Abstract
Vaccination reduces transmission of pathogens directly, by preventing individual infections, and indirectly, by reducing the probability of contact between infected individuals and susceptible ones. The potential combined impact of future dengue vaccines can be estimated using mathematical models of transmission. However, there is considerable uncertainty in the structure of models that accurately represent dengue transmission dynamics. Here, we review models that could be used to assess the impact of future dengue immunization programmes. We also review approaches that have been used to validate and parameterize models. A key parameter of all approaches is the basic reproduction number, R0, which can be used to determine the critical vaccination fraction to eliminate transmission. We review several methods that have been used to estimate this quantity. Finally, we discuss the characteristics of dengue vaccines that must be estimated to accurately assess their potential impact on dengue virus transmission.
Keywords: dengue, vaccine, theoretical model, review
1. Introduction
The goal of vaccination programmes is to protect entire populations against disease, not just individual vaccine recipients. The effectiveness of future dengue vaccines as public health tools will depend on the sum of the direct protection of vaccine recipients and the indirect protection afforded populations by reducing the number of individuals in a population that can transmit a pathogen and thus providing some protection even to unvaccinated individuals. If the level of immunity at the population scale is high enough, efficient transmission is no longer possible. To assess the potential impact of a vaccine, estimation of this threshold level of immunity, the critical vaccination fraction, is essential.
The critical vaccination threshold can be estimated using theoretical models of transmission and empirical data on transmission dynamics. It is closely related to the basic reproduction number (R0), the expected number of new cases caused by a single infectious individual in a completely susceptible population. R0 is an important indicator of transmissibility: if exceeding one, cases increase and an epidemic may occur and, if less than one, each case will not replace itself and the pathogen will eventually go extinct. R0 refers explicitly to this rate of transmission in a completely susceptible population. As infections occur, immunity accrues and transmission is reduced by a factor of 1-p where p is the proportion of population with acquired immunity. For vaccination programmes to be effective on the population scale, the goal is vaccinate a sufficient proportion of the population, the critical vaccination threshold (pc), to drive the reproductive rate to less than one:
| [1] |
This expression succinctly relates the infectiousness of a pathogen to the public health target of vaccination and can be used to guide immunization strategies. Theoretical predictions based on this relationship have been validated with empirical estimates for a number of vaccine-preventable diseases [1, 2].
Estimation of the basic reproduction number requires two elements: a transmission model that can be used to relate observations of transmission dynamics to the theoretical quantity R0 and empirical data to parameterize the components of the model. In the case of dengue, R0 estimation is complicated by the interaction of the 4 distinct serotypes of dengue virus. Infection by a particular serotype leads to decades of immunity to that serotype (though counter-examples have been observed). Infection with a particular serotype also leads to temporary cross-protection against infection by the others [3]. When cross-protective immunity wanes, the same formerly protective antibodies can lead to either reduced rates of infection with other serotypes or to enhanced infection with the potential for more severe disease and greater transmissibility[4-7]. Either of these antibody mechanisms can have important impacts on transmission dynamics as they potentially impact susceptibility or transmissibility of dengue in those with pre-existing immunity. However, other mechanisms have been offered to explain the observation that those with pre-existing immunity experienced increased rates of severe disease upon subsequent infection, including the immunopathogenesis through interaction with cellular immune responses, cross-reactive T cells and other immune cascades [8, 9]. Any dengue virus transmission model or estimate of the basic reproductive number must consider the impact of immune-mediated interactions between the dengue serotypes. Below we present a review of dengue virus transmission models that have incorporated immune interactions and discuss the importance of model design for the estimation of R0 and vaccine effectiveness. Finally, we outline the critical characteristics of vaccines that must be known to predict their impact on transmission dynamics.
2. Dengue transmission models
The basic form of most published dengue virus transmission models is the compartmental model, a stalwart of infectious disease modeling. In these models, people are characterized by their infection state as either susceptible, infectious, or having some type of immunity. Figure 1a shows the starting point for models of dengue and for much of the work done in infectious disease modeling, the Susceptible-Infectious-Recovered (SIR) model. One challenge in crafting a model of pathogen transmission is to define the relevant states of infection that characterize the population. Figure 1b-e presents four structures that have been used to model dengue virus transmission. Each box represents a type of individual defined by their infection status. The models themselves are systems of equations that describe the rates at which people transition between these states. The transitions represent events such as infection, progression to illness, recovery, birth, or death. There are many different ways to classify different states and different transition rates. The models identified by this review (Table 1) start from a similar basic structure, but vary significantly in the way they extend it, particularly relative to their incorporation of the vector population, permanent cross-protection or enhancement of infection, and transient cross-protection.
Figure 1.

Schematics of multiple dengue models. a. Basic Susceptible-Infectious-Recovered (SIR) model that forms the basis of most infectious disease modeling. b. An expansion of the SIR model to include primary infections, secondary infections, and individuals that have encountered only one serotype, the primary recovered individuals (1°R). The red arrow distinguishes the rate at which individuals with immunity to a single serotype acquire second infections from the rate at which people with no immunity acquire their first. c. Model including a short-term cross-immune class (1°CI). d. Model assuming that secondary infections have higher rates of mortality. e. Model explicitly including a vector population.
Table 1. Dengue transmission models.
| citation | study | Year | Number of serotypes* | cross-protection | enhancement | vector |
|---|---|---|---|---|---|---|
| [19] | Fischer | 1970 | 3 | susceptibility | disease | no |
| [31] | Newton | 1992 | 1 | NA | NA | yes |
| [14] | Focks | 1995 | 4 | susceptibility | disease | yes |
| [52] | Feng | 1997 | 2 | susceptibility | susceptibility | yes |
| [21, 53, 54] | Esteva | 1998, 1999, 2000 | 1 | NA | NA | yes |
| [20] | Ferguson | 1999 | 2 | none | infectiousness | no |
| [55] | Ferguson | 1999 | 4 | susceptibility | susceptibility | no |
| [56-58] | Pongsumpun | 2001, 2002, 2003 | 1 | NA | NA | yes |
| [34, 35] | Massad | 2001, 2003 | 1 | NA | NA | yes |
| [28] | Bartley | 2002 | 2 | susceptibility | infectiousness | yes |
| [59] | Derouich | 2003 | 2 | susceptibility | none | yes |
| [60] | Esteva | 2003 | 2 | susceptibility | susceptibility | yes |
| [61] | Kawaguchi | 2003 | 2 | susceptibility | disease | No |
| [39] | Luz | 2003 | 1 | NA | NA | Yes |
| [41] | Cummings | 2004 | 4 | susceptibility | infectiousness | No |
| [15] | Cummings | 2005 | 4 | none | infectiousness | No |
| [24] | Schwartz | 2005 | 4 | none | infectiousness | No |
| [32, 62] | Favier | 2005, 2006 | 1 | NA | NA | Yes |
| [27] | Adams | 2006 | 2 | susceptibility | susceptibility, infectiousness, disease | No |
| [12] | Adams | 2006 | 2 | susceptibility | susceptibility | No |
| [13] | Coutinho | 2006 | 1 | NA | NA | Yes |
| [63] | Tran | 2006 | 1 | NA | NA | Yes |
| [11] | Wearing | 2006 | 4 | susceptibility | susceptibility, infectiousness | Yes |
| [64] | Atkinson | 2007 | 1 | NA | NA | Yes |
| [65] | Billings | 2007 | 4 | none | infectiousness | No |
| [36] | Chowell | 2007 | 1 | NA | NA | Yes |
| [66] | Burattini | 2008 | 1 | NA | NA | Yes |
| [10, 40] | Nagao | 2008 | 4 | susceptibility, disease | susceptibility, infectiousness, disease | No |
| [40] | Billings | 2008 | 2 | NA | susceptibility, infectiousness | No |
| [67] | Bianco | 2009 | 4 | susceptibility | infectiousness | No |
| [68] | Adams | 2009 | 1 | NA | NA | Yes |
| [69] | Chikaki | 2009 | 4 | susceptibility | infectiousness | Yes |
| [70] | Medlock | 2009 | 1 | none | disease | Yes |
| [22] | Recker | 2009 | 4 | none | susceptibility, infectiousness | No |
| [26] | Wikramaratna | 2010 | 4 | none | infectiousness | No |
| [71] | Otero | 2010 | 1 | NA | NA | Yes |
denotes the total number of serotypes modeled
2.1 Vector population
The rate of infection of susceptibles (the force of infection, often denoted as λ) is regulated by the number of infectious individuals and the transmission coefficient (β), the rate at which infectious contacts are made between humans through contact with vectors. Most commonly, models combine the underlying process of vector contact, the dynamics of infection in the vector and subsequent transmission to other humans into one aggregate rate, representing the mean vector-mediated rate at which humans infect other humans. Other models (as in Figure 1e) treat the vector biting process more explicitly, including, for example, separate contact rates for transmission from humans to vectors and vice versa.
Vector population dynamics and behavior are important determinants of transmission. Both behavior and abundance are spatially and temporally heterogeneous and are essential to the transmission dynamics of dengue viruses. Seasonal fluctuations in disease, for example, are likely attributable to the influence of seasonal weather changes on the vector population. In models, this component is often omitted, sometimes incorporated as simple sine curves [10-13] and, rarely, modeled explicitly [14]. The importance of these differences and other assumptions about the vector partly depends on the objective of the model. Models in the literature differ in opinion about what level of detail in the representation of the vector population is necessary to model the transmission dynamics of dengue viruses and to potentially assess the impact of vaccines [10, 11, 15]. Many outcomes may be modeled reasonably without explicitly accounting for vector population dynamics, but for others, such as those incorporating vector control efforts, it is a critical component.
2.2 Long-term immunity
The presence of related serotypes complicates models of dengue virus transmission substantially. Exposure to a single serotype is generally assumed to stimulate lifelong immunity to that serotype [16]. Though there have been observations of counterexamples, most evidence suggests immunity lasts decades [17]. The effect of immunity to one serotype on infections of the other serotypes is less clear. The null hypothesis is that there is no influence at all, but there is substantial evidence that more severe disease, Dengue Hemorrhagic Fever (DHF) in particular, is associated with previous heterologous infection [9]. This interaction potentially affects long-term dynamics of incidence as the population level immunity to all serotypes influences the incidence of each serotype. As such, many models have been developed to explore the potential role of enhancement on dengue dynamics. This is accomplished by increasing the number of compartments to include unique compartments for each serotype and distinguish primary and secondary infections, allowing different parameterization for the unique transmission characteristics corresponding to each infection. Though there is less empirical evidence to support it, similar models have been used to investigate the potential role of long-term cross protection [12]. The role of short-term immunity is addressed in Section 2.3.
Enhancement of secondary infections may be associated with enhancement of disease, susceptibility, or infectiousness, each with its own impact on dynamics. Immunity acquired from prior infection may lead to more severe disease in secondary infections. Alone, enhancement of disease would have little effect on dynamics, but would influence the relationship between models and observed data, as severe cases are more likely to be detected by surveillance systems. Enhancement of susceptibility is the hypothesis that cross-reactive antibodies resulting from previous exposure increase the likelihood of infection when an individual is exposed to a second serotype. A specific mechanism has not been articulated for this hypothesis to our knowledge, however it is conceivable that immune enhancement reduces the infectious dose for those with pre-existing infections via increased uptake of antibody-virus complexes compared to free virus. Secondary infections may also result in higher viremia resulting in enhanced infectiousness of the human to the vector [18].
The earliest mathematical models of dengue virus transmission assumed enhancement of disease, but did not test the validity of that assumption [14, 19]. Later models, however, showed that enhancement of infectiousness can lead to persistence of more than one serotype and create complex inter-annual cyclic behavior of dengue incidence as has been observed in endemic areas [20]. Indeed, if there is any interaction between serotypes, some sort of enhancement is necessary for coexistence to occur: protection from secondary infections leads to competitive exclusion while enhancement leads to coexistence. This has been demonstrated several times in dengue virus transmission models for both enhancement of susceptibility [12, 21] and infectiousness [12, 15, 20]. Asynchronous multi-year cycling of serotypes is a common feature of endemic dengue dynamics and can be generated under certain types of enhancement [12, 15, 20]. Several authors have suggested that the large oscillations observed in simulations incorporating enhancement of susceptibility or infectiousness are inconsistent with observed data [10-12] due to the magnitude of oscillations in incidence observed in these models. The inclusion of enhancement of both susceptibility and transmissibility in a single model has been said to reduce the magnitude of oscillations and the strength of enhancement needed to induce oscillations [22].
Another study, of note for its direct use of empirical data to estimate parameters, used mathematical models to estimate the force of infection in an age-stratified cohort of Thai children [23]. Multiple assumptions were made in different model structures, but the best fitting modeling included enhancement of susceptibility.
Third or fourth infections add further complexity. The observation that tertiary and quaternary clinically apparent infections are rare [17] and neutralizing antibody titers to all four serotypes tend to be high following two infections has lead many modelers to assume that individuals are immune to all serotypes following infection with two serotypes [15, 20, 24, 25]. However, several authors have explored the inclusion of tertiary and quaternary infections in both simulations and analysis of data to estimate reproductive numbers [10, 26]. Simulations have shown that qualitative behavior of models that include tertiary and quaternary infections are similar to those that do not. A more important effect of their occurrence is that they may lead to biased reproductive number estimates.
2.3 Short-term immunity
Empirical data suggest that people infected by dengue viruses are protected from infection by another serotype for 2—9 months post-infection [4]. However, these studies included a limited number of individuals and used only viruses from two serotypes. The earliest dengue virus transmission model allowed for this temporary cross-protection by assuming that an individual could only be infected once per transmission season [19]. Others have incorporated cross-protection more explicitly in the models by adding a compartment of temporary, post-infection, cross-protection (such as in Figure 1c) [11, 14, 27, 28]. Some of these models used the combination of this transient cross-protection and subsequent enhancement of infectiousness or susceptibility to demonstrate multi-strain transmission patterns consistent with observed data [11, 27].
More recently, Nagao and Koelle [10] considered models that looked at other forms of cross-immunity. They hypothesized that short-term cross protection may protect an individual from disease but not infection; for a short period after primary infection, they may acquire immunity to heterologous serotypes without disease. The hypothesis is predicated on experimental data from primates showing that cross-protected individuals can seroconvert on exposure to heterologous virus. They use their model to replicate changing patterns in the average age of infection in Thailand, but recent work shows that those changes can be accounted for by demographic change alone [29]. The hypothesis of short-term clinical cross-protection has yet to be tested in a setting where its effects could be more directly assessed.
2.4 Model parameterization
Aside from structural components, there are also many parameters that must be estimated to build realistic dengue virus transmission models. All of the potential immune and enhancement effects described above must be characterized numerically in terms of their contribution to the force of infection. Key components, such as the infectiousness of individuals with asymptomatic infections, remain unresolved. More empirical data is needed to estimate the dynamics of the natural history of infection in vectors and humans to add to existing estimates [30]. Assumptions such as a constant rate of progression from the incubation stage to the infectious stage are often not valid and can lead to erroneous predictions [11].
2.5 Confronting current models with data
Current modeling efforts have relied heavily on long-term surveillance data to compare the performance of competing modeling frameworks. Model simulations are compared to this empirical data using metrics such as the frequency and amplitude of serotype-specific oscillations or the correlation and phase difference between each serotype. The small number of time series used for this purpose makes it easier to compare models in principal, but may reduce generalizability. There are also problems with resolving differences between models as competing models may explain the data equally well. Furthermore, these time series lack fine-scale human data that can better capture the complex effects of immunity discussed above.
2.6 Overall considerations
While the underlying mechanisms of serotype interaction remain unclear, the observed co-existence and complex asynchronous cycling appear to be evidence of some type of interaction. Though the effects of enhanced susceptibility and infectiousness are not identical, they produce similar outcomes as described in the various dengue virus transmission models. The observation that significant enhancement of transmission associated with secondary infections leads to very large oscillations in incidence seems to indicate that only subtle degrees of enhancement are consistent with observed data. Meanwhile, transient cross-protection, either clinical or completely protective may be critical to fitting observed data.
In the context of vaccine development, consideration of multiple serotypes is critical. Enhancement of susceptibility, infectiousness, and disease are all relevant to vaccine development as each may lead to greater disease when partial immunity is present in the population. A further complication of these interactions is that they confound the determination of R0 (Section 3) and thus the critical vaccination fraction, pc (Section 4).
3. R0 estimation
Multiple methods can be used to estimate R0, the basis for estimates of the critical vaccination fraction. Table 2 present estimates of the basic reproduction number of dengue found in the published literature. These estimates vary widely. Differences may be attributable to geographic or temporal variation in transmission or differences in the methods used for estimation. Estimates of R0 for dengue have been calculated using four types of data: 1) the rates of constituent processes in an individual transmission cycle, 2) the initial growth rates of single epidemics, 3) the final size of single outbreaks measured by either serology or clinically diagnosed cases, and 4) the age-specific rates of prevalence from either serological or case data. For each type of data, a theoretical model is fit using an empirical measure of epidemic growth. Below, we review the estimates appearing in the literature, the methods used to create them, and their potential limitations.
Table 2. R0 estimates.
| Citation | study | Number of serotypes* | cross-protection | enhancement | Median (range)* | data origin | method | |
|---|---|---|---|---|---|---|---|---|
| [38] | Koopman | 1991 | 1 | No | No | 1.3 (0, 2.4) | Mexico | final epidemic size |
| [31] | Newton | 1992 | 1 | No | No | 1.9 | hypothetical | parameterization |
| [33] | Marques | 1994 | 1 | No | No | 2.0 (1.6, 2.5) | Brazil | epidemic growth curve |
| [23] | Ferguson | 1999 | 4 | susceptibility | susceptibility | 4.8 (1.4, 8.5) | Thailand | age stratified seroprevalence |
| [35] | Massad | 2001 | 1 | No | No | 6.3 (3.6, 12.9) | Brazil | epidemic growth curve |
| [34] | Massad | 2003 | 1 | No | No | 4.8 (2.7, 11.6) | Brazil | epidemic growth curve |
| [32] | Favier | 2006 | 1 | None | No | 3.0 (2.0, 103) | Brazil | epidemic growth curve |
| [36] | Chowell | 2007 | 1 | No | No | 2.0 (0.5, 3.3) 2.0 95% CI (1.75, 2.23) | Mexico | epidemic growth curve |
| [72] | Coehlo | 2008 | 1 | No | No | 3.69 (2.26, 11.39) | Brazil | epidemic growth curve |
| [10] | Nagao | 2008 | 4 | Susceptibility, disease | susceptibility, infectiousness, disease | (4, 12) | Thailand | age distribution of cases |
| [73] | Degallier | 2009 | 1 | No | No | 5.17 (1.56, 22.89) | Brazil | fitting a SIR model to cases |
| [29] | Cummings | 2009 | 4 | susceptibility | susceptibility | (5.2, 6.7) | Thailand | age distribution of cases |
range is shown in parentheses. 95% CI is shown where indicated.
3.1 Constituent processes
A single transmission event is the result of numerous biological processes. One approach to estimating transmission rates is to break the overall process into constituent processes that can be empirically measured. For example, dengue transmission may be described as the product of the number of mosquitoes infected per infectious person and the number of people infected per infectious mosquito,
where m is the number of female mosquitoes per person, a is their biting rate, βHV and βHV are the probabilities of human-to-vector and vector-to-human transmission given an infectious bite, tIH and tIV are the durations of infectiousness in humans and vectors, and tLV is the average vector lifespan. Empirical estimates of each parameter allow direct calculation of R0 [31]. Unfortunately, many of these parameters are difficult to estimate, some are highly variable in both time and space, and the model formulation itself carries implicit assumptions.
3.2 Initial epidemic growth rate
During the early phase of an epidemic in a susceptible population, infections accumulate exponentially as a function of the force of infection, λ, which is directly related to R0. Thus, empirical data on epidemic growth can be used to estimate R0 [32-36]. These models principally differ in their treatment of the incubation and infectious periods, both critical factors to linking epidemic growth rates to R0 [11, 37].
Furthermore, all of the estimates using epidemic growth rates assume that the empirical data describe transmission in the absence of immunity in the host population. However, most of the outbreaks studied for this purpose have been in endemic areas where there is likely significant immunity within the population due to previous transmission [32-36].
The models may be refined to estimate the force of infection in endemic contexts, but require assumptions about serotype interactions and estimation of the level of underlying immunity. Assessment of underlying immunity in this context is particularly difficult as diagnostics distinguishing past and present infecting serotypes based on antibody responses is notoriously difficult for dengue viruses.
3.3 Final outbreak size
R0 can also be estimated by fitting the total number of people infected in a single epidemic with theoretical predictions from transmission models where R0 is a parameter [38]. Similar to modeling the force of infection, however, this implicitly assumes a naïve population, again not the case for the populations studied in published work. The difficulties in refining this model are the same: the role of serotype interaction remains unclear and assessing population-level immunity is difficult.
3.4 Age-specific prevalence
Another way to exploit early infections is via age-stratified seroprevalence studies with cohorts including ages surrounding the age of peak incidence. This allows specific determination of the serotypes of early exposures and estimation of the force of infection over previous years based on different exposure rates across age groups. In perhaps the most robust estimation of R0 to date, models with different structures, including strain-specific interaction (cross-protection or enhancement of susceptibility) and time-varying force of infection, were fitted using empirical data on age-specific exposure rates in a cohort of Thai children [23]. An advantage of using seroprevalence or seroconversion data is that it allows for a specific examination of the number of infections rather than purely symptomatic infections.
4. R0 estimates and the vaccination threshold
The studies discussed in Section 3 are summarized in Table 2 with accompanying R0 estimates, ranging from a non-invasive 0.5 up to a highly virulent 103 secondary infections produced per initial infection. Part of this variation is likely due to geographic and temporal variation in the force of infection. The force of infection is dependent on vector and host population densities and vector capacity, all factors that vary naturally in space and time. For instance, as temperatures increase, the extrinsic incubation period of dengue decreases, increasing the force of infection and thus R0. In particular, outbreaks may start under marginal transmission conditions and explode as the conditions improve. Estimating R0 from early epidemics may thus lead to consistent underestimation of overall transmission. Vector control may also be an important influence over time [10]. Transmission models used to create these estimates generally assume homogenous mixing of vectors and humans, but studies suggest that there is significant spatial and individual-level variation in biting rates [36, 39].
A further source of variation in the estimates results from assumptions regarding the immunological background of the population affected. Estimation based on initial epidemic growth and epidemic size has been accomplished under the assumption that there is no extant immunity in the human population. When immunity is present in the population and not accounted for, the force of infection and R0 may be underestimated because individuals with acquired immunity who are exposed to infectious vectors will not be infected. Previous exposure may also influence the dynamics via cross-protection or enhancement, another component that has yet to be addressed in these empirical models. The only empirical R0 model considering any type of serotype interaction estimated R0 between 4.3 and 5.8 for each strain [23]. Estimation of R0 in the endemic context is of particular importance since this is where most dengue virus infections occur and where vaccination may have the greatest impact.
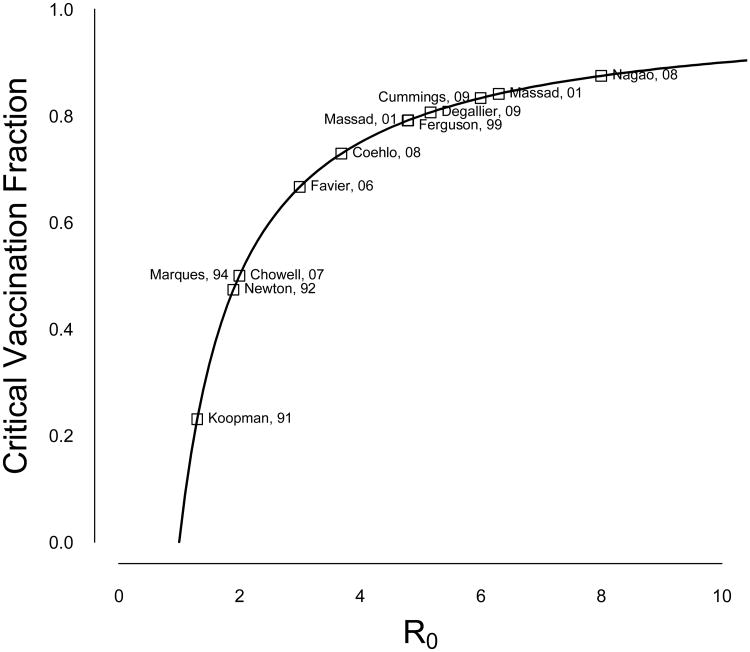
Figure 2 shows the relationship between R0 and the critical vaccination fraction, pc (Section 1), when R0 is greater than one. The accuracy of R0 estimates is particularly important at relatively low levels. For example, a difference in R0 between 10 and 11 translates to a 1% change in the critical vaccination threshold, but the difference between R0 equals 2 versus 3 is over 16%. The range of pc given the median estimates of all the studies is 9% to 91%, with the age-stratified study making a conservative estimate of 82% [23]. As described above, however, all of these estimates must be carefully assessed. Finally, it should be noted that these critical vaccination fractions represent the fraction of the population that must receive protective immunity, thus the proportion of the population that must be vaccinated could be higher depending upon vaccine efficacy.
Figure 2.
The effect of R0 on the critical vaccination fraction. Median estimates for each published R0 estimate are indicated.
5. Considerations for Estimating the Impact of Vaccines
Very few models have explicitly considered the potential impact of a vaccine. Since no effective vaccine exists, models that have considered the potential impact have been purely speculative about the action of vaccines and have represented vaccination in simple, abstract ways, for example, allowing conversion between susceptible and protected compartments without passing through infectious stages. Billings et al. [40] considered a two serotype model based on transmission in Southeast Asia and the use of a single monotypic vaccine or two monotypic vaccines imparting complete immunity. They suggest that two monotypic vaccines given in succession with high coverage could eradicate dengue despite the effects of enhancement. Cummings [41] investigated the effects of different potential dengue vaccine formulations on subsequent transmission in the presence of enhanced infectiousness in secondary infections. He found that even with imperfect efficacy, tetravalent vaccines resulted in decreased transmission.
To expand the description of vaccine action, more data on vaccine characteristics is needed. We have focused much of our discussion on the basic reproductive number, an important determinant of the potential impact of population-level vaccination. However, more information is critical for projecting the impact of immunization programmes and for strategizing their implementation to maximize that impact. Below, we discuss some of the necessary considerations for the development of effective vaccination models.
5.1 Direct and indirect protective effects of vaccination
The use of models to estimate the impact of vaccines on community-wide transmission requires detailed information on the impact of vaccination on an individual's risk of acquiring and transmitting infection. Vaccines may act to block infection, disease, further transmission, or all of the above in a single individual. The extent to which a vaccine performs each of these goals impacts its overall efficacy in reducing community-wide transmission through both direct and indirect effects. Halloran, Struchiner, and Longini [42] introduced a useful nomenclature to discuss vaccine efficacy against transmission and the disease process. They discuss three endpoints: VES, vaccine efficacy targeting susceptibility (or blocking infection), VEP, vaccine efficacy against progression of disease, and VEI, vaccine efficacy against infectiousness. Each of these components may be assessed empirically by conducting disease surveillance in vaccinees and their potential vector-mediated contacts, and comparing their infection rates to incidence in controls and their potential contacts. Each component may vary over time as a result of waning or boosting of immune responses. The link between the number of individuals immunized and the protection afforded to the individual can be estimated through these endpoints.
5.2 Non-protective immunity induced by vaccination
A major concern in dengue vaccine development is that vaccination may not afford complete protection, leading to enhancement of infection and increased disease severity or increased transmission [43]. This is partly dependent on the type of protection afforded by the vaccine (as discussed above) but also requires better understanding of the role of enhancement in population dynamics. There is little current evidence, but one study suggests that the risk of vaccinees is not elevated up to 8 years after vaccination with live attenuated vaccines {Chanthavanich, 2006 #559}. The disease severity and dynamics of viremia in vaccinees that do experience infection will be critical for estimating the population-level effects of vaccination.
5.3 Age-specific risk of severe disease
Vaccination programmes are designed to reduce the force of infection, often targeting the most susceptible population, usually children. By reducing the force of infection and shifting the age structure of susceptibles, vaccination may result in a shift in infections to older individuals. It is thus important to consider variation in the severity of illness across different age groups, something that is not well understood. One study found that severe illness resulting from secondary infection with dengue viruses declines with age [44]. However, another study found that some DHF symptoms were more severe in adults than children [45, 46] and primary infections appear to increase in severity as age increases [47]. If the risk of severe illness is age dependent, changes in the age of infection resulting from mass vaccination will alter the spectrum of disease. Depending on these effects and implementation plans, catch-up campaigns may be necessary to reduce a potential increase in disease in older individuals. Further research is needed to characterize the risk of severe illness, hospitalization, and death for multiple serotypes and settings and for both primary and secondary infections.
5.4 Variation in vaccine efficacy
The efficacy of many vaccines depends on both administration (dosage and timing) and the vaccinee (age and immune status). Successful dengue vaccines are likely to require multiple doses to confer complete protective immunity. Information on the performance of vaccines under different dosing regimens is necessary to quantitatively compare vaccination strategies. Prior natural exposure to dengue or other flaviviruses in vaccinees will also be important, as they may have increased or decreased chances of acquiring immunity upon vaccination. They may also have different side-effects because of the effects of cross-protection or enhancement.
6. Conclusions
The science of dengue virus transmission modeling is evolving. As the number of researchers in the field grows, we expect to see more innovative models and ways to test those models with empirical data. In just the past fifteen years, the field has tested several model elements and is beginning to reach consensus on several issues. There is growing consensus that short-term cross-protection is an important feature to include in the models and is helpful in matching temporal patterns of incidence. Because much of the current literature does not consider the role of short-term cross-protection or the presence of prior immunity in the populations studied, the force of infection and the basic reproduction number may be significantly underestimated. There is also growing consensus among dengue modelers that increased morbidity and mortality associated with secondary infections does not, on its own, have a large impact on the dynamics of dengue virus transmission at the population scale. Secondary infections may, however, play an important role in determining long-term dynamics via enhancement of susceptibility or infectiousness. Numerous studies suggest that at least one of these types of enhancement is important to the coexistence of multiple serotypes and the complex long-term dynamics of dengue.
Each of the models presented here represent formal hypotheses about the mechanisms that impact dengue virus transmission at the population level. Because of the lack of data on many aspects of transmission, we cannot yet definitively choose between these hypotheses. Methods to estimate the parameters of dynamical systems such as the models described here are developing quickly [48, 49]. Dialogue between theoreticians and empirical researchers can help identify the data that best distinguish the multiple hypotheses. Studies to measure specific aspects of transmission including household studies, cluster targeted transmission studies and large scale longitudinal studies could greatly improve our knowledge of dengue transmission. Collaboration between empiricists and theoreticians is one way that these studies might be best designed and implemented.
Models that include a more explicit representation of the vector and host populations have been described in recent years including individual based or agent-based models that include representation of each human host as an individual in a spatially explicit way or even individual vectors [50, 51]. These models offer exciting extensions of existing frameworks. Detailed data will be important and a limiter of growth of these detailed models, but these models may give us insight into fine spatial-scale interventions.
Current estimates of the basic reproduction number and by extension the critical vaccination threshold are widely disparate. This may represent true variability in R0 or, alternatively, differences in the methodology and assumptions. Estimates from multiple geographic settings using standardized methods are necessary to estimate the efforts required to control dengue globally. Preferably, estimates would be based on serological data instead of case data to more closely measure the actual number of infections. Though robust methods for estimating the basic reproduction number exist, the dengue community must decide on the best methods, collect appropriate data, and estimate R0 for settings across the globe.
Acknowledgments
This review was produced at the request of the WHO Initiative for Vaccine Research. Financial support for the work was provided by the Pediatric Dengue Vaccine Initiative. Derek Cummings holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund. DC also received support from the Gates Foundation Vaccine Modeling Initiative. One of the authors (J.H.) is a staff member of the World Health Organization, and he alone is responsible for the views expressed in this publication, which do not necessarily represent the decisions, policy or views of the World Health Organization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford University Press; 1991. [Google Scholar]
- 2.Wallinga J, Levy-Bruhl D, Gay NJ, Wachmann CH. Estimation of measles reproduction ratios and prospects for elimination of measles by vaccination in some Western European countries. Epidemiol Infect. 2001 Oct;127(2):281–95. doi: 10.1017/s095026880100601x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabin AB. Recent advances in our knowledge of dengue and sandfly fever. AmJTropMedHyg. 1955;4(2):198–207. doi: 10.4269/ajtmh.1955.4.198. [DOI] [PubMed] [Google Scholar]
- 4.Sabin AB. Research on dengue during World War II. AmJTropMedHyg. 1952;1(1):30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 5.Halstead SB. Observations related to pathogensis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale JBiolMed. 1970;42(5):350–62. [PMC free article] [PubMed] [Google Scholar]
- 6.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. AmJTropMedHyg. 1988;38(1):172–80. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 7.Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. Journal of Infectious Diseases. 2000;181(1):2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 8.Libraty DH, Endy TP, Houng HSH, Green S, Kalayanarooj S, Suntayakorn S, et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. Journal of Infectious Diseases. 2002;185(9):1213–21. doi: 10.1086/340365. [DOI] [PubMed] [Google Scholar]
- 9.Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clinical Microbiology Reviews. 2009 Oct;22(4):564–81. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagao Y, Koelle K. Decreases in dengue transmission may act to increase the incidence of dengue hemorrhagic fever. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2238–43. doi: 10.1073/pnas.0709029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wearing HJ, Rohani P. Ecological and immunological determinants of dengue epidemics. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(31):11802–7. doi: 10.1073/pnas.0602960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams B, Boots M. Modelling the relationship between antibody-dependent enhancement and immunological distance with application to dengue. J Theor Biol. 2006 Sep;242(2):337–46. doi: 10.1016/j.jtbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Coutinho FA, Burattini MN, Lopez LF, Massad E. Threshold conditions for a non-autonomous epidemic system describing the population dynamics of dengue. Bull Math Biol. 2006 Nov;68(8):2263–82. doi: 10.1007/s11538-006-9108-6. [DOI] [PubMed] [Google Scholar]
- 14.Focks DA, Daniels E, Haile DG, Keesling JE. A simulation model of the epidemiology of urban dengue fever: literature analysis, model development, preliminary validation, and samples of simulation results. Am J Trop Med Hyg. 1995 Nov;53(5):489–506. doi: 10.4269/ajtmh.1995.53.489. [DOI] [PubMed] [Google Scholar]
- 15.Cummings DAT, Schwartz IB, Billings L, Shaw LB, Burke DS. Dynamic effects of anti body-dependent enhancement on the fitness of viruses. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(42):15259–64. doi: 10.1073/pnas.0507320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gubler DJ, Suharyono W, Tan R, Abidin M, Sie A. Viraemia in patients with naturally acquired dengue infection. Bull World Health Organ. 1981;59(4):623–30. [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbons RV, Kalanarooj S, Jarman RG, Nisalak A, Vaughn DW, Endy TP, et al. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg. 2007 Nov;77(5):910–3. [PubMed] [Google Scholar]
- 18.Hay SI, Myers MF, Burke DS, Vaughn DW, Endy T, Ananda N, et al. Etiology of interepidemic periods of mosquito-borne disease. ProcNatlAcadSciUSA. 2000;97(16):9335–9. doi: 10.1073/pnas.97.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer DB, Halstead SB. Observations Related to Pathogenesis of Dengue Hemorrhagic Fever .5. Examination of Age Specific Sequential Infection Rates Using A Mathematical Model. Yale Journal of Biology and Medicine. 1970;42(5):329–&. [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson N, Anderson R, Gupta S. The effect of antibody-dependent enhancement on the transmission dynamics and persistence of multiple-strain pathogens. ProcNatlAcadSciUSA. 1999;96(2):790–4. doi: 10.1073/pnas.96.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteva L, Vargas C. Analysis of a dengue disease transmission model. Math Biosci. 1998 Jun;150(2):131–51. doi: 10.1016/s0025-5564(98)10003-2. [DOI] [PubMed] [Google Scholar]
- 22.Recker M, Blyuss KB, Simmons CP, Hien TT, Wills B, Farrar J, et al. Immunological serotype interactions and their effect on the epidemiological pattern of dengue. Proc Biol Sci. 2009 Jul 22;276(1667):2541–8. doi: 10.1098/rspb.2009.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson NM, Donnelly CA, Anderson RM. Transmission dynamics and epidemiology of dengue: insights from age-stratified sero-prevalence surveys. Philos Trans R Soc Lond B Biol Sci. 1999 Apr 29;354(1384):757–68. doi: 10.1098/rstb.1999.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz IB, Shaw LB, Cummings DA, Billings L, McCrary M, Burke DS. Chaotic desynchronization of multistrain diseases. Phys Rev E Stat Nonlin Soft Matter Phys. 2005 Dec;72(6 Pt 2):066201. doi: 10.1103/PhysRevE.72.066201. [DOI] [PubMed] [Google Scholar]
- 25.Adams B, Holmes EC, Zhang C, Mammen MP, Nimmannitya S, Kalayanarooj S, et al. Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok. Proc Natl Acad Sci U S A. 2006 Sep;103(38):14234–-9. doi: 10.1073/pnas.0602768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wikramaratna PS, Simmons CP, Gupta S, Recker M. The effects of tertiary and quaternary infections on the epidemiology of dengue. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams B, Holmes EC, Zhang C, Mammen MP, Nimmannitya S, Kalayanarooj S, et al. Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(38):14234–9. doi: 10.1073/pnas.0602768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartley LM, Donnelly CA, Garnett GP. The seasonal pattern of dengue in endemic areas: mathematical models of mechanisms. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002;96(4):387–97. doi: 10.1016/s0035-9203(02)90371-8. [DOI] [PubMed] [Google Scholar]
- 29.Cummings DA, Iamsirithaworn S, Lessler JT, McDermott A, Prasanthong R, Nisalak A, et al. The impact of the demographic transition on dengue in Thailand: insights from a statistical analysis and mathematical modeling. PLoS Med. 2009 Sep;6(9):e1000139. doi: 10.1371/journal.pmed.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishiura H, Halstead SB. Natural History of Dengue Virus (DENV)–1 and DENV-4 Infections: Reanalysis of Classic Studies. The Journal of Infectious Diseases. 2007;195:1007–13. doi: 10.1086/511825. [DOI] [PubMed] [Google Scholar]
- 31.Newton EA, Reiter P. A model of the transmission of dengue fever with an evaluation of the impact of ultra-low volume (ULV) insecticide applications on dengue epidemics. Am J Trop Med Hyg. 1992 Dec;47(6):709–20. doi: 10.4269/ajtmh.1992.47.709. [DOI] [PubMed] [Google Scholar]
- 32.Favier C, Degallier N, Rosa-Freitas MG, Boulanger JP, Costa Lima JR, Luitgards-Moura JF, et al. Early determination of the reproductive number for vector-borne diseases: the case of dengue in Brazil. Trop Med Int Health. 2006 Mar;11(3):332–40. doi: 10.1111/j.1365-3156.2006.01560.x. [DOI] [PubMed] [Google Scholar]
- 33.Marques CA, Forattini OP, Massad E. The Basic Reproduction Number for Dengue Fever in Sao-Paulo State, Brazil - 1990-1991 Epidemic. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1994;88(1):58–9. doi: 10.1016/0035-9203(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 34.Massad E, Burattini MN, Coutinho FA, Lopez LF. Dengue and the risk of urban yellow fever reintroduction in Sao Paulo State, Brazil. Rev Saude Publica. 2003 Aug;37(4):477–84. doi: 10.1590/s0034-89102003000400013. [DOI] [PubMed] [Google Scholar]
- 35.Massad E, Coutinho FA, Burattini MN, Lopez LF. The risk of yellow fever in a dengue-infested area. Trans R Soc Trop Med Hyg. 2001 Jul-Aug;95(4):370–4. doi: 10.1016/s0035-9203(01)90184-1. [DOI] [PubMed] [Google Scholar]
- 36.Chowell G, Ammon CE, Hengartner NW, Hyman JM. Estimation of the reproductive number of the Spanish flu epidemic in Geneva, Switzerland. Vaccine. 2006;24(44-46):6747–50. doi: 10.1016/j.vaccine.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 37.Chowell G, Nishiura H, Bettencourt LM. Comparative estimation of the reproduction number for pandemic influenza from daily case notification data. J R Soc Interface. 2007 Feb 22;4(12):155–66. doi: 10.1098/rsif.2006.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koopman JS, Prevots DR, Marin MAV, Dantes HG, Aquino MLZ, Longini IM, et al. Determinants and Predictors of Dengue Infection in Mexico. American Journal of Epidemiology. 1991;133(11):1168–78. doi: 10.1093/oxfordjournals.aje.a115829. [DOI] [PubMed] [Google Scholar]
- 39.Luz PM, Codeço CT, Massad E, Struchiner CJ. Uncertainties regarding dengue modeling in Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2003 Oct;98(7):871–8. [PubMed] [Google Scholar]
- 40.Billings L, Fiorillo A, Schwartz IB. Vaccinations in disease models with antibody-dependent enhancement. Math Biosci. 2008 Feb;211(2):265–81. doi: 10.1016/j.mbs.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Cummings DA. The spatial and temporal dynamics of dengue hemorrhagic fever incidence in Thailand. Baltimore, MD: The Johns Hopkins University; 2004. [Google Scholar]
- 42.Halloran ME, Struchiner CJ, Longini IM., Jr Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am J Epidemiol. 1997 Nov 15;146(10):789–803. doi: 10.1093/oxfordjournals.aje.a009196. [DOI] [PubMed] [Google Scholar]
- 43.Edelman R, Hombach J. “Guidelines for the clinical evaluation of dengue vaccines in endemic areas”: summary of a World Health Organization Technical Consultation. Vaccine. 2008 Aug 5;26(33):4113–9. doi: 10.1016/j.vaccine.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 44.Guzman MG, Kouri G, Bravo J, Valdes L, Vazquez S, Halstead SB. Effect of age on outcome of secondary dengue 2 infections. IntJInfectDis. 2002;6(2):118–24. doi: 10.1016/s1201-9712(02)90072-x. [DOI] [PubMed] [Google Scholar]
- 45.Wang CC, Lee IK, Su MC, Lin HI, Huang YC, Liu SF, et al. Differences in clinical and laboratory characteristics and disease severity between children and adults with dengue virus infection in Taiwan, 2002. Trans R Soc Trop Med Hyg. 2009 Sep;103(9):871–7. doi: 10.1016/j.trstmh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Wang CC, Huang ZS, Chiang PL, Chen CT, Wu HN. Analysis of the nucleoside triphosphatase, RNA triphosphatase, and unwinding activities of the helicase domain of dengue virus NS3 protein. FEBS Lett. 2009 Feb 18;583(4):691–6. doi: 10.1016/j.febslet.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Egger JR, Coleman PG. Age and clinical dengue illness. Emerg Infect Dis. 2007 Jun;13(6):924–5. doi: 10.3201/eid1306.070008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ionides EL, Breto C, King AA. Inference for nonlinear dynamical systems. Proc Natl Acad Sci U S A. 2006 Dec 5;103(49):18438–43. doi: 10.1073/pnas.0603181103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cauchemez S, Ferguson NM. Likelihood-based estimation of continuous-time epidemic models from time-series data: application to measles transmission in London. J R Soc Interface. 2008 Aug 6;5(25):885–97. doi: 10.1098/rsif.2007.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Otero MJ, Barmak D, Dorso CO, Solari HG, Natiello MA. Modeling dengue outbreaks. Mathematical Biosciences. 2011 May 5; doi: 10.1016/j.mbs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Magori K, Legros M, Puente ME, Focks DA, Scott TW, Lloyd AL, et al. Skeeter Buster: a stochastic, spatially explicit modeling tool for studying Aedes aegypti population replacement and population suppression strategies. PLoS neglected tropical diseases. 2009;3(9):e508. doi: 10.1371/journal.pntd.0000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng Z, Velasco-Hernández JX. Competitive exclusion in a vector-host model for the dengue fever. J Math Biol. 1997 May;35(5):523–44. doi: 10.1007/s002850050064. [DOI] [PubMed] [Google Scholar]
- 53.Esteva L, Vargas C. Influence of vertical and mechanical transmission on the dynamics of dengue disease. Math Biosci. 2000 Sep;167(1):51–64. doi: 10.1016/s0025-5564(00)00024-9. [DOI] [PubMed] [Google Scholar]
- 54.Esteva L, Vargas C. A model for dengue disease with variable human population. J Math Biol. 1999 Mar;38(3):220–40. doi: 10.1007/s002850050147. [DOI] [PubMed] [Google Scholar]
- 55.Ferguson NM, Donnelly CA, Anderson RM. Transmission dynamics and epidemiology of dengue: insights from age-stratified sero-prevalence surveys. PhilosTransRSocLond B BiolSci. 1999;354(1384):757–68. doi: 10.1098/rstb.1999.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pongsumpun P, Patanarapelert K, Sriprom M, Varamit S, Tang IM. Infection risk to travelers going to dengue fever endemic regions. Southeast Asian J Trop Med Public Health. 2004 Mar;35(1):155–9. [PubMed] [Google Scholar]
- 57.Pongsumpun P, Tang IM. A realistic age structured transmission model for dengue hemorrhagic fever in Thailand. Southeast Asian J Trop Med Public Health. 2001 Jun;32(2):336–40. [PubMed] [Google Scholar]
- 58.Pongsumpun P, Yoksan S, Tan IM. A comparison of the age distributions in the dengue hemorrhagic fever epidemics in Santiago de Cuba (1997) and Thailand (1998) Southeast Asian J Trop Med Public Health. 2002 Jun;33(2):255–8. [PubMed] [Google Scholar]
- 59.Derouich M, Boutayeb A, Twizell EH. A model of dengue fever. Biomed Eng Online. 2003 Feb;2:4. doi: 10.1186/1475-925X-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Esteva L, Vargas C. Coexistence of different serotypes of dengue virus. J Math Biol. 2003 Jan;46(1):31–47. doi: 10.1007/s00285-002-0168-4. [DOI] [PubMed] [Google Scholar]
- 61.Kawaguchi I, Sasaki A, Boots M. Why are dengue virus serotypes so distantly related? Enhancement and limiting serotype similarity between dengue virus strains. Proceedings of the Royal Society of London Series B-Biological Sciences. 2003;270(1530):2241–7. doi: 10.1098/rspb.2003.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Favier C, Schmit D, Müller-Graf CDM, Cazelles B, Degallier N, Mondet B, et al. Influence of spatial heterogeneity on an emerging infectious disease: the case of dengue epidemics. Proc Biol Sci. 2005 Jun;272(1568):1171–7. doi: 10.1098/rspb.2004.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tran A, Raffy M. On the dynamics of dengue epidemics from large-scale information. Theor Popul Biol. 2006 Feb;69(1):3–12. doi: 10.1016/j.tpb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 64.Atkinson MP, Su Z, Alphey N, Alphey LS, Coleman PG, Wein LM. Analyzing the control of mosquito-borne diseases by a dominant lethal genetic system. Proc Natl Acad Sci U S A. 2007 May;104(22):9540–5. doi: 10.1073/pnas.0610685104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Billings L, Schwartz IB, Shaw LB, McCrary M, Burke DS, Cummings DAT. Instabilities in multiserotype disease models with antibody-dependent enhancement. Journal of Theoretical Biology. 2007;246(1):18–27. doi: 10.1016/j.jtbi.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 66.Burattini MN, Chen M, Chow A, Coutinho FAB, Goh KT, Lopez LF, et al. Modelling the control strategies against dengue in Singapore. Epidemiol Infect. 2008 Mar;136(3):309–19. doi: 10.1017/S0950268807008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bianco S, Shaw LB, Schwartz IB. Epidemics with multistrain interactions: the interplay between cross immunity and antibody-dependent enhancement. Chaos. 2009 Dec;19(4):043123. doi: 10.1063/1.3270261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adams B, Kapan DD. Man bites mosquito: understanding the contribution of human movement to vector-borne disease dynamics. PLoS One. 2009;4(8):e6763. doi: 10.1371/journal.pone.0006763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chikaki E, Ishikawa H. A dengue transmission model in Thailand considering sequential infections with all four serotypes. J Infect Dev Ctries. 2009;3(9):711–22. doi: 10.3855/jidc.616. [DOI] [PubMed] [Google Scholar]
- 70.Medlock J, Luz PM, Struchiner CJ, Galvani AP. The impact of transgenic mosquitoes on dengue virulence to humans and mosquitoes. Am Nat. 2009 Oct;174(4):565–77. doi: 10.1086/605403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Otero M, Solari HG. Stochastic eco-epidemiological model of dengue disease transmission by Aedes aegypti mosquito. Math Biosci. 2010 Jan;223(1):32–46. doi: 10.1016/j.mbs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 72.Coelho GE, Burattini MN, Teixeira Mda G, Coutinho FA, Massad E. Dynamics of the 2006/2007 dengue outbreak in Brazil. Mem Inst Oswaldo Cruz. 2008 Sep;103(6):535–9. doi: 10.1590/s0074-02762008000600004. [DOI] [PubMed] [Google Scholar]
- 73.Degallier N, Favier C, Boulanger JP, Menkes C. Imported and autochthonous cases in the dynamics of dengue epidemics in Brazil. Rev Saude Publica. 2009 Feb;43(1):1–7. doi: 10.1590/s0034-89102009000100001. [DOI] [PubMed] [Google Scholar]