetes is increasing globally [1]. In 2008, 347 million people worldwide had diabetes, well above earlier estimations from the World Health Organization [2, 3]. Type 2 diabetes is caused by insulin resistance and β-cell dysfunction [4] and accounts for 90% of all cases of diabetes. Established risk factors for type 2 diabetes include age, family history, genetic variants, obesity and physical inactivity [5]. Public policies and clinical guidelines emphasize life-style modifications including healthy environments and social context that facilitate appropriate caloric consumption and physical activity [6-8]. Beyond those established diabetes risk factors, the role of environmental factors has gained considerable attention [9]. In 2011, the U.S. National Toxicological Program (NTP) conducted an international workshop review to comprehensively evaluate the epidemiologic and experimental evidence on the relationship of environmental chemicals with obesity, diabetes and metabolic syndrome (summary for diabetes in Table 1) [10].
Table 1.
Summary of the 2011 National Toxicology Program (NTP) workshop review of epidemiologic studies on environmental chemicals and diabetes [10, 12, 14, 66]
| Environmental Chemicals | No. studies included (prospective) | Year of publication of studies (range) | No. of subjects (range, median) | Age range of participants (years) | Adjusted relative risk range | Conclusions as reported by NTP workshop review |
|---|---|---|---|---|---|---|
| Arsenic (High levels)[12]* | 9 (1) | 1994-2010 | 235-706,314 (891) ** | ≥ 14 | 1.1-10.1 | Limited to sufficient evidence to support the association |
| Arsenic (Low-moderate levels) [12] | 19 (0) | 1984-2011 | 225-41,282 (660) ** | All ages | 0.9-2.8 | Insufficient data to support the association |
| POPs [66] | ||||||
| Trans-nonachlor | 5 (1) | 2006-2010 | 51-3,039 (658) | ≥18 | 2.0-8.1 | General positive pattern |
| DDE, DDT, or DDD | 14 (3) | 2005-2010 | 80-3,049 (543) | ≥18 | 0.7-12.3 | General positive pattern |
| PCBs | 12 (5) | 2005-2010 | 167-2,106 (471) | ≥18 | 0.8-5.5 | General positive pattern |
| Agent Orange/TCDD | 6 (0) | 1997-2008 | 169-1,499 (989) | 1.2-1.6 | General positive pattern | |
| Miscellaneous organochlorine POPs | 14 (2) | 1997-2010 | – | ≥18 | 0.8-35.7 | General positive pattern |
| POPs mixtures | 6 (2) | 2006-2010 | 180-2,106 (503) | ≥18 | 0.9-5.4 | General positive pattern |
| Brominated compounds | 4 (2) | 2006-2010 | 180-684 (410) | ≥18 | 0.5-2.7 | No positive pattern |
| PFAAs | 3 (0) | 2009-2010 | 474-13,141 (2,036) | ≥12 | 0.6-3.2 | No positive pattern |
| Organotins [10] | – | – | – | – | – | No epidemiological studies identified. Experimental animal studies were few but the quality was high. |
| Phthalates [10] | – | – | – | – | – | Three cross-sectional human studies reported some positive association between phthalates exposure and indirect evidence of insulin resistance including body weight, waist circumference, and HOMA but only one of them evaluated the association with diabetes. |
| Bisphenol A [10] | 2 (0) | 2008-2010 | 1,455-1,493 | – | 1.2-1.4 | Limited evidence to support the association especially when findings from experimental animal studies also considered. |
| Pesticides [10] | – | – | – | – | – | Limited evidence from epidemiology studies to reach conclusion. Case reports of hyperglycemia following poisoning with organophosphate pesticides; case reports showing T1D following poisoning with the banned rodenticide, Vacor. Numerous animal studies support the association between certain pesticides and hyperglycemia e.g., organophosphates, amitraz. |
| Maternal smoking during pregnancy [14] | T1D: 14 (6) T2D: 2(1) |
T1D:1997-2011 T2D: 2002-2011 |
T1D: 212-11,282 (1390) T2D: 4.945-73,381 |
T1D: All ages T2D: 14-47 |
T1D: 0.37-3.00 T2D: 1.14-4.02 |
No positive pattern between maternal or paternal smoking during pregnancy and T1D in offspring; insufficient data to reach conclusions on T2D. |
DDD: dichlorodiphenyldichloroethane, DDE dichlorodiphenyldichloroethane, DDT: dichlorodiphenyltrichloroethane, HOMA, homeostatic model assessment; NR: not reported, PCBs: polychlorinated biphenyls, PFAAs: perfluoroalkyl acids, POPs: persistent organic pollutants, TCDD: telrachlorodibenzo-p-dioxin
High arsenic and low-moderate arsenic levels were defined as ≥ and <150 μg/L in drinking water, respectively
Includes studies that report only no. of deaths rather than total number of subjects.
- Indicates not reported in the NTP report.
In reviewing the available evidence, the NTP considered consistency across populations, strength and temporality of the associations, and biological plausibility including extensive evaluation of the animal and mechanistic evidence. In addition to a report of the workshop review, several publications have summarized the findings of the NTP workshop review [11] including an overall paper for all the chemicals evaluated [10] and specific papers for arsenic [12], persistent organic pollutants [13], and maternal smoking [14]. Since the NTP workshop review, interest on the potential role of environmental chemicals in diabetes has continued to increase. In the present review, we used the NTP workshop review as a starting point (Table 1) and updated the new evidence available on the association between diabetes and the following environmental chemicals: arsenic, other metals, persistent organic pollutants, phthalates, and bisphenol A (BPA). For organotins, no new epidemiological studies have been published. Other environmental exposures with increasing evidence available to assess a potential association with diabetes such as exposure to tobacco smoke [15] and air pollution [16, 17] will not be evaluated in the present review.
Methods
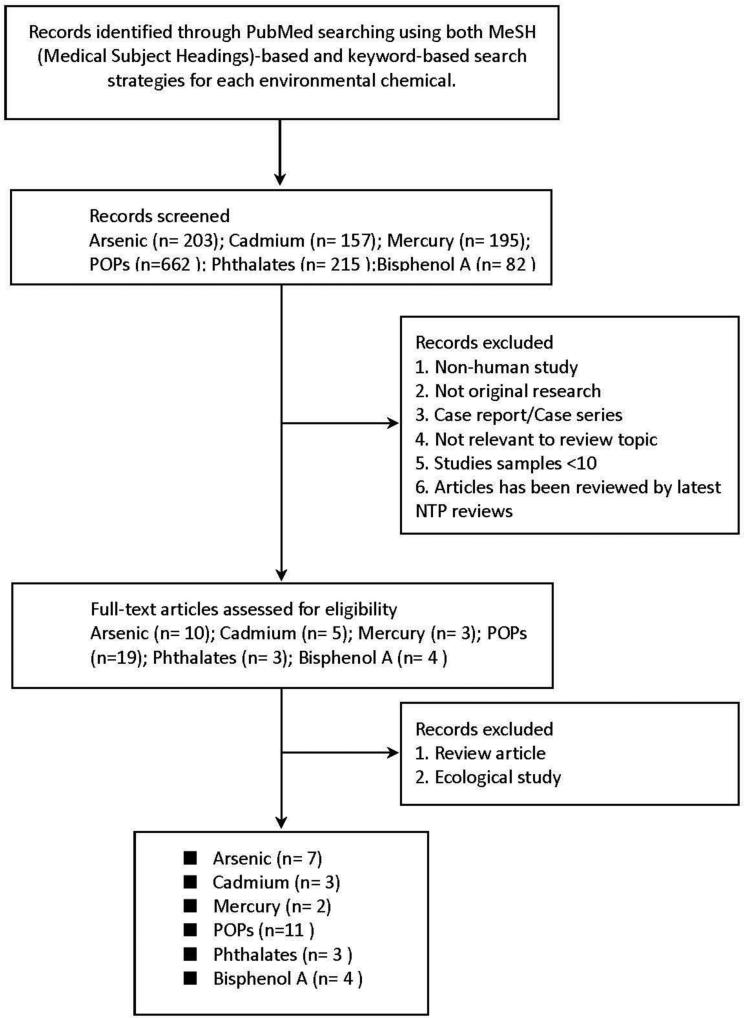
The systematic search and review processes were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement criteria, although the quality of the evidence and the risk of bias were not specifically evaluated in the current review [18]. To update the NTP workshop review, we searched MEDLINE for epidemiological studies investigating the association between those environmental chemicals and diabetes using the search strategy described in Table 2. The search period was January 1966 through May 2013. There were no language restrictions. We also manually reviewed the reference lists from relevant original research and the investigators’ files. Our exclusion criteria were: 1) publications containing no original research (reviews, editorials, non-research letters); 2) studies not carried out in humans; 3) exposures not measured at the individual level (e.g. ecological studies or studies measuring the chemical of interest at the community level); 4) case reports and case series; 5) studies not reporting the association between environmental chemicals and diabetes; and 6) studies previously reviewed by the NTP workshop review (Figure 1). Two authors, C.C. Kuo and K. Moon, independently abstracted data from the articles that met the selection criteria. We collected the following data for each study: authors, journal, year of publication, country, study design, study population; exposure assessment, diabetes diagnosis, measures of association, adjustment factors and other critical comments. For studies modeling chemical exposures both as continuous and categorical we reported categorical measures of association to allow for a more flexible evaluation of the dose-response relationship. For persistent organic pollutants, when multiple congeners were reported, we selected the congener with the weakest association, the congener with the maximum association and the congener with the median association. Disagreement was resolved by discussion between the two review authors, and if necessary, a third reviewer was involved.
Table 2.
PubMed search strategies for environmental chemicals and diabetes mellitus
| Database | PubMed |
|---|---|
| Date | Jun 06, 2013 |
| Strategy | We combined the results for each environmental chemical search strategy (#1 to #6 below) with the results for the diabetes search (#7 below) |
| #1. Arsenic [203] | “arsenic”[Mesh] OR arsenic |
| #2. Cadmium [157] | “cadmium”[Mesh] OR cadmium |
| #3. Mercury [195] | “mercury”[Mesh] OR mercury |
| #4. Persistent organic pollutants (POPs) [662] | “Polychlorinated Biphenyls”[Mesh] OR “Hydrocarbons, Chlorinated”[Mesh] OR “Dioxins”[Mesh] OR “Halogenated Diphenyl Ethers”[Mesh] OR “Polybrominated Biphenyls”[Mesh] OR “perfluorooctane sulfonic acid”[Substance Name] OR “perfluorooctanoic acid”[Substance Name] OR “Carbon Tetrachloride”[Mesh] “Polychlorinated Biphenyls” OR “chlorinated hydrocarbons” OR aldrin OR “carbon tetrachloride” OR chlordane OR chlordecone OR chlorobenzene* OR hexachlorobenzene OR chloroform OR ddt OR dichlorodiphenyltrichloroethane OR dichloroacetate OR “dichlorodiphenyl dichloroethylene” OR dichlorodiphenyldichloroethane OR dichloroethylenes OR dieldrin OR endrin OR “ethyl chloride” OR “ethylene dichlorides” OR heptachlor OR lindane OR hexachlorocyclohexane OR methoxychlor OR “methyl chloride” OR “methylene chloride” OR mirex OR mitotane OR “picryl chloride” OR polychloroterphenyl OR tetrachloroethylene OR toxaphene OR trichloroepoxypropane OR trichloroethane* OR trichloroethylene OR “vinyl chloride” OR “Dioxins” OR TCDD OR “Halogenated Diphenyl Ethers” OR “diphenyl ethers” OR PBDE* OR PCDE* OR “Polybrominated Biphenyls” OR “polybrominated biphenyls” OR Polybromobiphenyl* OR “polychlorinated biphenyls” OR Polychlorobiphenyl OR PCB OR “perfluorooctane sulfonic acid” OR “perfluorooctane sulfonic acid” OR pfosa OR 1763-23-1 OR “perfluorooctane sulfonate” OR “perfluorooctanoic acid” OR 335-67-1 OR “perfluorooctanoic acid” OR PFOA OR “pentadecafluorooctanoic acid” OR “perfluorooctanoyl chloride” OR “sodium perfluorooctanoate” OR “perfluorinated octanoic acid” OR “Carbon Tetrachloride” |
| #5. Phthalates [215] | “bisphenol A”[Substance Name] OR “bisphenol A” |
| #6. Bisphenol A [82] | “phthalic acid”[Substance Name] OR “Phthalic Acids”[Mesh] OR phthalate* OR “phthalic acid” OR phthalate* OR “phthalic acids” OR “dibutyl phthalate” OR “diethylhexyl phthalate” OR “o-phthalaldehyde” OR “phthalic anhydrides” OR phthalimides OR thalidomide |
| #7. Diabetes | “diabetes” OR “diabetes mellitus”[MeSH] |
The number in the brackets after each chemical is the number of articles identified based on a chemical-specific search strategy after combining the search strategy for this chemical with the search strategy for diabetes (#7).
Figure 1.
Flow diagram of study selection process.
Following the criteria proposed by the Office of Health Assessment and Translation (OHAT) at the National Toxicology Prevention (NTP) and similar criteria proposed by the 2004 Surgeon General Report on the health consequences of smoking [19, 20], we evaluated consistency, temporality, strength, dose-response, and biological plausibility (confounding) of the findings. Following this evaluation we classified the evidence for each environmental chemical and diabetes in four groups as modified from the Surgeon General Report [19]: sufficient evidence, suggestive but not sufficient evidence, and insufficient evidence to infer a relationship, and suggestive of no relationship. Similar categories have been proposed by OHAT: high level of evidence, moderate level of evidence, low level of evidence, and not classifiable [20].
Arsenic and Diabetes
Current perspectives
Inorganic arsenic in water and food are major global health problems [21, 22]. The toxicity and carcinogenicity of inorganic arsenic is well established [23, 24]. In recent years, increasing epidemiologic evidence from multiple countries supports the role of inorganic arsenic in the development of diabetes [25-30]. Experimental and mechanistic evidence provide additional support for arsenic diabetogenesis [31-33]. Arsenic could affect β-cell function and insulin sensitivity through several mechanisms including oxidative stress, glucose uptake and transport, gluconeogenesis, adipocyte differentiation, and calcium signaling [12, 34-36]. Arsenic could also act as an endocrine disrupter affecting the function of hormone receptors including glucocorticoid, androgen, estrogen, and thyroid hormone in cell culture and animal models [31, 32, 37]. Finally, arsenic could also impact diabetes through epigenetic mechanisms, including hyper- and hypo-methylation of diabetes related genes [38, 39].
In its evaluation of the association between arsenic and diabetes, the NTP workshop review distinguished between studies conducted in populations exposed to high arsenic levels in drinking water (≥150 μg/L) and studies conducted in populations exposed to low-moderate arsenic levels in drinking water (<150 μg/L) (Table 1). The NTP workshop review concluded that at high-chronic exposure levels, the epidemiological evidence was “limited to sufficient” to support the association between arsenic and diabetes. The studies at high levels of exposure, conducted in Taiwan and Bangladesh, were mostly consistent with an association between arsenic and diabetes. Despite the consistency in the findings, however, the level of evidence was considered “limited to sufficient” and not “sufficient” because most studies were cross-sectional, arsenic exposure was rarely measured at the individual level and some studies lacked appropriate definitions of diabetes and adjustment for confounders. At levels of arsenic in drinking water <150 μg/L, the NTP review concluded that the evidence was “insufficient” to conclude that arsenic is associated with diabetes because the findings were inconsistent and most studies were characterized by limited exposure and outcome assessment. All studies at low-moderate levels of exposure at the time of the NTP review were cross-sectional.
A total of seven studies meeting our inclusion criteria have been published following the NTP workshop in 2011, all of them in populations exposed to low-moderate arsenic levels in drinking water (Table 3). The studies were conducted in populations from Korea [40], Bangladesh [41], Cyprus [42], United States [43-45], and China [46]. Five studies were cross-sectional [40-43, 46], one study was case-control and two studies were prospective in design [44, 45]. Arsenic exposure was measured in drinking water at the household level in four studies [41, 42, 44, 46] and in urine in two studies from the USA and one study from South Korea [40, 43, 45]. Diabetes was defined by self-report [42] or by combining self-reported physician diagnosis [40, 41, 44, 46] and/or medication use [40, 43, 46] with at least fasting blood glucose level (FBG) [40, 41, 43, 44, 46], oral glucose tolerance test (OGTT) [43-45], or glycated hemoglobin(HbA1c) [43]) (Table 3) . All studies found a positive association between arsenic and diabetes, although only five of the associations were statistically significant (Table 3). Estimated relative risks comparing the highest vs. lowest arsenic exposure category ranged from 1.31 [40] to 1.90 [41] (median 1.55 [43]). The two studies that found no significant association between arsenic and diabetes were conducted in Cyprus (414 participants) and China (669 participants) [42, 46]. The study from South Korea measured only total arsenic in urine, limiting the interpretation of the study findings due to high seafood intake in the study population. The two prospective studies (nested case-control and case-cohort studies) were conducted in populations from the USA and found an association between arsenic and diabetes [44, 45]. These prospective studies provide critical evidence in support of the temporality criterion for causality. For future analyses, the consistency across populations in the two prospective studies and the four cross-sectional studies that report information in multiple categories of exposure could potentially facilitate the evaluation of the dose-response relationship through the use of dose-response meta-analyses. Overall, the evidence is suggestive but not sufficient to infer a causal relationship between inorganic arsenic exposure and diabetes at low-moderate levels of exposure. The major limitation is the small number and limited sample size of prospective studies.
Table 3.
Studies of metals and diabetes published since the National Toxicology Program Workshop [12]
| 1st author, year |
Design | Population | Men (%) |
Age Range (yrs) |
Diabetes Definition |
Exposure Assessment |
Exposure Categories |
N (Ca/NC) |
Relative Risk |
95% Confidence Interval |
Adjustment Factors |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arsenic | |||||||||||
| Kim 2011[40] | CS | South Korea (KNHANES 2008) | 46.9 | ≥20 | FBG ≥126 mg/dL, self-reported physician diagnosis, or diabetes medication | Urine total arsenic (μg/g creatinine) in a population with high seafood intake | Per doubling (mean 118.4 μg/g) | 1677 | 1.31 | 1.04-1.66 | Age, sex, body mass index, smoking, alcohol, education, hypertension, region, urban/rural, seafood consumption |
| Islam 2012[41] | CS | Bangladesh | 31.6 | >30 | FBG ≥126 mg/dL, self-reported physician diagnosis | Cumulative arsenic exposure via drinking water in tube wells (μg/L) | < 22 μg/L 23-32 33-261 ≥262 |
19/277 14/195 26/227 30/216 |
1.0 1.1 1.7 1.9 |
Reference 0.5-2.3 0.5-3.2 1.1-3.5 |
Age, sex, education, body mass index, family history of diabetes |
| Makris 2012 [42] | CS | Mammari, Cyprus | 47.3 | ≥18 | Self-reported physician diagnosis | Cumulative lifetime arsenic exposure via estimated regional drinking water (mg) | Quintiles (mg) 2nd vs. 1st 3rd vs. 1st 4th vs. 1st 5th vs. 1st |
317 NR NR NR NR |
0.72 1.79 0.77 1.86 |
0.09, 5.56 0.31, 10.26 0.11, 5.52 0.30, 11.59 |
Age, sex, smoking |
| Gribble 2012 [43] | CS | Arizona, Oklahoma, N/S Dakota, USA (Strong Heart Study) | 40.8 | 45-74 | FBG ≥126 mg/dL, OGTT ≥200 mg/dL, HbA1c ≥6.5% or diabetes medication | Baseline urine total arsenic (μg/L) in a population with low seafood intake | <7.9 μg/L 7.9-<14.1 14.1-<24.2 ≥24.2 |
413/558 492/506 503/467 531/454 |
1.00 1.26 1.38 1.55 p-trend |
Reference 1.14, 1.39 1.25, 1.54 1.39, 1.73 <0.001 |
Age, sex, urine creatinine, education, smoking, alcohol, body mass index |
| James 2013 [44] | CCO | San Luis Valley, Colorado USA (San Luis Valley Diabetes Study) | 46.1 | 20-74 | FBG ≥1 40 mg/dL, OGTT ≥200 mg/dL or self-reported diagnosis with medical record verification | Time-weighted average arsenic in residential drinking water (μg/L) | per 15 μg/L <4 μg/L ≥4 to <8 ≥8 to <20 ≥20 |
548 120 148 139 141 |
1.27 1.00 1.11 1.42 1.55 p-trend |
1.02,1.64 Reference 0.82, 1.95 0.94, 2.48 1.00, 2.51 0.07 |
Race, body mass index, physical activity |
| Kim 2013 [45] | NCC | Arizona USA (Southwestern American Indians) | NR | ≥25 | OGTT ≥200 mg/dL | Baseline urine total arsenic (μg/L) in a population with low seafood Baseline urine inorganic arsenic (μg/L) = total arsenic (arsenobetaine +MMA+DMA) |
Per doubling (median 21.1 μg/L) 6.6-15.3 μg/L 15.3-21.0 21.1-29.3 29.4-123.1 0.1-4.5 μg/L 4.6-6.9 7.0-9.4 9.5-36.0 |
300 300 300 |
1.11 1.00 ~1.3 ~2.3 ~1.3 p-trend 1.00 ~2.4 ~2.3 ~1.8 p-trend |
0.79, 1.57 Reference NR NR NR 0.12 Reference NR NR NR 0.06 |
Age, sex, body mass index, urine creatinine |
| Li 2013 [46] | CS | Inner Mongolia, China | 42.6 | Mean 49.7 | FBG ≥126 mg/dL, or diabetes medication | Arsenic in tube wells (μg/L) | <10 μg/L 10-50 >50 |
7/117 14/193 21/317 |
1.00 1.36 1.58 |
Reference 0.52, 3.57 0.58, 4.26 |
Age, sex, smoking, alcohol, body mass index, cumulative arsenic exposure (CAE)a |
|
Cadmium | |||||||||||
| Swaddiwudhipong 2010 [54] | CS | Thailand | 44.9 | ≥35 | FBG ≥126 mg/dL on two occasions, or diabetes medication | Urine cadmium (μg/g creatinine) | Continuous urine cadmium (mean 2.2 μg/g) | 5273 | 1.02 | 0.98, 1.05 | Age, alcohol, body mass index, hypertension, smoking |
| Barregard 2013 [55] | CO | Gothenburg, Sweden | 0 | 64 | Diabetes or impaired glucose tolerance Diabetes: FBG ≥110 mg/dL and/or OGTT ≥200 mg/dL on two occasions Impaired glucose tolerance: FBG <110 and OGTT 140 - <200 mg/dL |
Blood cadmium (μg/L) and urine cadmium (μg/g creatinine) | 1st quartile 2nd 4th 1st quartile 2nd 3rd |
Urine 62 70 67 56 Blood 57 79 71 51 |
1.0 1.0 0.8 1.2 1.0 0.7 0.9 2.2 |
Reference 0.5, 2.0 0.4, 1.6 0.5, 2.6 Reference 0.3, 1.5 0.4, 1.8 0.9, 6.1 |
Smoking (pack years), waist circumference, serum adiponectin |
| Moon 2013 [56] | CS | South Korea (KNHANES 2009-2010) | 49.9 | ≥30 | FBG ≥126 mg/dL, self-reported physician diagnosis, or diabetes medication | Blood cadmium (μg/L) | Quartiles (mean) 1st 0.55 μg/L 2nd 0.96 3rd 1.34 4th 2.11 |
78/718 72/722 88/710 95/701 |
1.00 0.77 0.85 0.90 |
Reference 0.54, 1.10 0.60, 1.20 0.63, 1.28 |
Age, sex, urban/rural, smoking, alcohol, regular exercise |
| Mercury | |||||||||||
| He 2013 [57] | CO | USA (CARDIA Study) | 47.7 | 20-32 | FBG ≥126 mg/dL, non-fasting blood glucose ≥200 mg/dL, OGTT ≥200 mg/dL, HbA1c ≥6.5%, or diabetes medication | Toenail mercury (μg/g) | <0.108 μg/g 0.108-0.174 0.175-0.265 0.266-0.424 >0.425 |
65/710 52/722 64/712 56/719 51/724 |
1.0 1.05 1.23 1.18 1.65 p-trend |
Reference 0.72, 1.53 0.85, 1.78 0.79, 1.77 1.07, 2.56 0.02 |
Age, sex, ethnicity, study center, body mass index, education, smoking, alcohol, physical activity, family history of diabetes, LCn-3PUFA intake, magnesium, toenail selenium. |
| Moon 2013 [56] | CS | South Korea (KNHANES 2009-2010) | 49.9 | ≥30 | FBG ≥126 mg/dL, self-reported physician diagnosis, or diabetes medication | Blood mercury (μg/L) | Quartiles (mean) 1st 2.10 μg/L 2nd 3.53 3rd 5.16 4th 9.62 |
84/712 83/714 78/717 88/708 |
1.00 1.16 1.04 1.08 |
Reference 0.83, 1.63 0.74, 1.48 0.76, 1.53 |
Age, sex, region, smoking, alcohol, regular exercise |
Abbreviations: CCO: Case-cohort study; CO: prospective cohort study; CS: cross-sectional study; NA, not associated; NCC: nested case-control; Ca/NC, cases/non cases; NR, not reported; FBG, fasting blood glucose; OGTT, oral glucose tolerance test; IFG, impaired fasting glucose, MMA, monomethylarsonic acid; DMA, dimethylarsinic acid; LCn-3PUFA, long-chain n-3 polyunsaturated fatty acid; Cumulative lifetime arsenic exposure (CLAEX) index =estimated median As concentration in contaminated water x daily water intake amount × duration
CAE(mg/L-yr)= the arsenic level in tube wells × duration of water exposure
Research needs – epidemiologic studies
Additional prospective studies at low-moderate levels of arsenic exposure through drinking water and food are needed to confirm the potential role of inorganic arsenic in diabetes development. It is also important to characterize pathways for diabetogenesis, including measures of insulin resistance and pancreatic ß-cell function. Insulin resistance has been evaluated in several human studies with no evidence of an association [43, 47], but pancreatic ß-cell function has not yet been evaluated. With the increasing awareness of arsenic in food and of the importance of food as source of arsenic, using biomarkers to estimate arsenic exposure is critical. Urine arsenic, including arsenic species, is the most widely use biomarker of inorganic arsenic exposure. Urine arsenic speciation also allows for the analysis of arsenic metabolism. Limitations of arsenic in urine include accounting for urine dilution and accounting for organic arsenicals of seafood origin. Arsenic speciation, moreover, is expensive and technically time-consuming. Developing advanced technology to measure arsenic efficiently should also be research priority.
Cadmium, Mercury and Diabetes
Current perspectives
Both cadmium and mercury are widespread in the environment and persistent in the food chain [48, 49]. Cadmium and mercury can induce hyperglycemia by altering pancreatic β-cell function via several pathways in experimental studies [50-52]. At the time of the 2011 NTP workshop, very little human data was available linking metals other than arsenic with diabetes.
For cadmium, a positive association between cadmium and diabetes in the National Health and Nutrition Examination Survey (NHANES III) was published in 2003 [53]. We have identified three additional publications investigating the association between cadmium and diabetes (Table 3). These studies were conducted in Thailand [54], Sweden [55], and South Korea [56]. Cadmium exposure was measured in urine [54], blood [56], or both blood and urine [55]. Diabetes was defined based on self-reported physician diagnosis and/or medication use [54, 56] plus FBG [54-56] or OGTT [55]. All studies were cross-sectional, although one study also conducted a prospective investigation over 5.4 years of follow-up [55]. None of them supported that cadmium exposure, as measured in blood and urine, contribute to the development of diabetes [54-56]. There is evidence from animal studies that cadmium can induce hyperglycemia and pancreatic toxicity [52]; however, additional work is needed to better understand the relevance of the dose levels and routes of administration used in experimental studies in the context of human exposure.
For mercury, two epidemiologic studies have evaluated its association with diabetes in the past year (Table 3) [56, 57]. The studies were conducted in young adults from four U.S. cities [57] and in a nationally representative sample of adults from South Korea [56]. Mercury exposure was measured in toenails [57] and blood [56]. The South Korean study was cross-sectional, measured mercury in blood and defined diabetes based on self-reported diagnosis, anti-diabetic medication use or FBG. The study did not support that mercury was associated with the prevalence of diabetes. The U.S. study was prospective and defined incident diabetes over 18 years of follow-up based on FBG, OGTT, or HbA1c. The hazard ratio for diabetes comparing the highest to lowest toenail mercury quintiles was 1.65 [95% CI: 1.07-2.56] [57]. While additional prospective studies are needed to evaluate the consistency of the association, this prospective study supports the possible role of mercury in diabetes development. Overall, however, the evidence is insufficient to infer an association between arsenic and mercury.
Research needs – epidemiologic studies
Since the NTP workshop, all recent reports have failed to confirm an association between cadmium and diabetes. Given these findings, initiating large-scale prospective studies might not be a current research priority. The only epidemiologic study that has found an association between cadmium and diabetes was conducted in NHANES III [53]. Since NHANES data are publically available, it will be important to replicate those analyses to confirm the findings in NHANES III as well as to evaluate the association in NHANES 1999+.
For mercury, additional prospective studies are needed to evaluate the association with diabetes. As the major sources of mercury in humans come from methylmercury exposure from seafood consumption [58], adjustment for nutrients (e.g., selenium, magnesium, n-3 fatty acids), lifestyle (seafood as marker of healthy diet), and other toxicants in seafood (persistent organic pollutants) represent important challenges. The most recent meta-analyses on seafood and diabetes found no association between seafood consumption and the risk of diabetes [59]. However, mercury exposure was not considered in any of the primary studies. Overall, well-designed prospective studies are warranted to evaluate the joint effect between mercury and other nutrients and toxicants and its impact on the risk of diabetes. Finally, how to appropriately evaluate joint effects across multiple metals that are potentially diabetogenic remains controversial. Multi-element analytical methods have become standard in metal assessment. Methods development for handling multi-exposures remains critical.
Persistent Organic Pollutants and Diabetes
Current perspectives
Humans have been extensively exposed to synthetic chemicals since World War II [60]. Of the human-made chemicals, persistent organic pollutants (POPs) may be the most widely distributed as they are semi-volatile, highly lipophilic, and resistant to photolytic, biological, and chemical degradation. POPs bioaccumulate in adipose tissues of both human and wildlife as well as in the food chain. They have a half-life ranging from months to years [61, 62]. Though POPs refer to a broad class of compounds, priority pollutants consist of pesticides (such as chlordane and dichlorodiphenyltrichloroethane (DDT)), industrial chemicals (such as polychlorinated biphenyls (PCBs) and hexachlorobenzene (HCB)), and unintended byproducts (such as dibenzodioxins and dibenzofurans) [63, 64]. The first epidemiological research reporting a relationship between dioxin and diabetes mellitus was published in 1997 [65]. By the time of the NTP workshop, more than 70 epidemiological studies had investigated the association between POPs and diabetes (Table 1) [10, 66]. Because of considerable heterogeneity across studies regarding exposure and outcome ascertainments, the NTP workshop review concluded that the data were insufficient to establish a causal relationship between POPs and diabetes. There was, however, suggestive evidence in support of a positive association among trans-nonachlor, dichlorodiphenyldichloroethane (DDE), DDT, dichlorodiphenyldichloroethane (DDD), PCBs, agent orange/ tetrachlorodibenzo-p-dioxin(TCDD), miscellaneous organochlorine compounds or POPs mixtures and type 2 diabetes [10, 13]. For brominated compounds and perfluoroalkyl acids (PFAA), the NTP workshop review concluded that there was no positive pattern of associations.
Our update review identified 11 additional publications meeting our inclusion criteria (Table 4). The studies were conducted in populations from Japan [67, 68], USA [69-73], Sweden [74], Finland [75], and Spain [62, 76]. Three studies focused on PCBs exposure [67, 69, 71] and eight studies evaluated a broader spectrum of POPs [62, 68, 70, 72-76]. Nine studies were cross-sectional [62, 67-72, 75, 76] and two studies were prospective cohort studies [73, 74]. Diabetes definitions combined self-reported physician diagnosis [62, 67-73, 76] and/or anti-diabetic medication use [62, 74, 75] with FBG [62, 71, 72, 74-76], OGTT [75], or HbA1c [67, 68, 70]. Overall, a significant positive association was consistently shown between organochlorine compounds and type 2 diabetes in studies worldwide. The two prospective studies evaluated the association between baseline organochlorine levels and diabetes development over five [74] and 19 [73] years of follow-up, providing strong inferences about the temporal precedence [73, 74]. One of the studies also conducted a meta-analysis of all prospective studies evaluating POPs and incident diabetes [73]. The pooled OR was 2.0 (95% CI 1.13-3.53) for HCB and 1.70 (95% CI 1.28-2.27) for total PCBs [73]. Two studies also analyzed the association between PCBs exposure and insulin resistance but no significant association was observed. This implies that PCBs may induce pancreatic β-cell dysfunction, although this hypothesis needs to be more thoroughly evaluated in human populations [69, 71]. In a population from Anniston, Alabama, the association between POPs and diabetes was only significant among women [72]. Additional research is needed to evaluate potential sex differences in diabetes susceptibility due to POPs exposures.
Table 4.
Studies of persistent organic pollutants (POPs) and diabetes published since the National Toxicology Program Workshop [66]
| 1st author, year |
Design | Population | Size | Men (%) |
Age Range (yrs) |
Diabetes Definition |
Exposure Assessment |
Exposure Modeling |
Chemical(s) (if PCBs, highest, lowest, & median risk) |
Relative Risk (highest, lowest, & median) |
95% Confidence Interval |
Adjustment Factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tanaka 2011 [67] | CS | Japan (Saku Control Obesity Program) | 117 | 50.4 | 40-64 | FBG ≥126 mg/dL, HbAlc ≥6.5%, self-reported physician diagnosis, or diabetes medication | Whole blood PCB 74, 99, 118, 138, 146, 153, 156, 163/164, 170, 180, 182/187 |
Per pg/g lipid | PCB 146 PCB 163/164 PCB 182/187 |
29.2 0.14 1.15 |
1.89, 451 0.03, 0.58 0.36, 3.64 |
Sex, age, body mass index |
| Persky 2011 [69] | CS | Capacitor manufacturing plant, Illinois, USA | 93 | 0 | Mean 64.3 | Self-reported physician diagnosis | Blood PCB 028, 052, 060, 066, 074, 099, 101, 105, 110, 118, 130, 137, 138, 146, 149, 153, 156, 157, 167, 170, 171, 172, 177, 178, 180, 183, 187, 189, 191, 193, 194, 195, 201, 203, 205, 206, 208, 209 |
Per log-transformed (wet weight, ng/mL) | Total PCBs Anti-estrogenic PCBs Estrogenic PCBs Dioxin-like PCBs Non-Dioxin-like PCBs |
4.4 12.3 3.4 10.0 4.0 |
p-values 0.02 <0.01 0.01 <0.01 0.03 |
Age, body mass index, triglycerides, total cholesterol; other variables (GGT, SHBG, hypertension, alcohol, DHEA sulfate, FSH, moderate/high CRP, and T3-uptake) included if p<0.05. |
| Lee 2011 [74] | CO | Uppsala, Sweden (PIVUS Study) | 725 | 48.3 | 70 | FBG ≥111.6 mg/dL or diabetes medication | Plasma PCB 74, 99, 105, 118, 138, 153, 156, 157, 170, 180, 189, 194, 206, 209 p,p′-DDE, trans-nonachlor, HCB, OCDD, BDE47 |
5th vs. 1st quintile (wet weight, pg/mL) | Total PCBs PCB 74 PCB 153 PCB 118 Total Pesticides p,p′-DDE trans-nonachlor HCB |
7.5 9.0 1.7 3.6 3.4 2.1 1.8 2.1 |
1.4, 38.8 1.0,78.6 0.5, 6.2 0.7, 18.8 1.0,11.7 0.7, 6.3 0.5, 6.8 0.6, 7.1 |
Sex, body mass index, smoking, exercise, alcohol, triglycerides, total cholesterol |
| Airaksinen 2011 [75] | CS | Helsinki, Finland (Helsinki Birth Cohort Study) | 1988 | 46.3 | 57-70 | FBG ≥126 mg/dL, OGTT ≥200 mg/dL or diabetes medication | Serum Oxychlordane, trans-nonachlor, p,p′-DDE, PCB153, BDE47, BDE153 | ≥90th vs. <10th percentile (ng/g lipid) | Oxychlordane trans-nonachlor p,p′-DDE BDE 153 BDE 47 PCB153 |
2.08 2.24 1.75 0.68 1.24 1.64 |
1.18, 3.69 1.25, 4.03 0.96, 3.19 0.36, 1.28 0.69, 2.23 0.92, 2.93 |
Sex, age, waist circumference, mean arterial pressure |
| Silverstone 2012 [72] | CO | Anniston, Alabama, USA (Anniston Community Health Survey) | 580 | 40.8 | 45-64 | FBG >125 mg/dL or self-reported physician diagnosis | Blood PCB 28, 44, 49, 52, 66, 74, 87, 99, 101, 105, 110, 118, 128, 138+158, 146, 153, 156, 157, 167, 170, 172, 180, 194, 149, 151, 170, 177, 178, 180, 183, 187, 189, 195, 196+203, 199, 206, 209, DDE |
per 1 SD wet-weight, ng/g) Quintiles (wet-weight, ng/g) | ΣPCBs DDE Mono-ortho Estrogenic Di, tri-, or tetra-ortho Ryanodine Mono-ortho TEQ ΣPCBs: 0.11-1.15 1.16-2.42 2.43-4.33 4.34-9.33 9.34-170 |
1.21 1.22 1.18 1.08 1.20 1.19 1.20 1.00 2.18 2.91 1.64 2.78 |
0.87, 1.69 0.85, 1.46 0.86, 1.62 0.80, 1.46 0.86, 1.68 0.85, 1.66 0.87, 2.02 Reference 0.98, 4.82 1.24, 6.83 0.64, 4.19 1.00, 7.73 |
Age, sex, body mass index, total lipids, race/ethnicity, family history of diabetes, lipid-lowering medication |
| Gasull 2012 [62] | CS | Catalonia, Spain (Catalan Health Interview Survey) | 886 | 42.9 | 18-74 | FBG ≥126 mg/dL, self-reported physician diagnosis, or diabetes medication | Serum o,p′-DDT, p,p′-DDT, o,p′-DDE, p,p′-DDE, o,p′-DDD, p,p′-DDD; PCB 28, 52, 101, 118, 138, 153, 180; PeCB, HCB, α-HCH, β-HCH, γ-HCH, and δ-HCH |
Highest vs. lowest quartile (wet-weight, ng/mL) 80th vs 20th percentile (wet-weight, ng/mL) |
Sum of PCBs HCB p,p′-DDT PCB 118 Sum of PCBs p,p′-DDT PCB 118 |
2.4 1.6 0.6 2.1 1.7 0.9 1.6 |
1.1, 5.4 0.7, 3.5 0.3, 1.2 1.0,4.4 1.1,2.6 0.7, 1.2 1.0, 2.4 |
Age, sex, body mass index, total cholesterol, triglycerides |
| Everett 2012 [70] | CS | USA (NHANES 1999-2004) | 2611 | NR | ≥20 | Self-reported physician diagnosis or HbAlc ≥6.5 % | Blood PCB 118, 126, 156, 169 2,3,4,7,8-PeCDF, 1,2,3,6,7,8-HxCDD 1,2,3,4,6,7,8-HpCDD, 1,2,3,4,6,7,8,9-OCDD |
Highest vs. lowest tertile (fg/g blood) | PCB 118 HpCDD HxCDD Dioxin TEQ |
3.53 1.73 2.25 3.08 |
1.64, 7.58 0.88, 3.40 1.16, 4.37 1.20, 7.90 |
Age, sex, race/ethnicity, education, poverty income ratio, body mass index, waist circumference, energy adjusted fruit and vegetable consumption, physical activity, family history of diabetes |
| Persky 2012 [71] | CS | Capacitor manufacturing plant employees and local residents, Illinois, USA | 63 | 100 | Mean 55.9 | Self-reported physician diagnosis | Blood PCB 028, 052, 056/060, 066, 074, 099, 101, 105, 110, 118, 130, 137, 138, 146, 149, 153, 156, 157, 167, 170, 171, 172, 177, 178, 180, 183, 187, 189, 191, 193, 194, 195, 201, 203, 205, 206, 208, 209 |
Log-transformed continuous (wet weight, ng/mL) | Total PCBs Total Lipid PCBs Dioxin-like PCBs Non-Dioxin-like PCBs Estrogenic PCBs Anti-estrogenic PCBs |
3.0 3.0 2.7 3.0 3.0 2.4 |
1.3, 7.0 1.3, 7.2 1.3, 5.8 1.3, 7.2 1.2, 7.5 1.2, 4.9 |
Age, body mass index, total lipids |
| Nakamoto 2012 [68] | CS | Japan | 2264 | 47.0 | 15-76 | Self-reported history of diabetes or HbAlc >6.1 % | Blood PCB 77, 81, 105, 114, 118, 123, 126, 156, 157, 167, 189 2,3,7,8-TeCDD, 1,2,3,7,8-PeCDD, 1,2,3,4,7,8-HxCDD, 1,2,3,6,7,8-HxCDD, 1,2,3,7,8,9-HxCDD, 1,2,3,4,6,7,8-HpCDD, OCDD, 2,3,7,8-TeCDF, 1,2,3,7,8-PeCDF, 2,3,4,7,8-PeCDF, 1,2,3,4,7,8-HxCDF, 1,2,3,6,7,8-HxCDF, 1,2,3,7,8,9-HxCDF, 2,3,4,6,7,8-HxCDF, 1,2,3,4,6,7,8-HpCDF, 1,2,3,4,7,8,9-HpCDF, OCDF |
Highest vs. lowest TEQ quartiles (pg/g lipid) | Total Dioxin PCDDs/ PCDFs Dioxin-like PCBs | 23 10 11 |
4.6, 430 2.9, 66 3.0, 76 |
Age, sex, smoking, alcohol, regional block, survey year, body mass index |
| Arrebola 2013 [76] | CS | Southern Spain | 386 | 51 | Median 52.0 | Self-reported history of diabetes and FBG ≥126 mg/dL | Adipose tissue & serum PCB 138, 153, 180, HCB, P-HCH, p,p′-DDE, TEXB-extract | Highest vs. lowest tertile (ng/g lipid) | ΣPCBs ΣOCs HCB β-HCH p,p′-DDE (tissue) p,p′-DDE (serum) |
1.03 1.81 1.91 1.81 4.44 1.69 |
0.35, 3.31 0.54, 6.09 0.54, 6.84 0.51, 6.45 1.03, 21.02 0.54, 5.22 |
Age, sex, body mass index, tissue origin |
| Wu 2013 [73] | NCC | USA (2 Nurses Health Study nested case control studies: NHL and breast cancer) | 1095 | 0 | Mean 58.6 | Self-reported diagnosis | Plasma PCB 118, 138, 153, 180, p,p′-DDE, p,p′-DDT, HCB ΔPCBs =PCB (118+138+153+180) Breast cancer study Total PCBs=ΣPCBs + other 18 PCBs NHL study Total PCBs=ΣPCBs + other 52 PCBs Total POPs=total PCBs+ DDT+ DDE+ HCB |
Highest vs. lowest tertile (ng/g lipid) | Breast cancer study: ΣPCBs Total PCBs Total POPs HCB PCB 138 PCB 118 NHL study: ΣPCBs Total PCBs Total POPs HCB PCB 180 p,p′-DDT |
1.21 1.30 1.56 2.76 0.97 1.32 0.98 0.79 1.55 3.52 0.68 1.01 |
0.43, 3.39 0.43, 3.93 0.48, 5.05 0.75, 10.1 0.33, 2.84 0.42, 4.20 0.26, 3.72 0.23, 2.71 0.49, 4.93 1.03, 12.1 0.19, 2.49 0.34, 3.07 |
Age, smoking, alcohol, physical activity, family history of diabetes, body mass index |
Abbreviations: CS, cross-sectional study; CO, prospective cohort study; NCC, nested case-control, NHL, non-Hodgkin lymphoma study; NR, not reported; FBG, fasting blood glucose; OGTT, oral glucose tolerance test; BDE, bromodiphenylether congeners; DDT, dichlorodiphenyltrichloroethane; DDE, dichlorodiphenyldichloroethene; DDD, dichlorodiphenyldichloroethane; PCB, polychlorinated biphenyls; PeCB, pentachlorobenzene; HCB, hexachlorobenzene; HCH, hexachlorocyclohexane; PeCDF, pentachlorodibenzofuran; HxCDD, hexachlorodibenzo-p-dioxin; HpCDD, heptachlorodibenzo-p-dioxin; OCDD, Octachlorodibenzo-p-dioxin; PCDD, polychlorinated dibenzo-p-dioxin; PCDF, polychlorinated dibenzo-furan; DL-PCB, dioxin-like PCB; TeCCD, tetrachorinated-dibenzo-p-dioxin; PeCDD, pentachlorodibenzo-p-dioxin; TeCDF, Tetrachlorodibenzofuran; HxCDF, hexachlorodibenzofuran; HpCDF, heptachlorodibenzofuran; OCDF, octachlorodibenzofuran; TEXB, total effective xenoestrogen burden.
For cross-sectional studies, reverse causality and disease progression bias could be important limitations. One longitudinal study found that diabetes may result in temporal increases in serum dioxin concentrations, especially among individuals with poor diabetes control [77]. A major challenge in POPs research is the evaluation and identification of specific compounds. Most studies tested at least five POPs but few studies have corrected for multiple testing [78]. Finally, standardization of POPs’ measurement (e.g., wet vs. dry deposition, toxic equivalency (TEQ) vs. measured values), adjustment or not for lipid levels, and evaluation of confounders, are ongoing challenges that require standardization in order to facilitate data comparison and meta-analysis. Overall, increasing and consistent evidence, including prospective studies [65, 73, 74, 79-87], supports the relationship between POPs and diabetes. Risk assessment of POPs should consider diabetes as a relevant outcome.
Research needs – epidemiologic studies
Given the large number of studies evaluating the association between POPs and diabetes, additional studies following similar study designs are unlikely to further advance the science. Meta-analyses of individual congeners, of congener groups according to their activity (e.g. estrogenic, dioxin-like), and of overall levels are strongly needed. The information obtained from the meta-analyses could increase our understanding on the critical compounds. Dose-response meta-analyses could also be useful. The evaluation of the potential impact of the reduction of POPs levels in diabetes risk is also critical to support the causality of the relationship.
Phthalate and Diabetes
Current perspectives
Phthalates are a family of man-made compounds used as plasticizers that provide flexibility and durability of plastics such as polyvinyl chloride (PVC) [88]. They are also used as solvents. The ubiquitous use of phthalate esters in plastics, personal care products such as cosmetics, medical devices, and food packaging leads to extensive exposure in general populations. In the US general population, about 75% of people have detectable phthalate metabolites in urine [89]. The first large epidemiological study linking phthalates and diabetes-related outcomes was published in 2007 using NHANES. Higher phthalates concentrations in urine were associated with increased abdominal obesity and insulin resistance [90]. Mechanistically, the close interplay between phthalates and peroxisome proliferator-activated receptor-alpha and gamma (PPARα and PPARγ) has been regarded as the main pathway to impair both glucose metabolism and beta-cell function [91-93]. Phthalates can behave as an endocrine disrupting chemical that directly activate PPARγ and promote adipogenesis [94-96]. However, the recent NTP workshop review suggested that there were insufficient data to reach conclusions on the role of phthalate and diabetes [10].
We identified three epidemiological studies published after the NTP workshop in 2011[88, 97, 98] (Table 5). The studies were conducted in Mexico [98], Sweden [97], and the USA [88]. Phthalate exposure was measured in the urine in two studies [88, 98] and in serum in one study [97]. Diabetes was defined based on self-reported physician diagnosis and fasting blood glucose in one study [97] and self-reported physician diagnosis in two studies [88, 98]. All studies were cross-sectional and consistently found borderline and/or statistically significant associations with some phthalates and diabetes. The estimated risks for total di-2-ethyl hexyl phthalate (DEHP) were ranged from 1.43 [88] to 1.64 [98]; for mono-ethyl phthalate (MEP) 0.89 [88] -1.28 [97] (median 1.02 [98]). Two studies focused on women [88, 98] and the other targeted an elder population [97] which limits the generalizability to general populations. Women might be more vulnerable to metabolic effects of phthalates as they have higher urine concentrations of certain phthalates [99]. The reasons for higher phthalate concentrations among women are unknown [99], but higher use of personal care products that contain phthalates could be involved [100]. No clear sex differences, however, have been found for blood phthalates [97, 99]. Given the lack of prospective evidence, the current evidence is suggestive but insufficient to support the relationship between phthalates and diabetes.
Table 5.
Studies of phthalates and diabetes published since the National Toxicology Program Workshop [10]
| 1st author, year |
Design | Population | Size | Men (%) |
Age Range (yrs) |
Diabetes Definition |
Exposure Assessment |
Exposure Modeling |
Chemical(s) with highest, lowest, & median risk |
Relative Risk |
95% CI | Adjustment Factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Svensson 2011 [98] | CS | Mexico | 221 | 0 | Mean 53.8 | Self-reported physician diagnosis | Urine MEP, MBP, MiBP, MBzP, MEHP, MEHHP, MEOHP, MECPP, MCPP |
Log-transformed continuous (Hg/L) | ΣDEHP MBzP MEP |
1.64 0.74 1.02 |
0.98, 2.73 0.55, 1.00 0.74, 1.39 |
Urine creatinine and education (MEP, MBP, MiBP, MBzP, and MEHP), Urine creatinine and age (MEHHP and MECPP); Urine creatinine and waist-hip ratio (MEHHP and ΣDEHP). |
| Lind 2012 [97] | CS | Uppsala, Sweden (PIVUS study) | 1016 | 48 | 70 | Self-reported diagnosis or FBG ≥126 mg/dL | Serum MEHP, MEP, MiBP, MMP |
Log-transformed continuous (ng/mL) Highest vs. lowest quintile (ng/mL) | MEHP MEP MiBP MMP MMP MEHP MiBP |
0.97 1.28 1.30 1.21 2.54 1.24 2.00 |
0.84, 1.13 0.97, 1.70 1.10, 1.55 1.00, 1.46 1.25, 5.13 0.61, 2.51 1.03, 3.88 |
Sex, serum cholesterol and triglycerides, body mass index, smoking, exercise, education |
| James-Todd 2012 [88] | CS | USA (NHANES 2001-2008) | 2350 | 0 | 20-79 | Self-reported physician diagnosis | Urine MEP, MnBP, MiBP, MBzP, and MCPP ΣDEHP=MEHP+ MEHHP+ MEOHP |
Highest vs. lowest quartile (ng/mL) | MBzP MEP ΣDEHP |
1.99 0.89 1.43 |
1.14, 3.49 0.48, 1.68 0.75, 2.75 |
Urine creatinine, age, race/ethnicity, education, poverty, fasting time, total caloric intake, total fat intake, smoking (not adjusted for MCPP & ΣDEHP), physical activity (not adjusted for MCPP), body mass index, waist circumference |
Abbreviations: Ca/NC, case/non-case; CI, confidence interval; CS, cross-sectional study; NR, not reported; FBG, fasting blood glucose; OGTT, oral glucose tolerance test; MEP, mono-ethyl phthalate; MBP, mono-n-butyl phthalate; MiBP, ,mono-isobutyl phthalate; MBzP, mono-benzyl phthalate, MEHP, mono(2-ethylhexyl)phthalate; MEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono-(2-ethyl-5-oxohexyl) phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MCPP, mono(3-carboxypropyl) phthalate; MMP, mono-methyl phthalate; MnBP, mono-n-butyl phthalate; DEHP, di-2-ethyl hexyl phthalate.
Research needs –epidemiologic studies
The weaknesses in the current epidemiological evidence include a lack of prospective studies and the use of self-reported diabetes diagnosis in most studies. In addition to prospective studies, there is need to evaluate exposure patterns and variation in phthalate biomarker concentrations over time using longitudinal designs. As most phthalates have relatively short half-lives in humans, more data are needed to determine the long-term stability/reproducibility of both urine and blood phthalates concentrations and the adequacy of those biomarkers to evaluate their relationship with chronic diseases [101]. Because phthalates are ubiquitous, reducing exposure is challenging from a public health practice perspective. Recently, the French government has passed a law to ban the use of tubes containing DEHP in pediatrics, neonatology, and maternity wards in hospitals [102].
Bisphenol A and Diabetes
Current perspectives
Bisphenol A (2, 2-Bis (4-hydroxyphenyl) propane; BPA) is widely used in polycarbonate plastic, epoxy resins, and food contact materials leading to potential exposure through food [103]. It was estimated over 8 billion pounds of BPA were produced with over 100 tons released into atmosphere each year [104, 105]. BPA exposure is ubiquitous and more than 90% of US populations have detectable BPA in urine [106]. Dietary exposure to BPA-contaminated food and beverages likely represents the major source of exposure for general populations [107], although it is clear that we do not understand all the sources of exposure to BPA, as evidenced by findings in medical devices [108] and thermal paper [109]. Mechanistically, BPA is often described as having estrogenic activity, but it also displays other biological activities and should not be considered only to act as an estrogen or even a selective estrogen receptor modulator. Depending on the system studied and the dose, BPA may exert multiple cellular and tissue-type specific effects [103]. Experimental studies using BPA at environmentally relevant levels have supported the role of BPA in the development of obesity and diabetes through the inhibition of adiponectin and impairment of pancreatic beta cell regulation [110, 111]. However, evidence in human is very limited and inconsistent. In 2011, the NTP 2011 workshop review did not make any conclusion on the relationship between BPA and diabetes due to scarce evidence [10].
Following the NTP workshop review, four additional publications have been published [112-115] (Table 6). These studies were all cross-sectional and conducted in representative populations from the USA [113, 114], South Korea [115], and Shanghai, China [112]. BPA exposure was measured in urine in all studies [112-115]. Diabetes was defined by self-reported physician diagnosis in one study [116], and by combining self-reported physician diagnosis and/or anti-diabetic medication use [112-114], with fasting blood glucose [112, 113] or hemoglobin A1c [113, 114]. The two studies conducted in the US based on NHANES 2003-2008 found estimated relative risks for diabetes of 1.08 per doubling of urine BPA concentration [114] and 1.68 in the highest quartile of BPA exposure to the lowest quartile [113]. In the NHANES study that evaluated the association between BPA and diabetes in each NHANES cycle separately (2003-2004, 2005-2006 and 2007-2008), the association was only identified in NHANES 2003-2004 (mean urine BPA 2.4 ng/mL) [114]. The association was not observed in NHANES 2005-2006 (mean urine BPA 1.7 ng/mL) or NHANES 2007-2008 (mean urine BPA 2.6 ng/mL) [113, 114, 117]. The studies from South Korea and China did not observe a positive association between BPA and diabetes; however, these studies restricted their study population to participants older than 40 years [112, 115]. In the study from Shanghai, China, no clear dose-response relationship with diabetes was observed across BPA quartiles [112]; however, BPA was significantly associated with obesity and insulin resistance [118]. The mean urine BPA concentration was lower in Shanghai compared to the studies in the US and Korea. Although no significant association between BPA and diabetes was found in the South Korean study, urine BPA levels were significantly higher among female participants with diabetes aged 50-59 years and among urban residents [116].
Table 6.
Studies of bisphenol A (BPA) and diabetes published since the National Toxicology Program Workshop [10]
| 1st author, year |
Design | Population | Men (%) |
Age Range (yrs) |
Diabetes Definition |
Exposure Assessment |
Exposure categories |
N (Ca/NC) |
Relative Risk |
95% CI | Adjustment Factors |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ning 2011 [112] | CS | Shanghai, China | 40 | ≥40 | FBG ≥126 mg/dL or history of diabetes | Urine Total conjugated and free BPA | Quartiles ≤0.47 ng/mL 0.48-0.81 0.82-1.43 > 1.43 |
257/591 296/574 250/600 284/571 |
1.00 1.30 1.09 1.37 |
Reference 1.03, 1.64 0.86, 1.39 1.08, 1.74 |
Age, sex, education, family history of diabetes, waist circumference, SBP, TG, hs-CRP, ALT, eGFR, albumin, total bilirubin |
| Silver 2011 [114] | CS | USA (NHANES 2003-2008) | 48.5 | Mean 46.5 | HbAlc ≥6.5 % or diabetes medication | Spot urine Total conjugated and free BPA | Per doubling (ng/mL) Pooled 03-04 cycle 05-06 cycle 07-08 cycle |
4389 1364 1363 1662 |
1.08 1.23 1.06 1.06 |
1.02, 1.16 1.07, 1.41 0.95, 1.19 0.91, 1.23 |
Age, age2, urine creatinine, sex, race-ethnicity, education, household income, body mass index, waist circumference, and smoking |
| Shankar 2011 [113] | CS | USA (NHANES 2003-2008) | 47.3 | Mean 45.0 | FBG ≥126 mg/dL, non- fasting blood glucose ≥200 mg/dL, HbAlc ≥6. 5 % or diabetes medication | Spot urine Total conjugated and free BPA | Quartiles <1.10 ng/mL 1.10-2.10 2.11-4.20 > 4.20 |
93/1028 98/807 109/868 123/841 |
1.00 1.42 1.48 1.68 |
Reference 1.03, 1.96 1.05, 2.08 1.22, 2.30 |
Age, sex, race/ethnicity, education, smoking, alcohol, body mass index, SBP and DBP, urine creatinine, total cholesterol |
| Kim 2013 [115] | CS | Korea (National Human Biomonitoring Survey) | 41.6 | 40-69 | Self-reported and doctor- diagnosed type 2 diabetes | Spot urine | Quartiles <1.36 ng/mL 1.36-2.14 2.15-3.32 > 3.32 |
19/283 23/280 23/280 34/268 |
1.00 1.23 1.17 1.71 |
Reference 0.62, 2.43 0.60, 2.28 0.89, 3.26 |
Urine creatinine, age, sex, body mass index, education, smoking, place of residence. |
Abbreviations: Ca/NC, case/non-case; CI, confidence interval; CS, cross-sectional study; NR, not reported; FBG, fasting blood glucose; OGTT, oral glucose tolerance test; SBP, systolic blood pressure; TG, triglyceride; hs-CRP, high-sensitivity C-reactive protein; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate.
Several of the studies conducted stratified analyses to assess overweight/obesity as a modifying factor for diabetes [113, 114] or insulin resistance [118]. Overall, there was a trend for larger adjusted odds ratios in people with a normal body mass index (less than 24 or 25 kg/m2) compared to those considered overweight or obese. This suggests that the metabolic effects of being overweight or obese may overwhelm any effects of BPA on diabetes or insulin resistance [118].
Given the lack of prospective evidence, the current epidemiological evidence is insufficient to support the relationship between BPA and diabetes. As noted during the discussions from the 2011 NTP workshop, the findings from the in vivo studies in laboratory animals are supportive of an effect of BPA on insulin sensitivity and glucose homeostasis, in particular suggesting a phenotype of insulin resistance. However, there are inconsistencies in the animal data. Understanding the basis for this lack of consistency is an important research need. Continued analysis of the existing literature is unlikely to clarify the sources of the observed heterogeneity because of variations in experimental design such as route of administration, dose levels tested, endpoints evaluated, life stage at exposure and assessment, species, sex, and diet.
Research needs – epidemiologic studies
Since all studies have been cross-sectional, more large-scale prospective studies are needed to evaluate the relationship between BPA and diabetes. Appropriate adjustment for potential confounders is a major challenge in the evaluation of the association between BPA and diabetes. As the main sources of BPA exposure are food and beverages in epoxy-coated cans, polycarbonate drinking bottles or other BPA-related packages, populations that tend to use more processed and tinned food may have higher BPA exposure [103, 119]. Adjustment for those relevant dietary factors and for underlying socioeconomic factors is generally difficult. One factor that complicates conducting and interpreting epidemiological studies of BPA, especially cross-sectional studies, is that there is considerable within person variability in urinary BPA concentrations [120-122] and thus a single spot urine sample may result in misclassification of BPA exposure. Other challenges in BPA epidemiologic research include BPA contamination of biospecimens that may occur during sample preparation or storage, background contamination from labware and/or the analytical technique employed [123].
Conclusion
Increasing evidence supports the role of environmental chemicals in diabetes development including arsenic and other metals, persistent organic pollutants, phthalates and bisphenol A. An important advance in recent years has been the increase number of prospective studies, especially for arsenic and persistent organic pollutants. However, the number of prospective studies remains small making it difficult to reach firm conclusions. Remaining questions include the evaluation of the dose-response relationship, the role of joint exposures, and effect modification with other comorbidities and genetic variants. Exposure and outcome assessment also remain critical aspects in study design to minimize misclassification. Exposure assessments with repeated measures are especially important as such an approach would not only minimize measurement error, but also help characterize exposure patterns for environmental chemicals. Overall, the evidence is suggestive but not sufficient to infer a causal association between some environmental chemicals and diabetes outcomes.
Acknowledgments
Supported by grants from the National Institute of Environmental Health Sciences (R01ES021367, P30ES03819) and the National Heart Lung and Blood Institute (R01HL090863).
Footnotes
Conflict of Interest
Chin-Chi Kuo declares that he has no conflict of interest.
Katherine Moon declares that she has no conflict of interest.
Kristina A. Thayer declares that she has no conflict of interest.
Ana Navas-Acien has received travel/accommodations expenses covered or reimbursed from the ADA for the ADA annual meeting.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.World Health Organization (WHO) Diabetes. 2013 [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) In: National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. CDC, editor. U.S. Department of Health and Human Services; Atlanta, GA: 2011. [Google Scholar]
- 5.John B, Buse KSP, Charles F, Burant . Type 2 Diabetes Mellitus. In: Shlomo Melmed KSP, Reed Larsen P, Kronenberg Henry M., editors. Williams Textbook of Endocrinology. Elsevier Saunders; Philadelphia, PA: 2011. pp. 1371–1435. [Google Scholar]
- 6.Handelsman Y, Mechanick JI, Blonde L, et al. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan: executive summary. Endocr Pract. 2011;17:287–302. doi: 10.4158/ep.17.2.287. [DOI] [PubMed] [Google Scholar]
- 7.Conditions TNCCfC . TYPE 2 DIABETES, National clinical guideline for management in primary and secondary care (update) Royal College of Physicians; London: 2008. [PubMed] [Google Scholar]
- 8.American Diabetes Association Standards of medical care in diabetes--2013. Diabetes Care 2013. 36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grandjean P. Exposure to Environmental Chemicals as a Risk Factor for Diabetes Development. In: Bourguignon J-P, Jégou B, Kerdelhué B, et al., editors. Multi-System Endocrine Disruption. Springer; Berlin Heidelberg: 2011. pp. 91–99. [Google Scholar]
- 10•.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120:779–89. doi: 10.1289/ehp.1104597. [This review article provides a thorough and transparent review of the role of environmental chemicals in diabetes and is the starting point for this review.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Toxicology Program (NTP) NTP Workshop: Role of environmental chemicals in the development of diabetes and obesity. 2011 [Google Scholar]
- 12•.Maull EA, Ahsan H, Edwards J, et al. Evaluation of the Association between Arsenic and Diabetes: A National Toxicology Program Workshop Review. Environ Health Perspect. 2012;120:1658–70. doi: 10.1289/ehp.1104579. [This article provides a thorough review of the role of arsenic in diabetes development from epidemiological and experimental perspectives. It also covered several open research challenges that warrant future research.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Taylor KW, Novak RF, Anderson HA, et al. Evaluation of the Association between Persistent Organic Pollutants (POPs) and Diabetes in Epidemiological Studies: A National Toxicology Program Workshop Review. Environ Health Perspect. 2013 doi: 10.1289/ehp.1205502. [This article provides a comprehensive review of the role of persistent organic pollutants in diabetes development and serves as a reference point for this review.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behl M, Rao D, Aagaard K, et al. Evaluation of the association between maternal smoking, childhood obesity, and metabolic disorders: a national toxicology program workshop review. Environ Health Perspect. 2013;121:170–80. doi: 10.1289/ehp.1205404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–64. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 16.Pearson JF, Bachireddy C, Shyamprasad S, et al. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes Care. 2010;33:2196–201. doi: 10.2337/dc10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes. 2012;61:3037–45. doi: 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 19.The 2004 United States Surgeon General's Report: The Health Consequences of Smoking. N S W Public Health Bull. 2004;15:107. [PubMed] [Google Scholar]
- 20.National Toxicology Program U.S. Department of Health and Human Services, editor. Draft OHAT approach for systematic review and evidence integration for literature-based health assessments -February 2013. 2013 [Google Scholar]
- 21.Navas-Acien A, Nachman KE. Public Health Responses to Arsenic in Rice and Other Foods. JAMA Intern Med. 2013:1–2. doi: 10.1001/jamainternmed.2013.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization (WHO) Arsenic. 2012 [Google Scholar]
- 23.World Health Organization (WHO) The International Programme on Chemical Safety. (IPCS), editor. Environmental Health Criteria Document 224. Arsenic and Arsenic Compounds. 2001 [Google Scholar]
- 24.International Agency for Research on Cancer (IARC) Arsenic in Drinking Water (Group 1) Vol. 84. Lyon; 2004. Summaries and Evaluations. [Google Scholar]
- 25.Tseng CH, Tai TY, Chong CK, et al. Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: a cohort study in arseniasis-hyperendemic villages in Taiwan. Environ Health Perspect. 2000;108:847–51. doi: 10.1289/ehp.00108847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman M, Tondel M, Ahmad SA, Axelson O. Diabetes mellitus associated with arsenic exposure in Bangladesh. Am J Epidemiol. 1998;148:198–203. doi: 10.1093/oxfordjournals.aje.a009624. [DOI] [PubMed] [Google Scholar]
- 27.Coronado-Gonzalez JA, Del Razo LM, Garcia-Vargas G, et al. Inorganic arsenic exposure and type 2 diabetes mellitus in Mexico. Environ Res. 2007;104:383–9. doi: 10.1016/j.envres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Rejoinder: Arsenic exposure and prevalence of type 2 diabetes: updated findings from the National Health Nutrition and Examination Survey, 2003-2006. Epidemiology. 2009;20:816–20. doi: 10.1097/EDE.0b013e3181afef88. discussion e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300:814–22. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- 30.Navas-Acien A, Silbergeld EK, Streeter RA, et al. Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ Health Perspect. 2006;114:641–8. doi: 10.1289/ehp.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davey JC, Nomikos AP, Wungjiranirun M, et al. Arsenic as an endocrine disruptor: arsenic disrupts retinoic acid receptor-and thyroid hormone receptor-mediated gene regulation and thyroid hormone-mediated amphibian tail metamorphosis. Environ Health Perspect. 2008;116:165–72. doi: 10.1289/ehp.10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davey JC, Bodwell JE, Gosse JA, Hamilton JW. Arsenic as an endocrine disruptor: effects of arsenic on estrogen receptor-mediated gene expression in vivo and in cell culture. Toxicol Sci. 2007;98:75–86. doi: 10.1093/toxsci/kfm013. [DOI] [PubMed] [Google Scholar]
- 33.Douillet C, Currier J, Saunders J, et al. Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol Appl Pharmacol. 2013;267:11–5. doi: 10.1016/j.taap.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tseng CH. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol Appl Pharmacol. 2004;197:67–83. doi: 10.1016/j.taap.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Diaz-Villasenor A, Burns AL, Hiriart M, et al. Arsenic-induced alteration in the expression of genes related to type 2 diabetes mellitus. Toxicol Appl Pharmacol. 2007;225:123–33. doi: 10.1016/j.taap.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Druwe IL, Vaillancourt RR. Influence of arsenate and arsenite on signal transduction pathways: an update. Arch Toxicol. 2010;84:585–96. doi: 10.1007/s00204-010-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodwell JE, Gosse JA, Nomikos AP, Hamilton JW. Arsenic disruption of steroid receptor gene activation: Complex dose-response effects are shared by several steroid receptors. Chem Res Toxicol. 2006;19:1619–29. doi: 10.1021/tx060122q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fry RC, Navasumrit P, Valiathan C, et al. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS Genet. 2007;3:e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smeester L, Rager JE, Bailey KA, et al. Epigenetic changes in individuals with arsenicosis. Chem Res Toxicol. 2011;24:165–7. doi: 10.1021/tx1004419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y, Lee BK. Association between urinary arsenic and diabetes mellitus in the Korean general population according to KNHANES 2008. Sci Total Environ. 2011;409:4054–62. doi: 10.1016/j.scitotenv.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Islam R, Khan I, Hassan SN, et al. Association between type 2 diabetes and chronic arsenic exposure in drinking water: a cross sectional study in Bangladesh. Environ Health. 2012;11:38. doi: 10.1186/1476-069X-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makris KC, Christophi CA, Paisi M, Ettinger AS. A preliminary assessment of low level arsenic exposure and diabetes mellitus in Cyprus. BMC Public Health. 2012;12:334. doi: 10.1186/1471-2458-12-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gribble MO, Howard BV, Umans JG, et al. Arsenic exposure, diabetes prevalence, and diabetes control in the Strong Heart Study. Am J Epidemiol. 2012;176:865–74. doi: 10.1093/aje/kws153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.James KA, Marshall JA, Hokanson JE, et al. A case-cohort study examining lifetime exposure to inorganic arsenic in drinking water and diabetes mellitus. Environ Res. 2013;123:33–8. doi: 10.1016/j.envres.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Kim NH, Mason CC, Nelson RG, et al. Arsenic Exposure and Incidence of Type 2 Diabetes in Southwestern American Indians. Am J Epidemiol. 2013 doi: 10.1093/aje/kws329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Li B, Xi S, et al. Prolonged environmental exposure of arsenic through drinking water on the risk of hypertension and type 2 diabetes. Environ Sci Pollut Res Int. 2013 doi: 10.1007/s11356-013-1768-9. [DOI] [PubMed] [Google Scholar]
- 47.Del Razo LM, Garcia-Vagras GG, Valenzuela OL, et al. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapan and Lagunera Regions in Mexico. Environmental Health. 2011;10:73. doi: 10.1186/1476-069X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization (WHO) Exposure to Cadmium: A major public health concern. Switzerland: 2010. [Google Scholar]
- 49.World Health Organization (WHO) Mercury and health. 2012 [Google Scholar]
- 50.Chen YW, Yang CY, Huang CF, et al. Heavy metals, islet function and diabetes development. Islets. 2009;1:169–76. doi: 10.4161/isl.1.3.9262. [DOI] [PubMed] [Google Scholar]
- 51.Chen YW, Huang CF, Yang CY, et al. Inorganic mercury causes pancreatic beta-cell death via the oxidative stress-induced apoptotic and necrotic pathways. Toxicol Appl Pharmacol. 2010;243:323–31. doi: 10.1016/j.taap.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 52.Edwards JR, Prozialeck WC. Cadmium, diabetes and chronic kidney disease. Toxicol Appl Pharmacol. 2009;238:289–93. doi: 10.1016/j.taap.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz GG, Il'yasova D, Ivanova A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care. 2003;26:468–70. doi: 10.2337/diacare.26.2.468. [DOI] [PubMed] [Google Scholar]
- 54.Swaddiwudhipong W, Mahasakpan P, Limpatanachote P, Krintratun S. Correlations of urinary cadmium with hypertension and diabetes in persons living in cadmium-contaminated villages in northwestern Thailand: A population study. Environ Res. 2010;110:612–6. doi: 10.1016/j.envres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Barregard L, Bergstrom G, Fagerberg B. Cadmium exposure in relation to insulin production, insulin sensitivity and type 2 diabetes: a cross-sectional and prospective study in women. Environ Res. 2013;121:104–9. doi: 10.1016/j.envres.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Moon SS. Association of lead, mercury and cadmium with diabetes in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009-2010. Diabet Med. 2013;30:e143–8. doi: 10.1111/dme.12103. [DOI] [PubMed] [Google Scholar]
- 57•.He K, Xun P, Liu K, et al. Mercury exposure in young adulthood and incidence of diabetes later in life: the CARDIA Trace Element Study. Diabetes Care. 2013;36:1584–9. doi: 10.2337/dc12-1842. [This large prospective study with a long-term follow-up period was conducted in young adults. Futhermore, the authors also evaluated insulin resistance and pancreatic -cell function longitudinally to explore potential pathophysiological mechanisms linking mercury exposure to diabetes development.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Selin NE, Sunderland EM, Knightes CD, Mason RP. Sources of mercury exposure for U.S. seafood consumers: implications for policy. Environ Health Perspect. 2010;118:137–43. doi: 10.1289/ehp.0900811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xun P, He K. Fish Consumption and Incidence of Diabetes: meta-analysis of data from 438,000 individuals in 12 independent prospective cohorts with an average 11-year follow-up. Diabetes Care. 2012;35:930–8. doi: 10.2337/dc11-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lind L, Lind PM. Can persistent organic pollutants and plastic-associated chemicals cause cardiovascular disease? J Intern Med. 2012;271:537–53. doi: 10.1111/j.1365-2796.2012.02536.x. [DOI] [PubMed] [Google Scholar]
- 61.Porta M, Puigdomenech E, Ballester F, et al. Monitoring concentrations of persistent organic pollutants in the general population: the international experience. Environ Int. 2008;34:546–61. doi: 10.1016/j.envint.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Gasull M, Pumarega J, Tellez-Plaza M, et al. Blood concentrations of persistent organic pollutants and prediabetes and diabetes in the general population of Catalonia. Environ Sci Technol. 2012;46:7799–810. doi: 10.1021/es300712g. [DOI] [PubMed] [Google Scholar]
- 63.European Commission. Persistent Organic Pollutants. 2012 [Google Scholar]
- 64.World Health Organization (WHO) Persistent organic pollutants (POPs). Children's Health and the Environment; WHO Training Package for the Health Sector. 2008 [Google Scholar]
- 65.Henriksen GL, Ketchum NS, Michalek JE, Swaby JA. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology. 1997;8:252–8. doi: 10.1097/00001648-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 66.Taylor KW, Novak RF, Anderson HA, et al. Evaluation of the Association between Persistent Organic Pollutants (POPs) and Diabetes in Epidemiological Studies: A National Toxicology Program Workshop Review. Environ Health Perspect. 2013;121:774–83. doi: 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanaka T, Morita A, Kato M, et al. Congener-specific polychlorinated biphenyls and the prevalence of diabetes in the Saku Control Obesity Program (SCOP). Endocr J. 2011;58:589–96. doi: 10.1507/endocrj.k10e-361. [DOI] [PubMed] [Google Scholar]
- 68.Nakamoto M, Arisawa K, Uemura H, et al. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health. 2012 doi: 10.1007/s00420-012-0819-8. [DOI] [PubMed] [Google Scholar]
- 69.Persky V, Piorkowski J, Turyk M, et al. Associations of polychlorinated biphenyl exposure and endogenous hormones with diabetes in post-menopausal women previously employed at a capacitor manufacturing plant. Environ Res. 2011;111:817–24. doi: 10.1016/j.envres.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 70.Everett CJ, Thompson OM. Associations of dioxins, furans and dioxin-like PCBs with diabetes and pre-diabetes: is the toxic equivalency approach useful? Environ Res. 2012;118:107–11. doi: 10.1016/j.envres.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 71.Persky V, Piorkowski J, Turyk M, et al. Polychlorinated biphenyl exposure, diabetes and endogenous hormones: a cross-sectional study in men previously employed at a capacitor manufacturing plant. Environ Health. 2012;11:57. doi: 10.1186/1476-069X-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silverstone AE, Rosenbaum PF, Weinstock RS, et al. Polychlorinated biphenyl (PCB) exposure and diabetes: results from the Anniston Community Health Survey. Environ Health Perspect. 2012;120:727–32. doi: 10.1289/ehp.1104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73•.Wu H, Bertrand KA, Choi AL, et al. Persistent organic pollutants and type 2 diabetes: a prospective analysis in the nurses' health study and meta-analysis. Environ Health Perspect. 2013;121:153–61. doi: 10.1289/ehp.1205248. [This study combined two nested case-control studies to evaluate the association between persistent organic pollutants (POPs) exposure and incident diabetes with an 18-year follow up. The authors also conducted a meta-analysis to summarize recent prospective evidence regarding POPs and incident diabetes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee DH, Lind PM, Jacobs DR, Jr, et al. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care. 2011;34:1778–84. doi: 10.2337/dc10-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Airaksinen R, Rantakokko P, Eriksson JG, et al. Association between type 2 diabetes and exposure to persistent organic pollutants. Diabetes Care. 2011;34:1972–9. doi: 10.2337/dc10-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arrebola JP, Pumarega J, Gasull M, et al. Adipose tissue concentrations of persistent organic pollutants and prevalence of type 2 diabetes in adults from Southern Spain. Environ Res. 2013;122:31–7. doi: 10.1016/j.envres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 77.Kerger BD, Scott PK, Pavuk M, et al. Re-analysis of Ranch Hand study supports reverse causation hypothesis between dioxin and diabetes. Crit Rev Toxicol. 2012;42:669–87. doi: 10.3109/10408444.2012.694095. [DOI] [PubMed] [Google Scholar]
- 78.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee DH, Steffes MW, Sjodin A, et al. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PloS one. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turyk M, Anderson H, Knobeloch L, et al. Organochlorine exposure and incidence of diabetes in a cohort of Great Lakes sport fish consumers. Environ Health Perspect. 2009;117:1076–1082. doi: 10.1289/ehp.0800281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rignell-Hydbom A, Lidfeldt J, Kiviranta H, et al. Exposure to p,p'-DDE: a risk factor for type 2 diabetes. PLoS One. 2009;4:e7503. doi: 10.1371/journal.pone.0007503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang SL, Tsai PC, Yang CY, Leon Guo Y. Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the Yucheng cohort. Diabetes Care. 2008;31:1574–9. doi: 10.2337/dc07-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vasiliu O, Cameron L, Gardiner J, et al. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiol. 2006;17:352–9. doi: 10.1097/01.ede.0000220553.84350.c5. [DOI] [PubMed] [Google Scholar]
- 84.AFHS [16 September 2012];An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides: 2002 Follow-up Examination Results May 2002 to March 2005. 2005 http://www.dtic.mil/cgibin/GetTRDoc?Location=U2&doc=GetTRDoc.pdf&AD=ADA438835.
- 85.Kang HK, Dalager NA, Needham LL, et al. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med. 2006;49:875–84. doi: 10.1002/ajim.20385. [DOI] [PubMed] [Google Scholar]
- 86.Michalek JE, Pavuk M. Diabetes and cancer in veterans of Operation Ranch Hand after adjustment for calendar period, days of spraying, and time spent in Southeast Asia. J Occup Environ Med. 2008;50:330–40. doi: 10.1097/JOM.0b013e31815f889b. [DOI] [PubMed] [Google Scholar]
- 87.Steenland K, Calvert G, Ketchum N, Michalek J. Dioxin and diabetes mellitus: an analysis of the combined NIOSH and Ranch Hand data. Occup Environ Med. 2001;58:641–8. doi: 10.1136/oem.58.10.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.James-Todd T, Stahlhut R, Meeker JD, et al. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001-2008. Environ Health Perspect. 2012;120:1307–13. doi: 10.1289/ehp.1104717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hauser R, Meeker JD, Park S, et al. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112:1734–40. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stahlhut RW, van Wijngaarden E, Dye TD, et al. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115:876–82. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Casals-Casas C, Feige JN, Desvergne B. Interference of pollutants with PPARs: endocrine disruption meets metabolism. Int J Obes (Lond) 2008;32(Suppl 6):S53–61. doi: 10.1038/ijo.2008.207. [DOI] [PubMed] [Google Scholar]
- 92.Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci. 2003;74:297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 93.Hectors TL, Vanparys C, Pereira-Fernandes A, et al. Evaluation of the INS-1 832/13 cell line as a beta-cell based screening system to assess pollutant effects on beta-cell function. PLoS One. 2013;8:e60030. doi: 10.1371/journal.pone.0060030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feige JN, Gelman L, Rossi D, et al. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem. 2007;282:19152–66. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- 96.Latini G, Scoditti E, Verrotti A, et al. Peroxisome proliferator-activated receptors as mediators of phthalate-induced effects in the male and female reproductive tract: epidemiological and experimental evidence. PPAR Res. 2008;2008:359267. doi: 10.1155/2008/359267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lind PM, Zethelius B, Lind L. Circulating levels of phthalate metabolites are associated with prevalent diabetes in the elderly. Diabetes Care. 2012;35:1519–24. doi: 10.2337/dc11-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Svensson K, Hernandez-Ramirez RU, Burguete-Garcia A, et al. Phthalate exposure associated with self-reported diabetes among Mexican women. Environ Res. 2011;111:792–6. doi: 10.1016/j.envres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ Health Perspect. 2004;112:331–8. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parlett LE, Calafat AM, Swan SH. Women's exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol. 2013;23:197–206. doi: 10.1038/jes.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect. 2002;110:515–8. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Global Agricultural Information Network (GAIN) French National Assembly supports BPA Ban Bill hastens implementation. USDA Foreign Agricultural Service; 2012. [Google Scholar]
- 103.Joint food and agriculture organization of the united nations and world health organization (FAO/WHO) Joint food and agriculture organization of the united nations and world health organization (FAO/WHO) expert meeting to review toxicological and health aspects of bisphenol a: Final report, including report of stakeholder meeting on bisphenol A, 1-5 November 2010. Ottawa, Canada: 2011. [Google Scholar]
- 104.Vandenberg LN, Maffini MV, Sonnenschein C, et al. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vandenberg LN, Chahoud I, Heindel JJ, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–70. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Calafat AM, Ye X, Wong LY, et al. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lakind JS, Naiman DQ. Daily intake of bisphenol A and potential sources of exposure: 2005-2006 National Health and Nutrition Examination Survey. J Expo Sci Environ Epidemiol. 2011;21:272–9. doi: 10.1038/jes.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Calafat AM, Weuve J, Ye X, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009;117:639–44. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Biedermann S, Tschudin P, Grob K. Transfer of bisphenol A from thermal printer paper to the skin. Anal Bioanal Chem. 2010;398:571–6. doi: 10.1007/s00216-010-3936-9. [DOI] [PubMed] [Google Scholar]
- 110.Hugo ER, Brandebourg TD, Woo JG, et al. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116:1642–7. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alonso-Magdalena P, Morimoto S, Ripoll C, et al. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114:106–12. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ning G, Bi Y, Wang T, et al. Relationship of urinary bisphenol A concentration to risk for prevalent type 2 diabetes in Chinese adults: a cross-sectional analysis. Ann Intern Med. 2011;155:368–74. doi: 10.7326/0003-4819-155-6-201109200-00005. [DOI] [PubMed] [Google Scholar]
- 113.Shankar A, Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab. 2011;96:3822–6. doi: 10.1210/jc.2011-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Silver MK, O'Neill MS, Sowers MR, Park SK. Urinary bisphenol A and type-2 diabetes in U.S. adults: data from NHANES 2003-2008. PLoS One. 2011;6:e26868. doi: 10.1371/journal.pone.0026868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim K, Park H. Association between urinary concentrations of bisphenol A and type 2 diabetes in Korean adults: A population-based cross-sectional study. Int J Hyg Environ Health. 2013;216:467–71. doi: 10.1016/j.ijheh.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 116.Kim K, Park H. Association between urinary concentrations of bisphenol A and type 2 diabetes in Korean adults: a population-based cross-sectional study. Int J Hyg Environ Health. 2012;216:467–71. doi: 10.1016/j.ijheh.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 117.Lang IA, Galloway TS, Scarlett A, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–10. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 118.Wang T, Li M, Chen B, et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:E223–7. doi: 10.1210/jc.2011-1989. [DOI] [PubMed] [Google Scholar]
- 119.Magliano DJ, Lyons JG. Bisphenol A and diabetes, insulin resistance, cardiovascular disease and obesity: controversy in a (plastic) cup? J Clin Endocrinol Metab. 2013;98:502–4. doi: 10.1210/jc.2012-3058. [DOI] [PubMed] [Google Scholar]
- 120.Braun JM, Kalkbrenner AE, Calafat AM, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119:131–7. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Braun JM, Smith KW, Williams PL, et al. Variability of Urinary Phthalate Metabolite and Bisphenol A Concentrations before and during Pregnancy. Environmental health perspectives. 2012 doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mahalingaiah S, Meeker JD, Pearson KR, et al. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect. 2008;116:173–8. doi: 10.1289/ehp.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ye X, Zhou X, Hennings R, et al. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: an elusive laboratory challenge. Environ Health Perspect. 2013;121:283–6. doi: 10.1289/ehp.1206093. [DOI] [PMC free article] [PubMed] [Google Scholar]