SUMMARY
Sex steroid hormones are associated with chronic diseases and mortality with risk associations that differ between racial and ethnic groups. However, it is currently unclear whether sex steroid hormone levels differ between black and white men. The aim of this study was to assess racial variation in circulating testosterone, free testosterone, sex hormone-binding globulin (SHBG) and estradiol levels in men. We searched PubMed for articles comparing circulating hormones in black and white men. A meta-analysis was performed using weighted mean differences (WMD) to compare hormones levels between black and white men. Fifteen eligible studies were identified; three did not report adjusted means. After age adjustment, free testosterone levels were significantly higher in black than in white men (WMD = 4.07 pg/mL, 95% CI 1.26, 6.88). Depending on the free testosterone concentration in white men, this WMD translates into a racial difference ranging from 2.5 to 4.9%. Total testosterone (WMD = 0.10 ng/mL, 95% CI −0.02, 0.22), estradiol (WMD = 0.67 pg/mL, 95% CI −0.04, 1.38) and SHBG (WMD = −0.45 nmol/L, 95% CI −1.75, 0.85) concentrations did not differ comparing blacks with whites. After adjustment for age, black men have a modestly but significantly 2.5 to 4.9% higher free testosterone level than white men. Based on previous studies on effects of sex steroid hormones on risk of chronic diseases or mortality, this modest difference is unlikely to explain racial differences in disease risk.
Keywords: meta-analysis, racial variation, sex steroid hormones, SHBG
INTRODUCTION
Sex steroid hormones influence the development of primary and secondary sex characteristics as well as many other biological processes during the lifetime of a man. Circulating levels of steroid hormones change as men age; testosterone concentrations decrease (Orwoll et al., 2006), whereas estradiol levels increase (Jasuja et al., 2013). Abnormally low hormone levels of testosterone and/or high concentrations of estradiol are associated with a number of chronic diseases, such as diabetes mellitus (Ding et al., 2006) and cardiovascular disease (Ruige et al., 2010). Androgens influence the development and growth of prostate cancer, however, the long postulated association between steroid hormone concentrations and risk of prostate cancer is less than clear (Ross et al., 1986; Ellis & Nyborg, 1992; Kehinde et al., 2006; Roddam et al., 2008; Muller et al., 2012). The incidence and prevalence of several chronic diseases vary between different racial and ethnic groups, for example, Hispanics and blacks having a higher prevalence of diabetes (National Center for Health Statistics, 2012), blacks having a higher incidence of prostate cancer (Siegel et al., 2013) and mortality from cardiovascular disease (National Center for Health Statistics, 2012), and whites having a higher incidence of osteoporosis (Looker et al., 2012). Racial variation in circulating levels of sex hormones might, in part, explain these health disparities. However, the results on racial variation in circulating steroid hormone levels in previous studies were not consistent (Ross et al., 1986; Ellis & Nyborg, 1992; Wright et al., 1995; Kehinde et al., 2006).
To explore possible explanations of racial variation in diseases, knowledge about racial variation in circulating sex steroid hormones is essential. To the best of our knowledge, no meta-analysis has been conducted to date to determine racial variation in circulating levels of different sex steroid hormones between black and white men.
METHODS
Study identification and selection
Articles were searched in the PubMed database through May 2013 with keywords in combination as listed below: steroid hormones, testosterone, free testosterone, sex hormone-binding globulin (SHBG), racial, race and ethnicities. We did not include ‘estradiol’ in our original search strategy as our starting point was differences in testosterone concentration. However, adding ‘estradiol’ to our search terms added one more publication of potential importance to our analysis (Abd Elmageed et al., 2013), which was ineligible because the authors did not provide means and standard deviations or confidence intervals. Also, the reference lists of already known articles were examined for other eligible studies based on the above-mentioned key words. All studies provided with a title and abstract were screened by two independent reviewers (A.R., S.R.) to select reports for full textual review. Disagreement between them was resolved by consensus. Studies were included:
If the included participants were men.
If they reported at least one of the following hormones: testosterone, free testosterone, estradiol, SHBG. We selected these hormones for the following reasons: (i) testosterone is the major male androgen, (ii) free testosterone is a measure of bioavailable testosterone, (iii) estradiol is the major oestrogen in men and (iv) SHBG is the major carrier of testosterone and estradiol in the peripheral circulation.
If they reported on differences in circulating levels of the above-mentioned hormones and SHGB in male healthy study participants. Thus, studies including on men with prostate cancer or other diseases that may affect hormone levels (such as benign prostatic hyperplasia or other cancers) were excluded (e.g. Mohler et al., 2004).
If races/ethnicities included in the study were referred to as ‘black’, ‘African-American’, ‘Non-Hispanic black’, ‘white’, ‘non-Hispanic white’ or ‘Caucasian’. We did not include men of Hispanic or Asian origin.
If data have (partly) been published twice, only data of the first publication were used. This refers to two publications: (i) Nyante et al. (2012) shows hormone data that were previously published by Rohrmann et al. (2007). We used the data published by Rohrmann et al. (2007). (ii) We used the information published by Orwoll et al. (2006); the 2010 publication (Orwoll et al., 2010) is at least partly based on the same study cohort and therefore not included in our analysis.
If publications presented the levels of hormones in arithmetic or geometric means with corresponding standard error, standard deviation or confidence interval. Studies reporting only medians were excluded (Asbell et al., 2000; Litman et al., 2006; Tsai et al., 2006) as recommended by the Cochrane Collaboration (Higgins & Green, 2011).
If the study was published in English.
Case reports, editorials and reviews were excluded.
Data extraction
For each included study, data were extracted with regard to: year of publication, race (blacks and whites), number of participants, sex of participants (only men included), sex steroid hormones levels presented either in geometric means or in arithmetic means (testosterone, free testosterone, estradiol, SHBG), age of participants (≥18 years), country of study and whether the reported mean concentrations were adjusted for potential confounding factors (unadjusted, age-adjusted or multivariable-adjusted results). Data were extracted from tables and text.
Where geometric means were presented in studies (5 of 15), they were transformed into arithmetic means using large sample approximation. In a further step, all units were converted into the same units; total testosterone in ng/mL, free testosterone and estradiol in pg/mL and SHBG in nmol/L. For the results of Rohrmann et al. (2007), the difference in total and free testosterone levels, which was not significant when comparing the geometric mean, became statistically significant after transformation in arithmetic means. For two studies (Wu et al., 1995; Gapstur et al., 2002), the authors were contacted for more information. If necessary, the standard deviations were approximated for the different adjustment levels using the standard deviation from the unadjusted level (Ellis & Nyborg, 1992) and multivariable-adjusted level (Wu et al., 1995) respectively.
Different information was available from the 15 studies concerning hormone types measured and the extent to which the means were adjusted for potential confounders, thus we report unadjusted-, age- and multivariable-adjusted results in this meta-analysis.
Statistical analyses
The results of the included studies were presented measuring the absolute difference in means between hormone levels in black and white men. This method, called weighted mean difference (WMD), is used to compare continuous variables with the same units accounting for sample size (Higgins & Green, 2011). Thus, results of WMD represent the absolute difference in the given units of hormones and SHBG. Standard deviations (SD), which are needed to calculate mean difference, were obtained from tables or calculated from standard errors and confidence intervals (Higgins et al., 2008; Higgins & Green, 2011). For the test of heterogeneity of the WMD across the studies, the I2 statistic was used. The I2 statistic describes the percentage of total variation across studies that is caused by heterogeneity and thus, is an appropriate tool to assess inconsistency across studies (Higgins et al., 2003). Two subgroups were used for sensitivity analysis; firstly, only studies that could be used in all three models (unadjusted, age and multivariable adjustment) were included in the analysis for testosterone and secondly, only studies with young men were included. We computed the per cent difference in hormone concentrations between black and white men by dividing the WMD from the meta-analysis by the hormone concentration in white men and multiplying by 100. To get a possible range in the per cent difference, we compute this figure for the study with the lowest and the highest hormone concentration in white men. The meta-analysis was done using STATA version 11 (Stata Corporation, College Station, TX, USA).
RESULTS
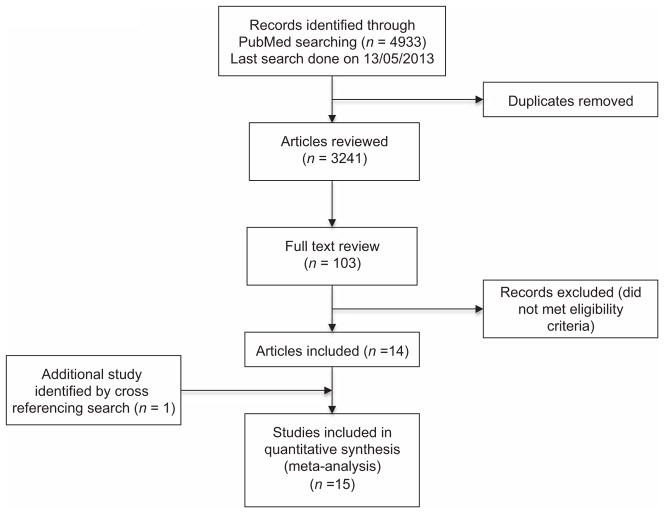
Through the electronic database search (PubMed), a total of 4933 studies were identified for potential inclusion (Fig. 1). After a full text review of 103 studies, 14 studies were included (Ross et al., 1986; Ellis & Nyborg, 1992; Wright et al., 1995; Wu et al., 1995; Eastham et al., 1998; Platz et al., 2000; Ukkola et al., 2001; Winters et al., 2001; Gapstur et al., 2002; Cheng et al., 2005; Hoffman et al., 2005; Orwoll et al., 2006; Rohrmann et al., 2007; Nyante et al., 2012) and a search of references led to one further study to include (Ettinger et al., 1997). Overall 15 studies, all conducted in US populations, were identified (Fig. 1). Table 1 shows the characteristics of the study populations included in these reports. From Table 1, it is evident that the type of adjustment made in these different studies is very diverse, such that not all of them included, for example, body mass index (BMI) in their analysis. Some studies described potential confounders (Table 1), but did not provide multivariable-adjusted mean levels of sex steroid hormones (Ross et al., 1986; Ellis & Nyborg, 1992; Wright et al., 1995; Eastham et al., 1998; Ukkola et al., 2001; Winters et al., 2001; Hoffman et al., 2005). Therefore, Table 1 shows whether the studies reported unadjusted, age-adjusted or multivariable-adjusted hormone and SHBG concentrations. A funnel plot was conducted giving a hint of publication bias in the unadjusted models (data not shown).
Figure 1.
Consort diagram for search strategy.
Table 1.
Characteristics of the study population, study type and confounders
| Reference | Race a | N | Age (mean) |
Confounders examined |
Study Population |
Total Testosterone |
Free Testosterone |
Estradiol | SHBG |
|---|---|---|---|---|---|---|---|---|---|
| Cheng et al (2005) | B | 129 | 63.3 | Age | Multiethnic Cohort Study | Age-adjusted | – | – | – |
| W | 159 | 60.9 | |||||||
| Eastham et al (1998) | B | 45 | 64.5 | Age, serum level of PSAb | Prostate biopsy patients | Unadjusted | – | – | – |
| W | 82 | 64.9 | |||||||
| Ellis et al (1992) | B | 525 | 38.34 | Age, weight | Centers for Disease Control: male army enlistees | Unadjusted | – | – | – |
| W | 3564 | 38.37 | Age-adjusted | ||||||
| Multivariable-adjusted | |||||||||
| Ettinger et al (1997) | B | 109 | 30.7 | None | Cohort from Kaiser Permanente in Northern California | Unadjusted | Unadjusted | – | Unadjusted |
| W | 114 | 31.3 | |||||||
| Gapstur et al (2002) | B | 482 | 28.4 | Age, weight, waist circumference | CARDIA male hormone study, USA | Unadjusted | Unadjusted | – | Unadjusted |
| W | 692 | 28.8 | Age-adjusted | Age-adjusted | Age-adjusted | ||||
| Multivariable-adjusted | Multivariable-adjusted | Multivariable-adjusted | |||||||
| Hoffman et al (2005) | B | 20 | 36.8 | Age, weight, height, waist circumference, hip circumference, VAT, subcutaneous adipose tissueb | New York metropolitan area | Unadjusted | – | – | Unadjusted |
| W | 21 | 44 | |||||||
| Nyante et al. (2012) | B | 183 | 48 c | Age, race/ethicity, BMI, waist circumference, smoking, alcohol | NHANES 1999–2004 f | Multivariable-adjusted | Multivariable-adjusted | Multivariable-adjusted | Multivariable-adjusted |
| W | 503 | ||||||||
| Orwoll et al (2006) | B | 236 | 73.2 d | BMI, health status, smoking, alcohol, age | Osteoporotic Fractures in Men Cohort Study (Mr. OS) | Unadjusted | Unadjusted | Unadjusted | Unadjusted |
| W | 2009 | Age-adjusted | Age-adjusted | Age-adjusted | Age-adjusted | ||||
| Multivariable-adjusted | Multivariable-adjusted | Multivariable-adjusted | Multivariable-adjusted | ||||||
| Platz et al (2000) | B | 43 | 55 | Age | Health Professionals Follow-Up Study, USA | Age-adjusted | – | Age-adjusted | Age-adjusted |
| W | 55 | 54.4 | |||||||
| Rohrmann et al (2007) | B | 363 | 29.5 e | Age, percent body fat, alcohol, smoking, physical activity, and sampling weights applied | NHANES III f | Age-adjusted | Age-adjusted | Age-adjusted | Age-adjusted |
| 56.2 e | Multivariable-adjusted | Multivariable-adjusted | Multivariable-adjusted | Multivariable-adjusted | |||||
| 73.4 e | |||||||||
| W | 674 | 31.8 e | |||||||
| 55.9 e | |||||||||
| 74.4 e | |||||||||
| Ross et al (1986) | B | 50 | 20.6 | Health, height, weight, use of cigarettes, alcohol, licit and illicit drugs | Volunteers from 2 universities in Los Angeles | Unadjusted | Unadjusted | Unadjusted | Unadjusted |
| W | 50 | 19.9 | |||||||
| Ukkola et al (2001) | B | 95 | 33.9 | Age, BMI, smoking, caffeine, caloriesb | HERITAGE Family Study, multicenter cohort study, USA | Unadjusted | – | Unadjusted | Unadjusted |
| W | 215 | 36.3 | |||||||
| Winters et al (2001) | B | 23 | 19.8 | Age, weight, BMI, waist-to-hip ratio, fasting insulin levelsb | Students from University of Pittsburgh recruited by advertisement | Unadjusted | Unadjusted | Unadjusted | Unadjusted |
| W | 23 | 21.5 | |||||||
| Wright et al (1995) | B | 16 | 27 | Age, weight, BMIb | General Clinical Research Center of the Medical University of South California | Unadjusted | – | Unadjusted | Unadjusted |
| W | 17 | 27 | |||||||
| Wu et al (1995) | B | 306 | 69.9 c | Age, BMI, physical activity | Controls from a multicenter case-control study of prostate cancer: USA and Canada | Age-adjusted | Age-adjusted | – | Age-adjusted Multivariable-adjusted |
| W | 402 | Multivariable-adjusted | Multivariable-adjusted |
B = Black, W = White
Confounders were assessed in the study but not used to compute adjusted hormone concentrations
Total median age for all ethnic groups included in the study
Total mean age for all ethnic groups included in the study
Median age for different age categories (20–44 yr, 45–69 yr, 70+ yr)
National Health and Nutrition Examination Survey.
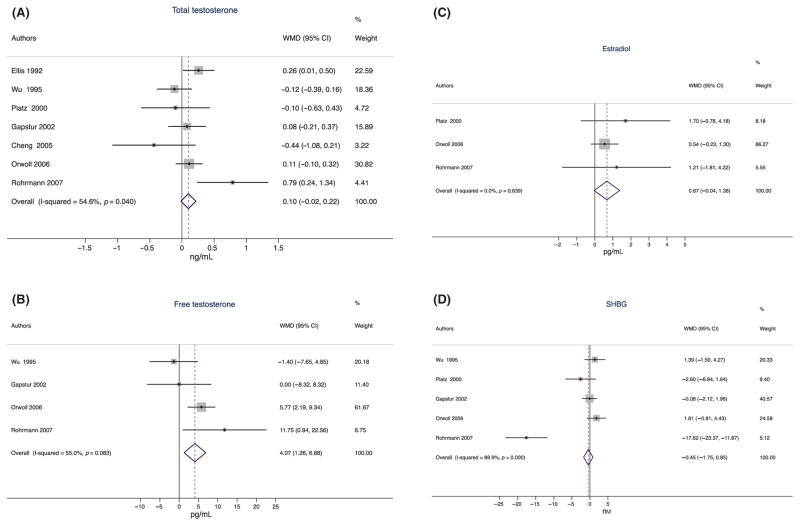
Black men had significantly higher unadjusted (WMD = 0.27 ng/mL, 95% CI 0.16–0.39), and multivariable-adjusted (WMD = 0.27 ng/mL, 95% CI 0.15–0.38) total testosterone levels than white men (Table 2), but age-adjusted concentrations did not differ (WMD = 0.10 ng/mL, 95% CI −0.02 to 0.22, Fig. 1a). However, the variation in WMD attributable to heterogeneity in the results across the studies was high with 60% (unadjusted), 55% (age adjusted) and 60% (multivariable adjusted). As the meta-analyses using age-adjusted and multivariable-adjusted results included different subsets of studies, we performed sensitivity analyses for total testosterone with studies that each reported unadjusted, age- and multivariable-adjusted results (Ellis & Nyborg, 1992; Gapstur et al., 2002; Orwoll et al., 2006; Rohrmann et al., 2007). In this subset of studies, similar, although not identical inferences were found as for overall: black men had higher testosterone levels than white men (unadjusted: WMD = 0.19 ng/mL, 95% CI 0.06–0.31; age-adjusted WMD = 0.19 ng/mL, 95% CI 0.06–0.33; multivariable-adjusted WMD = 0.24 ng/mL, 95% CI 0.10–0.37). Restricting to this subset of studies, variation in the WMD attributable to heterogeneity was lower with 35% for the unadjusted results and 49% for the age-adjusted results. A second sensitivity analysis with only younger men in the studies (Ross et al., 1986; Wright et al., 1995; Ettinger et al., 1997; Winters et al., 2001; Gapstur et al., 2002) showed similar unadjusted and multivariable-adjusted compared results to all men (data not shown).
Table 2.
Differences in serum hormones between black and white men in a meta-analyses using unadjusted and multivariable-adjusted data (age-adjusted results are shown in Figure 2)
| Type of adjustment | Analyte | Studies (n) | WMD | 95% CI | I-squared (%) | p-value |
|---|---|---|---|---|---|---|
| Unadjusted | Total T (ng/ml) | 10 | 0.27 | 0.16, 0.39 | 59.9 | 0.005 |
| Multivariable | 6 | 0.27 | 0.15, 0.38 | 53.6 | 0.056 | |
| Unadjusted | Free T (pg/ml) | 5 | 4.77 | 1.89, 7.66 | 25.4 | 0.258 |
| Multivariable | 5 | 5.66 | 2.91, 8.42 | 38.9 | 0.162 | |
| Unadjusted | Estradiol (pg/ml) | 5 | 1.26 | 0.62, 1.90 | 64.2 | 0.016 |
| Multivariable | 3 | 0.75 | 0.02, 1.49 | 91.3 | <0.001 | |
| Unadjusted | SHBG (nmol/l) | 8 | −0.25 | −1.24, 0.74 | 83.0 | <0.001 |
| Multivariable | 5 | 0.36 | −0.92, 1.65 | 88.7 | <0.001 |
WMD = weighted mean difference.
In contrast to total testosterone, we observed statistically significantly higher unadjusted (WMD = 4.77 pg/mL, 95% CI 1.89–7.66; Table 2), age-adjusted (WMD = 4.07 pg/mL, 95% CI 1.26–6.88; Fig. 2B) and multivariable-adjusted (WMD = 5.99 pg/ mL, 95% CI 2.91–8.42; Table 2) free testosterone concentration in black than in white men. Using the lowest (Orwoll et al., 2006) and highest (Gapstur et al., 2002) free testosterone concentrations in white men in the studies included in our analysis, these sizes of differences would equate to black men having a 3.0–5.8% (unadjusted), 2.5–4.9% (age-adjusted) and 3.5–6.8% (multivariable-adjusted) higher free testosterone levels than white men.
Figure 2.
Meta-analysis of age-adjusted differences between black and white men in (a) total testosterone, (b) free testosterone, (c) estradiol and (d) SHBG concentration. This figure represents the weighted mean difference (WMD) in total testosterone, free testosterone, estradiol and sex hormone-binding globuline (SHBG) concentrations between black and white men.
Unadjusted and multivariable-adjusted estradiol concentrations were significantly higher in black than in white men (Table 2); age-adjusted estradiol concentrations did not differ (Fig. 2C). SHBG concentration did not statistically significantly differ between black and white men (Table 2; Fig. 2d), although the variation in the WMD caused by heterogeneity was high (83, 90 and 89%, respectively).
DISCUSSION
The aim of this study was to evaluate whether sex steroid hormone levels differ in adult black and white men. We did this using different approaches to take into account factors that affect circulating hormone concentrations. Adjusting only for age, we did not observe statistically significant differences in total testosterone concentrations between black and white men, but free testosterone levels were significantly although modestly higher in blacks than in whites. In the multivariable-adjusted models, total testosterone was also significantly higher in black than white men, but the magnitude of the racial difference was very heterogeneous between the studies, as were the factors adjusted for (see Tables 1 and 2). To evaluate how much of the difference in results was because of a different subset of studies in the meta-analyses of the unadjusted, age-adjusted and multivariable-adjusted results, we performed sensitivity analyses including only those studies that each provided all three estimates. The results in this subset of studies were similar to those overall; however, the differences between black and white men were statistically significant, irrespective of the level of adjustment. This may be because of the smaller heterogeneity in this sample.
Only free testosterone differed between black and white men in all models. Using the actual free testosterone concentration of the studies included in our analysis and the result of our meta-analysis, we estimate that black men have a 2.5–4.9% higher free testosterone level than white men. To evaluate whether this percentage difference in free testosterone concentrations between black and white men may have implications on health, we set this figure in relation to results of a previous NHANES III analysis that examined the association between sex steroid hormone concentrations and all-cause mortality (Menke et al., 2010). Given that, in this study (Menke et al., 2010), we only detected modestly increased mortality when comparing the extremes of the distribution of free testosterone level, we conclude that a 2.5–4.9% difference in concentration between black and white men is unlikely to explain the all-cause mortality disparity (Cullen et al., 2012). At lower free testosterone concentration, we cannot rule out that the small racial difference in concentration might account for a small racial difference in all-cause mortality. The influence of steroid hormones on the risk of chronic diseases and mortality is complex and estimates based on one measurement in adulthood very likely do not adequately reflect these associations. This is particularly true as hormone levels change over time and the changes might differ by race/ethnicity.
We presented unadjusted, age-adjusted and multivariable-adjusted differences in hormone concentrations. The unadjusted levels (or age-adjusted levels) are the concentration the men (or the men if they were the same age) actually experience and thus, could be an explanation of why men of a certain racial or ethnic group have a higher risk of a certain disease than others, irrespective of the reasons for the differences in hormone levels by race. The levels taking into account factors influencing hormone levels, such as obesity, and the prevalence of which differ by race, help us to understand whether there may be inherent differences (e.g. genetic) in hormone levels between black and white men.
A high degree of heterogeneity of the results among the studies was observed including when using the unadjusted and adjusted hormone levels. There are numerous explanations of the observed heterogeneity in the results. In general, there may be residual confounding and the extent of confounding may differ from study to study. Concerning multivariable adjustment, the set of possible confounders adjusted for in studies varied greatly from study to study (Table 1). For example, studies took into account body weight (Ellis & Nyborg, 1992), BMI (Wu et al., 1995; Orwoll et al., 2006), per cent of body fat (Rohrmann et al., 2007) or waist circumference (Gapstur et al., 2002). Black men had a statistically significant higher estradiol level compared with white men, although the results for the age-adjusted model were only borderline statistically significant. Only three studies presented results with age (Platz et al., 2000; Orwoll et al., 2006; Rohrmann et al., 2007) and multivariable (Orwoll et al., 2006; Rohrmann et al., 2007; Nyante et al., 2012) adjustment for estradiol. It is well-known that circulating estradiol concentration is especially affected by differences in body fat mass as testosterone is converted into estradiol in fat tissue (Schneider et al., 1979), and, thus, differences between studies might well be explained by differences in body composition between men with different ethnic background. However, all studies included in the multivariable analysis of differences in free testosterone levels included BMI or body weight and height, leading to a slightly larger WMD than only adjusting for age. Other factors taken into account in some of the studies were, for example, smoking, physical activity, alcohol consumption or health status. Thus, results of the meta-analysis should be interpreted with caution and future studies should pay special attention to possible confounders such as obesity, smoking or physical activity when addressing whether there are differences in hormone levels between blacks and whites beyond those factors for which their prevalences are known to differ by race.
In our analysis, we included studies that were published between 1986 and 2013. Factors that are associated with sex steroid hormone and SHBG concentrations, in particular the prevalences of obesity and of cigarette smoking, have changed in this time period. Between 1988–1994 (NHANES III) and 1999–2000, the age-adjusted prevalence of obesity increased from 22.9 to 30.5%. Similar increases occurred for men in all age groups and all racial/ethnic groups (Flegal et al., 2002). The increase in the prevalence of obesity until 2009–2010 was also similar among racial/ethnic groups, with comparable prevalence rates among non-Hispanic black (38.8%) compared with non-Hispanic white men (36.2%) (Flegal et al., 2012). In contrast to obesity, the prevalence of current smoking had decreased in US men from 30.8% in 1988–1994 to 25.6% in 2007–2008 (Huffman et al., 2012). Interestingly, a lower prevalence of smoking among black adolescents has been reported, but at some point in young adulthood, the pattern is reversed with higher smoking prevalence among black than white men (Kandel et al., 2011). Therefore, secular trends in the prevalence need to be considered in potential confounders for the analysis and interpretation of the results.
None of the studies included in our analyses controlled for environmental or geographic factors. Some studies found that the grade of urbanization may influence testosterone level. For example, South African men had higher testosterone levels when living in urban than in rural areas (Gray et al., 2006). Also, significantly higher levels of testosterone in men living in Western industrialized societies versus men in pre-industrial societies were found (Kehinde et al., 2006). However, these hormone differences are may be because of higher energy intake because men in urban areas were found to have increased BMI compared with men in rural areas (Gray et al., 2006). As discussed, some of the studies included in this meta-analysis adjusted for body fatness (see Table 1).
Few studies (Orwoll et al., 2010) have examined differences in hormone levels between different subgroups of white or black populations. Little is known about steroid hormone concentrations in healthy black men outside the United States (e.g. Campbell et al., 2006; Giton et al., 2011; Lukas et al., 2004). One small study, conducted in the United States showed similar testosterone and SHBG concentrations among middle-aged black men of different origin (Chen et al., 2004).
Further limitations concern the design of the studies included in this meta-analysis. Most studies were conducted to assess racial difference in serum hormone levels; other studies instead set the primary focus on the associations between circulating hormone levels and disease outcomes such as bone density, PSA, prostate cancer and visceral adipose tissue. Recruitment of study participants was very different such that the studies did not select random/representative samples from their target population and, therefore, it might be questionable whether the results can be extrapolated back to the general population. Only two of the studies used a nationally representative sample (Rohrmann et al., 2007; Nyante et al., 2012). Three studies (Asbell et al., 2000; Litman et al., 2006; Tsai et al., 2006) only reported median hormone concentrations, which cannot be used for meta-analyses. The largest one (Litman et al., 2006) with 538 black and 710 white men reported no racial/ethnic differences in testosterone or SHBG concentration. Also, Asbell et al. (2000) observed no difference between black and white men, but in the study by Tsai et al. (2006), including 238 African-Americans and 412 Caucasians, total and bioavailable testosterone as well as total and bioavailable estradiol, but not SHGB concentrations, were statistically significantly higher in African-American than Caucasian men. Thus, results of our meta-analyses could have been different, if it was possible to include these three studies. Because of the explorative character of this meta-analysis, only a few quality criteria of including studies into the meta-analyses were defined in advance. Furthermore, we found a hint of a publication bias, although interpretation is difficult because of the small number of studies. There may be also other causes of asymmetry.
Differences in the methods used to measure circulating hormone concentrations, for example, sensitivity and detection limits, may also have influenced the results. Sex steroid hormones were mostly measured by chemiluminescent immunoassays or radioimmunoassay (DeVane et al., 1975; Anderson et al., 1976; Ross et al., 1986), another study did not describe the method used (Ellis & Nyborg, 1992). None of these studies used mass spectrometry to determine circulating levels of testosterone and estradiol, which is considered to be gold standard for their determination (Huhtaniemi et al., 2012). As shown in a European study, using immunoassays may lead to unreliable measurements of estradiol in men, whereas testosterone can be measured reasonably well using immunoassays (Huhtaniemi et al., 2012). Free testosterone can be examined in two ways, by serum extraction followed by equilibrium dialysis (Pardridge & Mietus, 1979) or by calculation from concentrations of SHBG, testosterone and albumin and the laws of mass action (Sodergard et al., 1982). Most studies used mass action equation. Whether morning samples were used for testosterone measurements is not sufficiently described. Despite these various methodological approaches results are still comparable because of the fact that differences between black and white men were calculated within a study.
In summary, this meta-analysis supports statistically significant differences in circulating level of free testosterone, but not other steroid hormones, between black and white men when taking into account age. However, it is unlikely that small differences between black and white men as observed in this study may account for racial differences in mortality rates at least in the normal range of free testosterone concentration. There is still little evidence about determinants of racial differences in steroid hormone levels. On the one hand there are direct effects of genetics on hormone metabolism (Ahn et al., 2009), but it has not been addressed in much detail how strongly this differs by race/ethnicity (Lunn et al., 1999; Zeigler-Johnson et al., 2004). As discussed above, lifestyle and anthropometry are determinants of circulating hormone levels and the prevalences of these factors may differ by race. To our knowledge, this is the first meta-analysis comparing steroid hormone levels between races and therefore it may contribute to further knowledge about associations of disease incidence and mortality and hormonal influence. However, given the heterogeneous study designs and sparse results existing to data, further large studies with a special attention to possible confounders are recommended to illuminate these associations in more detail and also clarify which factors most strongly affect and influence possible associations between circulating hormones and chronic diseases.
Acknowledgments
The authors thank Dr. Anna Wu and Dr. Susan Gapstur for providing additional information. Part of this research was funded by the Maryland Cigarette Restitution Fund at Johns Hopkins.
References
- Abd Elmageed ZY, Moroz K, Srivastav SK, Fang Z, Crawford BE, Moparty K, et al. High circulating estrogens and selective expression of ERβ in prostate tumors of Americans: implications for racial disparity of prostate cancer. Carcinogenesis. 2013;34:2017–2023. doi: 10.1093/carcin/bgt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Schumacher FR, Berndt SI, Pfeiffer R, Albanes D, Andriole GL, et al. Quantitative trait loci predicting circulating sex steroid hormones in men from the NCI-Breast and Prostate Cancer Cohort Consortium (BPC3) Hum Mol Genet. 2009;18:3749–3757. doi: 10.1093/hmg/ddp302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DC, Hopper BR, Lasley BL, Yen SS. A simple method for the assay of eight steroids in small volumes of plasma. Steroids. 1976;28:179–196. doi: 10.1016/0039-128x(76)90108-2. [DOI] [PubMed] [Google Scholar]
- Asbell SO, Raimane KC, Montesano AT, Zeitzer KL, Asbell MD, Vijayakumar S. Prostate-specific antigen and androgens in African-American and white normal subjects and prostate cancer patients. J Natl Med Assoc. 2000;92:445–449. [PMC free article] [PubMed] [Google Scholar]
- Campbell B, Leslie P, Campbell K. Age-related changes in testosterone and SHBG among turkana males. Am J Hum Biol. 2006;18:71–82. doi: 10.1002/ajhb.20468. [DOI] [PubMed] [Google Scholar]
- Chen AC, MacChia RJ, Conway F, Magai C, Desai M, Neugut AI. Prostate-specific antigen, sex steroid hormones, and the insulin-like growth factor axis in U.S.-born, Jamaican, and Haitian black men: a pilot study. Urology. 2004;64:522–527. doi: 10.1016/j.urology.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Cheng I, Yu MC, Koh WP, Pike MC, Kolonel LN, Henderson BE, et al. Comparison of prostate-specific antigen and hormone levels among men in Singapore and the United States. Cancer Epidemiol Biomarkers Prev. 2005;14:1692–1696. doi: 10.1158/1055-9965.EPI-04-0864. [DOI] [PubMed] [Google Scholar]
- Cullen MR, Cummins C, Fuchs VR. Geographic and racial variation in premature mortality in the U.S.: analyzing the disparities. PLoS ONE. 2012;7:e32930. doi: 10.1371/journal.pone.0032930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVane GW, Czekala NM, Judd HL, Yen SS. Circulating gonadotropins, estrogens, and androgens in polycystic ovarian disease. Am J Obstet Gynecol. 1975;121:496–500. doi: 10.1016/0002-9378(75)90081-2. [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- Eastham JA, May RA, Whatley T, Crow A, Venable DD, Sartor O. Clinical characteristics and biopsy specimen features in African-American and white men without prostate cancer. J Natl Cancer Inst. 1998;90:756–760. doi: 10.1093/jnci/90.10.756. [DOI] [PubMed] [Google Scholar]
- Ellis L, Nyborg H. Racial/ethnic variations in male testosterone levels: a probable contributor to group differences in health. Steroids. 1992;57:72–75. doi: 10.1016/0039-128x(92)90032-5. [DOI] [PubMed] [Google Scholar]
- Ettinger B, Sidney S, Cummings SR, Libanati C, Bikle DD, Tekawa IS, et al. Racial differences in bone density between young adult black and white subjects persist after adjustment for anthropometric, lifestyle, and biochemical differences. J Clin Endocrinol Metab. 1997;82:429–434. doi: 10.1210/jcem.82.2.3732. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among us adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among us adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Gapstur SM, Gann PH, Kopp P, Colangelo L, Longcope C, Liu K. Serum androgen concentrations in young men: a longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol Biomarkers Prev. 2002;11:1041–1047. [PubMed] [Google Scholar]
- Giton F, Fiet J, Cornu JN, Cussenot O, Belanger A, Urien S, et al. Serum sex steroids measured in middle-aged European and African-Caribbean men by gas chromatography-mass spectrometry. Eur J Endocrinol. 2011;165:917–924. doi: 10.1530/EJE-11-0551. [DOI] [PubMed] [Google Scholar]
- Gray PB, Kruger A, Huisman HW, Wissing MP, Vorster HH. Predictors of South African male testosterone levels: the THUSA study. Am J Hum Biol. 2006;18:123–132. doi: 10.1002/ajhb.20471. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions Version 5.1.0. The Cochrane Collaboration; 2011. [updated march 2011] Available at: www.cochrane-handbook.org. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat Med. 2008;27:6072–6092. doi: 10.1002/sim.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DJ, Wang Z, Gallagher D, Heymsfield SB. Comparison of visceral adipose tissue mass in adult African Americans and whites. Obes Res. 2005;13:66–74. doi: 10.1038/oby.2005.9. [DOI] [PubMed] [Google Scholar]
- Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd-Jones DM. Cardiovascular health behavior and health factor changes (1988–2008) and projections to 2020: results from the national health and nutrition examination surveys. Circulation. 2012;125:2595–2602. doi: 10.1161/CIRCULATIONAHA.111.070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi IT, Tajar A, Lee DM, O’Neill TW, Finn JD, Bartfai G, et al. Comparison of serum testosterone and estradiol measurements in 3174 european men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. Eur J Endocrinol. 2012;166:983–991. doi: 10.1530/EJE-11-1051. [DOI] [PubMed] [Google Scholar]
- Jasuja GK, Travison TG, Davda M, Murabito JM, Basaria S, Zhang A, et al. Age trends in estradiol and estrone levels measured using liquid chromatography tandem mass spectrometry in community-dwelling men of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 2013;68:733–740. doi: 10.1093/gerona/gls216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D, Schaffran C, Hu MC, Thomas Y. Age-related differences in cigarette smoking among whites and African-Americans: evidence for the crossover hypothesis. Drug Alcohol Depend. 2011;118:280–287. doi: 10.1016/j.drugalcdep.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehinde EO, Akanji AO, Memon A, Bashir AA, Daar AS, Al-Awadi KA, et al. Prostate cancer risk: the significance of differences in age related changes in serum conjugated and unconjugated steroid hormone concentrations between Arab and Caucasian men. Int Urol Nephrol. 2006;38:33–44. doi: 10.1007/s11255-005-3619-1. [DOI] [PubMed] [Google Scholar]
- Litman HJ, Bhasin S, Link CL, Araujo AB, McKinlay JB. Serum androgen levels in black, hispanic, and white men. J Clin Endocrinol Metab. 2006;91:4326–4334. doi: 10.1210/jc.2006-0037. [DOI] [PubMed] [Google Scholar]
- Looker AC, Borrud LG, Dawson-Hughes B, Shepherd JA, Wright NC. Osteoporosis or low bone mass at the femur neck or lumbar spine in older adults: United states, 2005–2008. NCHS Data Brief. 2012;93:1–8. [PubMed] [Google Scholar]
- Lukas WD, Campbell BC, Ellison PT. Testosterone, aging, and body composition in men from Harare, Zimbabwe. Am J Hum Biol. 2004;16:704–712. doi: 10.1002/ajhb.20083. [DOI] [PubMed] [Google Scholar]
- Lunn RM, Bell DA, Mohler JL, Taylor JA. Prostate cancer risk and polymorphism in 17 hydroxylase (CYP17) and steroid reductase (SRD5a2) Carcinogenesis. 1999;20:1727–1731. doi: 10.1093/carcin/20.9.1727. [DOI] [PubMed] [Google Scholar]
- Menke A, Guallar E, Rohrmann S, Nelson WG, Rifai N, Kanarek N, et al. Sex steroid hormone concentrations and risk of death in us men. Am J Epidemiol. 2010;171:583–592. doi: 10.1093/aje/kwp415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler JL, Gaston KE, Moore DT, Schell MJ, Cohen BL, Weaver C, et al. Racial differences in prostate androgen levels in men with clinically localized prostate cancer. J Urol. 2004;171:2277–2280. doi: 10.1097/01.ju.0000127739.88383.79. [DOI] [PubMed] [Google Scholar]
- Muller RL, Gerber L, Moreira DM, Andriole G, Castro-Santamaria R, Freedland SJ. Serum testosterone and dihydrotestosterone and prostate cancer risk in the placebo arm of the reduction by Dutasteride of Prostate Cancer Events trial. Eur Urol. 2012;62:757–764. doi: 10.1016/j.eururo.2012.05.025. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Health, United States, 2012; With Special Feature on Emergency Care. Hyattsville, MD: 2013. [PubMed] [Google Scholar]
- Nyante SJ, Graubard BI, Li Y, McQuillan GM, Platz EA, Rohrmann S, et al. Trends in sex hormone concentrations in US males: 1988–1991 to 1999–2004. Int J Androl. 2012;35:456–466. doi: 10.1111/j.1365-2605.2011.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, Barrett-Connor E, et al. Testosterone and estradiol among older men. J Clin Endocrinol Metab. 2006;91:1336–1344. doi: 10.1210/jc.2005-1830. [DOI] [PubMed] [Google Scholar]
- Orwoll ES, Nielson CM, Labrie F, Barrett-Connor E, Cauley JA, Cummings SR, et al. Evidence for geographical and racial variation in serum sex steroid levels in older men. J Clin Endocrinol Metab. 2010;95:E151–E160. doi: 10.1210/jc.2009-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM, Mietus LJ. Transport of steroid hormones through the rat blood-brain barrier. Primary role of albumin-bound hormone. J Clin Invest. 1979;64:145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz EA, Rimm EB, Willett WC, Kantoff PW, Giovannucci E. Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals. J Natl Cancer Inst. 2000;92:2009–2017. doi: 10.1093/jnci/92.24.2009. [DOI] [PubMed] [Google Scholar]
- Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab. 2007;92:2519–2525. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- Ross R, Bernstein L, Judd H, Hanisch R, Pike M, Henderson B. Serum testosterone levels in healthy young black and white men. J Natl Cancer Inst. 1986;76:45–48. [PubMed] [Google Scholar]
- Ruige JB, Mahmoud AM, De Bacquer D, Kaufman JM. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2010;97:870–875. doi: 10.1136/hrt.2010.210757. [DOI] [PubMed] [Google Scholar]
- Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48:633–638. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Tsai CJ, Cohn BA, Cirillo PM, Feldman D, Stanczyk FZ, Whittemore AS. Sex steroid hormones in young manhood and the risk of subsequent prostate cancer: a longitudinal study in African-Americans and Caucasians (United States) Cancer Causes Control. 2006;17:1237–1244. doi: 10.1007/s10552-006-0052-4. [DOI] [PubMed] [Google Scholar]
- Ukkola O, Gagnon J, Rankinen T, Thompson PA, Hong Y, Leon AS, et al. Age, body mass index, race and other determinants of steroid hormone variability: the HERITAGE Family Study. Eur J Endocrinol. 2001;145:1–9. doi: 10.1530/eje.0.1450001. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Brufsky A, Weissfeld J, Trump DL, Dyky MA, Hadeed V. Testosterone, sex hormone-binding globulin, and body composition in young adult African American and Caucasian men. Metabolism. 2001;50:1242–1247. doi: 10.1053/meta.2001.26714. [DOI] [PubMed] [Google Scholar]
- Wright NM, Renault J, Willi S, Veldhuis JD, Pandey JP, Gordon L, et al. Greater secretion of growth hormone in black than in white men: possible factor in greater bone mineral density–a clinical research center study. J Clin Endocrinol Metab. 1995;80:2291–2297. doi: 10.1210/jcem.80.8.7543111. [DOI] [PubMed] [Google Scholar]
- Wu AH, Whittemore AS, Kolonel LN, John EM, Gallagher RP, West DW, et al. Serum androgens and sex hormone-binding globulins in relation to lifestyle factors in older African-American, white, and Asian men in the United States and Canada. Cancer Epidemiol Biomarkers Prev. 1995;4:735–741. [PubMed] [Google Scholar]
- Zeigler-Johnson C, Friebel T, Walker AH, Wang Y, Spangler E, Panossian S, et al. CYP3a4, CYP3a5, and CYP3a43 genotypes and haplotypes in the etiology and severity of prostate cancer. Cancer Res. 2004;64:8461–8467. doi: 10.1158/0008-5472.CAN-04-1651. [DOI] [PubMed] [Google Scholar]