Abstract
Vertebrates have evolved a powerful vascular system that involves close interactions between blood vessels and target tissues. Vascular biology had been mostly focused on the study of blood vessels for decades, which has generated large bodies of knowledge on vascular cell development, function and pathology. We argue that the prime time has arrived for vascular research on vessel-tissue interactions, especially target tissue regulation of vessel development. The central nervous system (CNS) requires a highly efficient vascular system for oxygen and nutrient transport as well as waste disposal. Therefore, neurovascular interaction is an excellent entry point to understanding target tissue regulation of blood vessel development. In this review, we summarize signaling pathways that transmit information from neural cells to blood vessels during development and the mechanisms by which they regulate each step of CNS angiogenesis. We also review important mechanisms of neural regulation of blood-brain barrier establishment and maturation, highlighting different functions of neural progenitor cells and pericytes. Finally, we evaluate potential contribution of malfunctioning neurovascular signaling to the development of brain vascular diseases and discuss how neurovascular interactions could be involved in brain tumor angiogenesis.
Introduction
Evolution of advanced vascular systems is driven by the needs to efficiently deliver nutrients and oxygen to target tissues throughout the body of multicellular organisms. Given that blood vessels are designed to provide logistics to all tissues, it is not surprising that they are under tight regulation by target tissues that they serve. Target tissues therefore typically have special molecular machinery that modulates blood vessel function in response to different physiological states. Target tissues are also intimately involved in blood vessel network formation during development. Much of vascular biology has been focused on the study of blood vessels themselves (such as endothelial cells and mural cells), and as a result has accumulated large bodies of knowledge on vascular cell development, function and pathology. However, we argue that it is impossible to gain a comprehensive understanding of vascular systems without insight into vessel-tissue interactions, especially target tissue regulation of blood vessel development. Supported by the literature, we reason that animals are equipped with signaling pathways dedicated to establishing close vessel-tissue interactions during development and their dys-regulation underlies a significant number of vascular diseases. Genetically accessible organisms as well as new molecular tools are beginning to allow us to explore these interactions, providing novel perspectives on vascular biology. Of note, the central nervous system (CNS) consumes much more energy per unit volume of tissue than the rest of the body, and requires a highly efficient vascular system for oxygen and nutrient transport as well as waste disposal. Therefore, neurovascular interaction is an excellent entry point to understanding target tissue regulation of blood vessel development.
Vascular and nervous systems share a variety of features at the molecular and cellular levels. Molecular approaches have identified common cues that guide both vessels and nerves during development. For example, axon guidance cues semaphorins and netrins have been found to restrict vessels to intersomitic regions during embryonic development (Gu et al., 2005; Lu et al., 2004). At the cellular level, growth cones of axons and vascular tip cells share common morphological features, with filopodial and lamellopodial projections believed to generate the force needed for extending axons and vessels (Tam and Watts, 2010). Vascular and neural cells form a neurovascular unit that maintains brain homeostasis and its dysfunction contributes to progression of brain diseases. Given such a close relationship at the cellular level, vascular and nervous systems must have bidirectional communication to coordinate their functions. In fact, vascular cells play important roles in regulating neurogenesis by forming “vascular niche”, a unique anatomical structure within which both embryonic and adult neural progenitor cells divide and self-renew (Palmer et al., 2000; Shen et al., 2004). However, it still remains elusive how neural cells signal to vascular components during angiogenesis and, in general, how the neurovascular unit functions. We argue that the nature of neural-to-vascular signaling has fundamental implications for understanding tissue-specific regulation of blood vessels, which presumably show distinct properties to meet the special needs of different target tissues. In addition, we reason that many brain vascular disorders, both at the developmental and adult stage, can likely be attributed (at least partly) to neural cell dysfunction that disrupts such neural-to-vascular signaling.
Vessel ingression and initial vascular patterning
During embryonic brain development, vascularization begins with the invasion of blood vessels from the perineural vascular plexus (PVP) on the pial surface (Risau, 1993) (Fig. 1). The invasion of vessels is probably independent of neuronal migration, since vascular patterning is unaffected in reeler mice and SRK rats in which laminar organization is severely disrupted (Stubbs et al., 2009). Shortly after sprouting from the PVP, capillaries elongate radially into brain parenchyma, in parallel to neuroepithelial cells. Such close interactions between vessels and neural cells resemble that of the retinal vasculature, where blood vessels can be perfectly overlaid on astrocyte processes, suggesting that astrocytes are a major template for retinal angiogenesis (Dorrell et al., 2002; Gerhardt et al., 2003). This intimate neural cell association with blood vessels also raises a possible role of cell-cell contact and/or localized signaling at the neurovascular interface in mediating blood vessel development. For example, retinal astrocytes express R-cadherin and functional blockage of this adhesion molecule prevents normal vascular network formation (Dorrell et al., 2002). This suggests that astrocytes may guide vessel migration through specific cell adhesion molecules (such as R-cadherin) together with expression of VEGF isoforms. Binding to R-cadherin on the astrocytes may stabilize filopodia of endothelial cells and the resulting adhesion complexes may direct their migration, in a process similar to axonal growth (Doherty et al., 2000). Some groups including our lab also observed radial glia directly contacting endothelial cells during brain development and our experiments suggest that this contact is required for modulating certain molecular pathways in cortical endothelial cells (discussed below) (Gerhardt et al., 2004; Ma et al., 2013). It will be interesting to further investigate the molecular underpinning of such close interactions and its function in shaping the CNS vasculature. In addition to vessels penetrating from the pial surface, vessels can populate the brain from the periventricular zone (Vasudevan et al., 2008; Stubbs et al., 2009). Vasculatures from these two sources connect poorly, leaving the cortical intermediate zone the lowest vascular density and rendering this region susceptible to ischemia risks (Volpe et al., 2005). Interestingly, there is some evidence that endothelial cells from these two origins differ in transcription factor expression (Vasudevan et al., 2008), suggesting that angiogenesis can follow different genetic/developmental programs in different parts of the brain. It also suggests that neurovascular interaction is region specific during development.
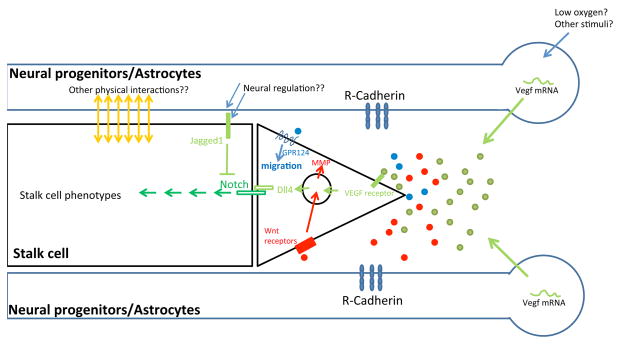
Figure 1. Neural regulation of initial vessel ingression.
The blue rectangles on the top and bottom represent radial glia fibers or astrocytic processes, while the circles to the right represent cell bodies of neural progenitor cells or astrocytes. The black rectangle and triangle in the middle represent endothelial stalk and tip cells, respectively. R-Cadherin from neural cells potentially stabilizes filopodia from tip cells. Low oxygen levels or other stimuli may up-regulate Vegf gene expression within neural cells. Secreted VEGF proteins (green dots) then induce endothelial Dll4 expression, which is critical for tip cell selection and stalk cell development. Other Notch ligand such as Jagged1 has opposite functions to Dll4, suggesting that a balance between Dll4 and Jagged1 may determine tip cell number. Neural progenitor cells also secrete Wnt ligands (red dots), which stimulate endothelial cell migration probably by up-regulating MMP levels. Unidentified diffusible factors (blue dots) from neural cells may bind to GPR124 on endothelial cells to regulate Cdc42-dependent cell migration (in the forebrain).
At the cellular level, vessel migration is achieved by specialized cell types called tip cells. Determining molecular mechanisms that control tip cell behavior is thus the first step towards understanding how neural cells may guide migration of new endothelial cells under diverse physiological conditions. Genetic evidence shows that VEGF plays important roles in filopodial extension from tip cells as well as subsequent angiogenic sprouting in the retina and the brain (Gerhardt et al., 2003; Ruhrberg et al., 2002). VEGF expression is believed to be up-regulated by hypoxia due to neuronal activities or when neural tissues attempt to meet high metabolic demands. In cultured astrocytes, this is supported by experiments showing that hypoxia increases VEGF transcript levels (Stones et al., 1995). However, the proof for hypoxia-induced VEGF expression in embryonic CNS is still lacking, partly due to difficulties in measuring oxygen levels in embryos. As the first identified angiogenic factor, VEGF has multiple isoforms produced by alternative splicing from a single gene. These isoforms differ in domains conferring heparin-binding ability and therefore VEGF isoforms can bind to extracellular matrix with different affinity, forming a gradient in vivo. Indeed, each VEGF isoform has a distinct function in mediating CNS angiogenesis. Elegant genetic engineering that produced mice each with a single isoform showed that heparin bound VEGF is necessary for normal vessel branching morphogenesis (Ruhrberg et al., 2002), and a proper VEGF protein gradient is likely responsible for this function. Similarly in the retina, the extracellular matrix bound VEGF164 isoform provides a gradient to which tip cells respond for regulating their migration, while diffusible VEGF120 controls stalk cell proliferation (Gerhardt et al., 2003). Intriguingly, systematic examination of VEGF isoform expression pattern within the brain showed that relative levels of VEGF isoforms are not significantly changed at different developmental stages (Ng et al., 2001), suggesting the importance of maintaining a proper distribution of VEGF proteins in the brain. It remains unclear whether the constant VEGF isoform ratio is retained in different parts of the brain and how this relates to vascular patterning. Conditional deletion of the VEGF gene provides the most direct evidence for neural cell secretion of VEGF in controlling initial vessel ingression. Conditional knockout of the VEGF gene using Nestin-cre causes reduced vessel density and neural apoptosis, probably resulting from insufficient blood supply (Raab et al., 2004). In the mutants, blood vessels are unable to ingress into the forebrain from the PVP and are not directed towards the ventricular zone in the hindbrain. This supports the hypothesis that neural progenitor cells produce high levels of VEGF (Breier et al., 1992; Breier et al., 1995), which guide blood vessels toward the ventricular zone. However, it is unclear how neural progenitor cells up-regulate VEGF during development. One widely believed model is that hypoxia drives VEGF expression via HIF-1alpha, which is a major oxygen detector in animals. But conditional removal of HIF-1alpha from neural cells showed normal vessel density at early developmental stages, although vessel density becomes reduced in later embryos (Tomita et al., 2003), concomitant with a decrease in VEGF expression shortly before birth. Since Tomita et al. used a Nestin-cre expressed at a similar stage as Raab et al., this suggests that not all VEGF expression may be dependent on HIF-1alpha. Therefore, more experiments are needed to definitively implicate the molecular pathway that regulates VEGF expression during development.
The Wnt signaling pathways play a diversity of roles in development and physiology. Wnt ligands are highly expressed in the developing neural tube and downstream molecules of Wnt signaling are enriched in CNS but not non-CNS endothelial cells during development (Daneman et al., 2009). Genetic removal of both Wnt7a and Wnt7b ligands results in a poorly vascularized spinal cord, and similar defects are observed in mutants that lack beta-catenin or other Wnt signaling components (Daneman et al., 2009; Stenman et al., 2008). This suggests that canonical Wnt signaling is required for initiating CNS angiogenesis. Neural progenitor cells are the cell type secreting Wnt ligands, as it was shown that Wnt ligand expression in the ventricular zone correlates with beta-catenin activation in CNS endothelial cells. As both Wnt and VEGF are secreted by neural progenitor cells to control brain angiogenesis, how then does Wnt coordinate with VEGF? Since vessels fail to invade neural tissue after Wnt signaling disruption, it is possible that Wnt ligands function as a factor for endothelial cell migration. In vitro assay suggests that Wnt7a promotes brain endothelial cell migration (Daneman et al., 2009). This is consistent with our observation that MMP2 is up regulated when Wnt signaling is ectopically elevated within endothelial cells. It is known that Wnt signaling can induce MMP expression and regulate T cell transmigration (Wu B et al., 2007). Thus, we propose that neural progenitor cells produce a gradient of VEGF protein by expressing a fixed ratio of VEGF isoforms. The vessels, in response, extend filopodia from tip cells and increase stalk cell proliferation. Canonical Wnt ligand from progenitor cells, on the other hand, induces endothelial expression of MMPs, likely among other genes, to promote extracelluar matrix degradation and facilitate migration. This is consistent with findings that precise regulation of MMP activity is crucial for the balance between vessel basement membrane breakdown and stabilization (Saunders et al., 2006).
Given the paramount importance of tip cells in shaping the vascular pattern, it is necessary to first understand the molecular mechanisms that select tip cells among endothelial cell population at the leading front of the vasculature. It should be noted that most experiments on tip cell selection have been performed with the retinal vasculature as a model, for molecular markers can label retinal tip cells unambiguously. Notch and Dll4 are highly expressed in the front of the retinal vasculature in response to VEGF gradients, suggesting their functions in tip cell fate determination (Hellstrom et al., 2007). Both genetic and pharmacological experiments showed that endothelial cells low in Notch activity are more likely to become tip cells while those high in Notch activity are prevented from becoming tip cells and become stalk cell instead (Hellstrom et al., 2007; Lobov et al, 2007; Suchting et al., 2007). A model has been proposed that neural tissue-secreted VEGF stimulates a subpopulation of endothelial cells to up regulate Dll4 and these cells enriched in Dll4 become tip cells. On the other hand, Dll4 in prospective tip cells activates Notch signaling in neighboring cells that become stalk cells. Although this conclusion is based on data from the retina, it could also be generalized to brain vessels because similar vascular phenotypes were observed in the hindbrain in genetic loss-of-function experiments (Suchting et al., 2007). Surprisingly, another ligand for Notch, Jagged1 has a positive role in regulating retinal angiogenesis, by promoting tip cell formation, endothelial cell proliferation and mural cell coverage (Benedito et al., 2009). It was shown that endothelial cell specific deletion of Jagged1 results in elated level of Hey1, which is a downstream target of Notch signaling. This suggests that normally Jagged1 inhibits Notch signaling activity within endothelial cells. Therefore, the balance between Jagged1 and Dll4 levels can determine the number of tip cells. Since Dll4 is highly expressed in tip cells where Jagged1 is absent, one possibility is that Dll4 and Jagged1 expression may be differentially regulated by neural cells. If so, it would be interesting to speculate that neural cells may regulate tip cell number by producing specific factors that change Dll4 and Jagged1 ratio under different physiological conditions. Similarly, other neural tissue secreted factors like TGF-beta superfamily members can activate the endothelial TGF-beta/BMP/Smad signaling pathway that is known to interact with Notch/Dll4 signaling to orchestrate stalk cell phenotypes (Moya et al., 2012). Neural tissues may thus also regulate tip cell number through TGF-beta/BMP signaling.
One way of expanding our understanding in neural regulation of vascular development is to identify and study novel receptors on CNS-specific vascular cells, followed by determining intercellular signaling that mediates the function of such receptors in controlling vessel development. Recently, G protein-coupled receptor (GPCR) GPR124, a gene initially identified in the tumor vasculature, was found expressed in CNS endothelial cells and pericytes with critical functions in CNS-specific angiogenesis (Kuhnert et al., 2010; Anderson et al., 2011; Cullen et al., 2011). Gpr124 null mutants are embryonically lethal and show forebrain and neural tube specific vascular defects. Blood vessels at early embryonic stage fail to invade neural tissues. In vitro experiments demonstrated that GPR124 is required to mediate Cdc42-dependent migration in response to unidentified forebrain-derived diffusible molecules (Kuhnert et al., 2010). This finding suggests that CNS angiogenesis may involve novel molecules for mediating intercellular signaling from brain to endothelial cells. Further experiments including identifying the ligand(s) for GPR124 may shed new light on the mechanisms of CNS-specific angiogenesis and of neural regulation of this process.
Vessel stabilization and remodeling
In principle, vascular patterning may arise not only by guided migration but also by remodeling of existing vessels (Fig. 2). During the remodeling process, the vasculature eliminates some branches as well as stabilizes others. Regression can happen if blood vessels are not well stabilized during this process. At the molecular level, vessel regression occurs most likely due to endothelial cell death. So survival factors provided by neural cells may explain how neural tissues regulate vessel regression/stabilization. VEGF has been implicated in the regulation of endothelial cell survival in addition to its functions in tip cell formation and migration. In an experimental model where neonatal retina is exposed to high oxygen level condition, VEGF expression from neuroglia is down-regulated by the hyperoxia. This is followed by capillary regression via endothelial cell apoptosis. Exogenous supply of VEGF can prevent apoptosis and rescue the regression phenotype (Alon et al., 1995). Later studies using conditional knockout alleles provide direct evidence for VEGF function as a survival factor in vivo. Removal of VEGF from only the retinal astrocytes has minor effects on vessel development but profound effects on vessel stabilization under hyperoxic condition, suggesting that VEGF from astrocytes mainly function to stabilize vessels (Scott et al., 2010). However, we cannot exclude the possibility that astrocyte deletion of VEGF may be compensated for by VEGF up-regulation of other cell types so that initial angiogenesis is not severely damaged. Alternatively, other factors may compensate for the loss of VEGF and guide initial vessel migration in the retina. Nevertheless, this suggests that neural tissues do produce VEGF to serve as a guidance cue for initial vascular migration, which can in addition function as a survival factor for endothelial cells. Furthermore, VEGF can influence vessel stability by co-operating with other molecules. For example, it was found that VEGF switches function of angiopoietin-2 from anti-angiogenic to pro-angiogenic (Lobov et al., 2002). In the presence of VEGF, angiopoietin-2 stimulates endothelial cell proliferation, basement membrane remodeling, and new vessel sprouts in the pupillary membrane. However, when VEGF signaling is inhibited, angiopoietin-2 promotes cell death and vessel regression. Angiopoietin-1 is another blood vessel growth factor that has been studied for long time, and its receptor Tie-2 is highly expressed in endothelial cells. Genetic inactivation of either angiopoietin-1 or Tie-2 severely disrupts vascular remodeling after normal vessel sprouting is finished (Suri et al., 1996; Sato et al., 1995). Overexpression of angiopoietin-1 in mice leads to increased vascularization, possibly due to decreased pruning and reduced regression (Thurston et al., 1999). On the other hand, angiopoietin-1 mutants show poor association between endothelial cells and surrounding extracellular matrix. Therefore, angiopoietin-1 is probably involved in vessel stabilization by promoting a strong vessel-matrix interaction. Interestingly, it was found that astrocytes can secrete angiopoeitin-1 in vitro, suggesting one possible mechanism that neural cells may utilize for regulating vessel remodeling in vivo (Lee at al., 2003).
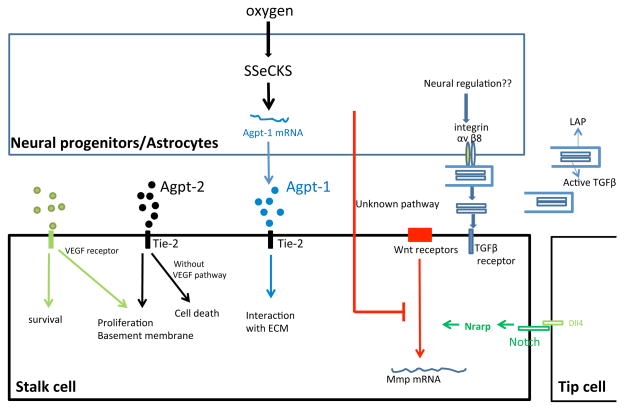
Figure 2. Regulation of vessel stabilization and remodeling by neural cells.
The blue rectangle on the top represents neural cells while the black rectangle on the bottom represents endothelial cells. VEGF and Angiopoietin-2 (Agpt-2) from neural cells control endothelial cell survival either separately or by synergistic actions. SSeCKS in astrocytes stimulates Angiopoietin-1 (Agpt-1) production under hyperoxia, and Agpt-1 enhances interactions between endothelial cells and the extracellular matrix (ECM). Our data suggest that unknown pathways from radial glia stabilize nascent blood vessels by suppressing endothelial Wnt signaling during later embryonic cortical development. In contrast, evidence from the retina suggests that Notch signaling pathway positively regulates Wnt signaling by Nrarp in endothelial cells and that Wnt signaling is essential for vessel stability. Latent TGFβ is widely expressed in the brain, and neural progenitor cells can localize latent TGFβ activation via integrin αvβ8 so that active TGFβ is released locally (within the vicinity of progenitor cells) to regulate blood vessel stabilization.
Another well understood example of vascular remodeling is hyaloid vessel regression. It is long known that hyaloid vascular networks are removed shortly after birth by vessel regression that requires the participation of macrophages (Lang et al., 1993). Later studies showed that macrophages induce capillary regression by activating apoptosis of vascular cells in a cell cycle dependent manner. Genetic ablation of lymphomyeloid transcription factor PU.1 in mice showed that absence of macrophages in the mutants results in persistent hyaloid vessel networks in the postnatal eye, confirming that regression is dependent on macrophages. Furthermore, deletion of canonical Wnt signaling molecules within hyaloid endothelial cells also causes a persistent postnatal vessel network, suggesting that the canonical Wnt pathway is required for hyaloid vessel regression. Additional experiments showed that Wnt7b ligand is expressed only in macrophages and is required for the regression. Put together, this shows that macrophages secrete the Wnt7b ligand, which then promotes vessel regression probably by activating apoptotic pathways within endothelial cells (Lobov et al., 2007). This suggests that signals from tissue-specific cell types play important roles in vascular network stabilization and remodeling. Wnt signaling also interacts with other pathways to control angiogenesis and vessel stability. In the mouse retina as well as in the zebrafish intersegmental vasculature, Dll4-induced Nrarp expression down-regulates Notch signaling and promotes Wnt signaling in endothelial stalk cells (Phng et al., 2009). Genetic deletion of Nrarp in the mouse retina results in decreased stalk cell proliferation and vessel regression marked by the presence of collagen empty sleeves. Lef1 and Ctnnb1 knockouts also have the similar phenotypes. Therefore, Nrarp functions as a molecular link between Notch and Wnt signaling in orchestrating angiogenesis including vessel stability. Altogether, it seems that canonical Wnt signaling may play multiple and sometimes opposite roles in angiogenesis, depending on specific tissues and interactions with other pathways. This raises the question of how Wnt pathways regulate brain angiogenesis. Wnt reporter lines show that endothelial cells are high in canonical Wnt signaling in both the hindbrain and cerebral cortex at early stages of brain development but the level decreases later in development (Liebner et al., 2005; Ma et al., 2013). This suggests neural mechanisms that shut down Wnt signaling to avoid potential vessel destabilization/regression observed in hyloid vessels. Consistent with this idea, our group found that genetic ablation of radial glial cells leads to vessel regression, concomitant with ectopic activation of Wnt signaling in endothelial cells. This suggests that radial glial progenitors in the late embryonic cortex signal to nascent blood vessels and regulate vessel stabilization via suppression of canonical Wnt signaling. Interestingly, our acute primary co-culture experiments suggested that neural progenitors suppress endothelial Wnt signaling only when the two cell types are in very close proximity (Ma et al., 2013). We are now pursuing the mechanisms that underlie these intercellular interactions. In short, we have provided an explanation for the dual role of Wnt signaling in brain angiogenesis, from a perspective of neural-to-vascular signaling. Wnt ligands likely facilitate endothelial cell migration by up regulating MMPs, among other genes, to degrade extracellular matrix components. Later during development, when blood vessels are being stabilized, radial glial cells may down-regulate endothelial Wnt pathways to suppress MMP activities.
TGF-beta pathways also control a variety of developmental and physiological processes and genetic studies suggest that it plays important roles in angiogenesis. Unlike many other common ligands, TGF-beta is secreted in a latent form that is generated by binding of TGF-beta to a latency-associated peptide (LAP). Regulated processing is necessary for the release of bioactive TGF-beta molecules from LAP. TGF-beta activation involves molecules such as thrombospondin-1, MMPs, and integrins alphavbeta6 and alphavbeta8. Integrins alphav and beta8 are particularly relevant to brain vascular research because null mutants for each have extensive cerebral hemorrhage and alteration of extracellular matrix, which are very similar to phenotypes from mutations in TGF-beta signaling (Zhu et al., 2002; McCarty et al., 2002). In vitro data showed that alphav and beta8 are paired to bind the RGD domain of LAP, followed by MMP14 cleavage of LAP to release active TGF-beta (Mu et al., 2002). Genetic mutation of RGD domain in mice has a similar vascular phenotype as TGF-beta1/3 null mutant, suggesting that in vivo TGF-beta activation probably functions via this mechanism to regulate angiogenesis (Yang et al., 2007). The same mechanism also acts in the retina to regulate angiogenesis as genetic data demonstrate that alphavbeta8 integrin in neural cells is essential for retinal vessel development. Deletion of the integrins specifically from neural cells results in intraretinal hemorrhage and reduced vessel sprout formation in the secondary plexus. The phenotypes, at least partly, correlate with diminished alphavbeta8 mediated activation of TGF-beta (Hirota et al., 2011; Arnold et al., 2012). Earlier work in the postnatal brain also found that astrocytic alphavbeta8 localizes latent TGF-beta and subsequently activates it to regulate the function of endothelial cells in close proximity (Cambier et al., 2005). These studies suggest a regulatory link between neural cells and blood vessels: integrins expressed in glial cells controls activation of TGF-beta ligands that then bind to endothelial cells to stabilize vessels. We reason that one purpose of using integrin-dependent TGF-beta activation as a mechanism to regulate vessel stability may be to gain the power of localized control over endothelial cells. Since TGF-beta ligands may be expressed widely in CNS, only certain neural cell types or cells in certain locales would localize TGF-beta using alphavbeta8 followed by its activation to stabilize surrounding vessels. Consistent with this reasoning, beta8 was found to be required only in neural progenitor cells but not in neurons or endothelial cells for regulating CNS angiogenesis (Proctor et al., 2005). However, it remains unclear how neural progenitor cells control integrin dependent TGF-beta activation at the molecular level.
Pericytes in CNS angiogenesis
A neurovascular unit consists of endothelial cells, astrocytes, neurons and pericytes. Known as a major supporting cell type, pericytes are in direct contact with endothelial cells and ensheathe the capillary wall. Endothelial cell-secreted PDGF-B binds to PDGFR-B expressed on pericytes, controlling pericytes proliferation, migration and recruitment to vessels. In the developing CNS, endothelial cells of sprouting vessels release PDGF-B that attracts PDGFR-B positive pericyte progenitors. Mouse genetics show that both PDGF-B and PDGFR-B null mutants appear to be defective in this process, causing loss of pericytes (Hellstrom et al., 1999). This results in microaneurysm in the cerebral cortex and later data also showed endothelial cell hyperplasia, suggesting that pericytes control endothelial cell proliferation (Lindahl et al, 1997). This is consistent with previous in vitro results showing inhibition of endothelial cell proliferation by pericytes (Armulik et al., 2005). Loss of pericytes also causes redistribution of adherens and tight junction proteins, implying that pericytes may contribute to vascular permeability (see further discussion in next section). In the adult brain, loss of pericytes causes reductions in microcirculation and age-dependent vascular damage even before neuronal degenerative changes (Bell et al., 2010). Given the importance of pericytes in neurovascular function, it is not surprising that complex signaling pathways from neural cells control pericyte recruitment, differentiation and function. It seems that endothelial cells are the primary cell types responsible for pericyte recruitment by secreting PDGF-B, but during later development and adulthood neural cells start to regulate crucial aspects of pericyte differentiation and interaction with endothelial cells. An elegant study using a brain endothelial cell specific Cre driver show that Smad4 deletion from EC causes impaired physical association between pericytes and brain endothelial cells, suggesting that TGF-beta/BMP ligands (presumably secreted and processed by neural cells) tightly control such interactions (Li et al., 2011). More recently, astrocyte specific deletion of laminin was found to lead to breakdown of the blood-brain barrier (BBB) (Yao et al., 2013). This could be due to defective astrocyte regulation of the BBB or secondary effects due to pericyte problems. Indeed, further studies found that, in the absence of laminin, pericyte marker is down regulated, suggesting that normally astrocytic signals are required for pericyte differentiation. Thus, pericytes are a key player in brain angiogenesis and are also under direct or indirect regulation by neural cells.
Blood-brain barrier development
BBB is a specialized structure present in the brain vasculature. Outside the nervous system, blood vessels are generally leaky to allow substance exchange between blood and tissues. BBB restricts such flow into brain tissues to maintain CNS homeostasis, by preventing toxins and pathogens from entering so that normal neural communication is not disrupted. BBB accomplishes this by acquiring tight cell junctions, special transporters and different exocytosis rates along endothelial cells that line brain vessels. Compromise in this barrier is associated with and contributes to a variety of diseases including stroke and brain trauma. There are several excellent reviews on this topic (e.g. Zlokovic et al., 2008). Here we focus on neural signals that contribute to BBB establishment and maintenance (Fig. 3).
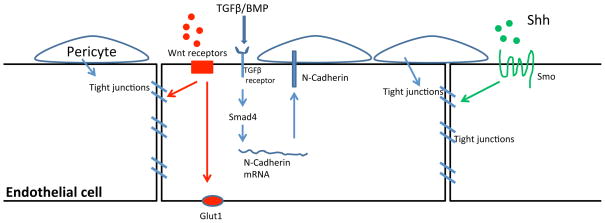
Figure 3. Regulation of blood-brain barrier (BBB) establishment and maturation by neural signals.
Neural progenitor cells secrete Wnt and Sonic hedgehog (Shh) ligands that activate formation of tight junctions and expression of BBB specific transporters (such as Glut1) within endothelial cells. Pericytes are important for maintaining endothelial tight junction and other BBB properties, but unlikely to be responsible for inducing BBB. Perictye-endothelial cell interaction is mediated by N-Cadherin, which is positively regulated by TGFβ/BMP/Smad4 signaling within endothelial cells.
Several decades ago, transplant experiments showed that BBB is not an intrinsic endothelial cell property but is rather induced by astrocytes when endothelial cells invade neural tissues (Stewart et al., 1981). Later co-culture experiments also suggest that astrocytes induce barrier properties of non-neural endothelial cells (Hayashi et al., 1997). However, barrier property in vivo is established during embryogenesis and astrocytes are largely absent in the developing brain so that they are unlikely to induce BBB in vivo. Mouse genetics offers new approaches to study BBB at the molecular level. Since BBB is a CNS-specific feature, CNS endothelial cell differentiation must involve unique processes that confer barrier properties. So it will be important to understand the molecular pathways that control CNS endothelial cell differentiation. Besides regulating vessel ingression into neural cells, canonical Wnt signaling is necessary for CNS-specific vasculature differentiation. Wnt7a/b double knockout as well as endothelial specific removal of beta-catenin result in hemorrhage throughout the neural tube as well as reduced expression of Glut-1, a hallmark of adult BBB (Daneman et al., 2009; Stenman et al, 2008). In vitro experiments showed that Wnt ligands can induce expression of tight endothelial cell junction markers, one of the characteristics of mature BBB, and that inhibition of endothelial beta-catenin signaling abolishes these effects (Liebner et al., 2005). Since Wnt signaling plays essential roles in brain development, Wnt function in BBB development suggests that other pathways of brain development may have similar roles in endothelial cell differentiation. Recently, hedgehog pathway was also found to be important for BBB integrity (Alvarez et al., 2011). In the mouse developing brain, neural cells secrete hedgehog ligands while brain endothelial cells express hedgehog receptors. Shh−/− mutants show reduced expression of junctional proteins essential for BBB. In accordance, endothelial cell specific deletion of smoothened, a receptor for Shh, has a similar phenotype at embryogenesis. Therefore, it seems that brain morphogens such as Wnt and Shh control CNS endothelial cell differentiation and BBB establishment during development and may also be required for BBB maintenance later in adulthood.
Since astrocytes are unlikely the primary cell types that induce BBB properties, what could be the cell types that perform this task? It has been found that pericyte recruitment to blood vessels in the brain is temporally correlated with the onset of barrier properties (Daneman et al., 2010). Mice defective in pericyte generation show increased vascular permeability and impaired tight junction as well as vesicle trafficking. Expression of extracellular matrix components together with that of MMP9 is also altered and these changes likely contribute vascular permeability. Interestingly, gene expression levels of many BBB markers are not changed in the absence of pericytes, suggesting that pericytes may not be responsible for inducing BBB but rather contribute to BBB maturation. Therefore, it is likely that neural progenitor cells, which outnumber postmitotic neurons during early development, induce most BBB molecular markers, which is then followed by pericyte coverage. Indeed, co-culture experiments using neural progenitor cells and brain microvascular endothelial cells have shown that neural progenitor cells induce barrier properties in endothelial cells (Weidenfeller et al., 2007). Such conclusions, however, need to be validated in vivo using animal models. In addition, the brain has the most numerous pericytes compared to other tissues, suggesting that neural progenitor signals may also be involved in pericyte generation. Multiple TGF-beta ligands have been found expressed in the brain during development and mutations in TGF-beta pathways such as Endoglin, ALK1 and Smad4 have been linked to human vascular diseases (McAllister et al., 1994; Johnson et al., 1996; Berg et al, 1997; Gallione et al., 2004). To evaluate specific role of TGF-beta signaling, Li et al took advantage of a brain endothelial cell specific Cre driver to delete Smad4 protein, an indispensible component for TGF-beta signaling. The deletion causes hemorrhage in many parts of the brain and also disrupts pericyte-endothelial cell interaction. Although Evan blue extravasation shows that the mutant brain is very leaky, this could result from secondary effects of impairment in endothelial cell differentiation and/or pericyte-endothelial cell interaction. At the molecular level, it was shown that N-Cadherin is a target for Smad4 signaling. Interestingly, the up-regulation of N-Cadherin by Smad4 requires Notch signaling, which suggests that cooperation of these two pathways control proper pericyte-endothelial cell interaction (Li et al., 2011). It is known that Notch signaling in stalk cells is activated by tip cell Dll4 expression, which is in turn induced by VEGF from neural cells. Thus, it is possible that, besides VEGF, neural cells also secrete TGF-beta that, in cooperation with Notch signaling, facilitates pericyte-endothelial cell interaction in the brain.
Although astrocytes are unlikely to induce BBB properties, they are likely to contribute to BBB maturation, given the intimate association between astrocytes and vascular cells (Abbott et al., 2006). For example, it was suggested that oxygen tension of astrocytes regulates BBB maturation (Lee et al., 2003). This elegant study used in vitro approaches and revealed that Src-suppressed C-kinase substrate (SSeCKS) function decreases VEGF and activates angiopoeitin-1 expression within astrocytes. Furthermore, conditioned medium from SSeCKS-overexpressing astrocytes stimulate expression of BBB marker genes such as ZO-1, ZO-2 and Claudin-1. In addition, oxygen levels of astrocytes were found to affect SSeCKS levels as hypoxia down-regulates while reoxygenation up-regulates SSeCKS expression. This study thus potentially connects neural cell physiology to blood vessel differentiation. Despite of such a powerful in vitro system, however, the conclusion stills need to be confirmed in vivo using genetically tractable models. It also remains an intriguing question whether similar intercellular interactions exist between other neural cells and blood vessels and at other developmental stages. Such questions could be addressed by using inducible cell type-specific Cre drivers to delete candidate genes from specific neural cells, followed by analysis of vascular phenotypes. Lastly, cultured endothelial cells also express receptors for small molecules that function as neurotransmitters and modulators in the brain, suggesting rapid and complex signaling within the neurovascular unit in response to neural activity. From the viewpoint of brain physiology, it makes sense that BBB is modulated by neural activity so that transient opening or tightening of the barrier can control substance passage between blood and brain tissues to meet metabolic needs at short time intervals (Abbott et al., 2006). Recently, a breakthrough study identified Mfsd2a (major facilitator super family domain containing 2a), which is specifically expressed in endothelial cells, as a key regulator of BBB establishment and function (Ben-Zvi et al., 2014). Further investigation of how Mfsd2a expression or activity is regulated may suggest novel intercellular signaling in BBB development and function.
Disease
As we have discussed above, neural signaling is critical to initiate and maintain a functional vascular system in the brain throughout life. Thus, any disruption of such communication may lead to brain vascular disorders. In addition, neurological disorders could also change blood vessel functions and subsequently confound the symptoms.
Germinal matrix hemorrhage/intraventricular hemorrhage (GMH/IVH)
GMH/IVH is a major neonatal disease that retains a high incidence rate of 20% in premature newborns (Heuchan et al., 2002). Germinal matrix is a transient brain structure present during embryonic development and is a very neurogenic region, so to meet metabolic demands, it may have a more rigorous angiogenesis than other parts of the brain. Indeed, high expression of VEGF and Angiopoeitin-2 are found in the germinal matrix, and they are triggered by hypoxia (Mu et al., 2003). This is consistent with chemical detection of hypoxia in this region (Ballabh et al., 2010). Pericytes are important vascular support cells required for BBB maturation and vessel integrity. Quantification of immunostainings showed that the germinal matrix has the least number of pericytes in comparison to the cortex and the white matter in human fetuses, premature infants, and rabbit pups (Braun et al., 2007). Therefore, paucity of pericytes, active angiogenesis, and remodeling may contribute to the instability and fragility of germinal matrix vessels, making vasculature prone to hemorrhage. As a result, anti-angiogenesis treatment has been devised to prevent or alleviate GMH/IVH. For example, prenatal suppression of VEGF with cerecoxib or VEGFR-2 inhibitor ZD6474 can reduce the incidence of experimentally induced GMH/IVH in animal models (Ballabh et al., 2007). However, such strategy only emphasizes the contribution of active angiogenesis to the disease and neglects the fact that vessel instability is also a major factor. In addition, the long-term effects of such angiogenic suppression are unclear and subject to further evaluation. Therefore, we propose that it is necessary to develop new intervention strategies by stabilizing pre-existent vessels instead of preventing new vascular sprouts. Mouse molecular genetics will likely be helpful in generating useful models for further basic and therapeutic research on GMH/IVH.
Cerebral cavernous malformation (CCM)
CCM mainly affects the brain and is characterized by enlarged vessels associated with brain hemorrhage. It is caused by genetic mutations in one of the three genes, CCM1, CCM2 and CCM3 (Bergametti et al., 2005). Mice with mutations in any of the three genes have vascular malfunctions similar to human CCM symptoms (Boulday et al., 2011). Interestingly, CCM3 mutation in glia cells was found to contribute to the disease, suggesting that normal neural signaling/neurovascular interaction is critical for vascular functions (Louvi et al., 2011). Neural specific removal of CCM3 using gfap- and emx1-cre shows vascular phenotypes characterized by disorganized and dilated vessels and other lesions similar to CCM. This highlights the importance of non-cell-autonomous effects in the progression of vascular diseases. Despite RNA sequencing data revealing changes in genes for cytoskeleton remodeling, detailed molecular mechanisms, however, still remain unknown. Recently, deregulated endothelial-to-mesenchymal transition (EndMT) was also found to contribute to the progression of CCM pathogenesis (Maddaluno et al., 2013). Endothelial cell specific deletion of CCM1 gene causes CCM-like vascular abnormalities in mice, concomitant with a switch from VE-Cadherin to N-Cadherin, a hallmark for EndMT. Further experiments demonstrated that CCM1-deficient endothelial cells isolated from the brain, but not from the lung, show up-regulation of EndMT markers. This suggests that CCM1 is required to suppress EndTM within brain tissues, which raises the possibility that brain tissues may intrinsically promote EndMT.
Angiogenesis in glioblastoma
Glioblastoma (GBM), the most common brain tumor, remains one of the most deadly human cancers. Current therapy only ameliorates GBM modestly and thus much more needs to be understood for developing new drugs. The emerging concept of tumor stem cells offers a new avenue both for understanding and controlling cancers including GBM (Clarke et al., 2006). Brain tumor stem cells (BTSCs) are a subtype of tumor cells with higher propagation capacity, partly due to strong angiogenic ability (Bao et al., 2006). Indeed, BTSCs reside in a perivascular niche primarily consists of endothelial cells (Calabrese et al., 2007). In vitro, BTSCs can physically interact with endothelial cells, suggesting that cross talks between these two cells types are important for the tumor microenvironment. Endothelial cells maintain BTSC self-renewal and promote tumor propagation capacity in vivo. Given the importance of vascular contribution to BTSC biology, it is an attractive strategy to target blood vessels for brain tumor intervention. Although BTSCs are different from neural stem or progenitor cells, they share fundamental properties so that knowledge in the stem cell field may shed lights on BSTCs. Since BTSCs are characterized by a strong drive for inducing angiogenesis, they are reminiscent of neural progenitor cells that produce different angiogenic factors for recruiting blood vessels during embryogenesis (as discussed in the first part of this review). Like neural progenitor cells, BTSCs secrete VEGF to stimulate angiogenesis (Bao et al., 2006). It was found that stem cell-like glioma cells have elevated VEGF levels that are further increased by hypoxia. On the other hand, VEGF-neutralizing or VEGFR-2 blocking antibodies added to BTSC conditioned medium inhibit endothelial cell proliferation and vascular tube formation, strongly suggesting a role of BTSC-produced VEGF in tumor blood vessel regulation. Stromal-derived factor-1 (SDF-1) or CXCL12 is another angiogenic factor produced by both BTSCs and endothelial cells. It was shown that CXCL12 can activate its receptor CXCL4 in glioma and increase the expression of VEGF (Yang et al., 2005). This autocrine/paracrine loop has not been observed between neural progenitor cells and blood vessels, suggesting that BTSCs have distinct properties that drive angiogenesis. TGF-beta, as an important regulator of normal angiogenesis, is also expressed in glioma cells including those with BTSC features (Qiu et al., 2011). Glioma cell conditioned medium has been found to promote endothelial cell tube formation in a TGF-beta dependent manner (Pen et al., 2008), suggesting that tumor-produced TGF-beta may play a role in angiogenesis. Interestingly, beta8 integrin has also been found to control GBM angiogenesis and invasiveness (Tchaicha et al., 2011). Manipulation of beta8 integrin protein levels within glioma changed their angiogenic and invasive properties, concomitant with changes in latent TGF-beta activation. For example, overexpression of beta8 in gliomas that normally have low levels of beta8 reduced hemorrhage around the tumor in vivo, suggesting a similar role of this integrin in regulating blood vessel stabilization as during normal brain development. Further studies of how neural cells, especially neural progenitor cells, signal to vascular cells may reveal more similarities between normal brain and BTSC-induced angiogenesis and may facilitate efforts at controlling brain tumor.
Acknowledgments
This work was supported by an NIH grant NS076729 to Z. H and an AHA pre-doctoral fellowship award 14PRE19080006 to S.M.
Contributor Information
Shang Ma, Email: ma5@wisc.edu.
Zhen Huang, Email: z.huang@neurology.wisc.edu.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature Rev. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Pan L, Yang XM, Hughes VC, Walls JR, Dominguez MG, Simmons MV, Burfeind P, Xue Y, Wei Y, MacDonald LE, Thurston G, Daly C, Lin HC, Economides AN, Valenzuela DM, Murphy AJ, Yancopoulos GD, Gale NW. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein–coupled receptor. Proc Natl Acad Sci USA. 2011;108:2807–2812. doi: 10.1073/pnas.1019761108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Arnold TD, Ferrero GM, Qiu H, Phan IT, Akhurst RJ, Huang EJ, Reichardt LF. Defective retinal vascular endothelial cell development as a consequence of impaired integrin alphaVbeta8-mediated activation of transforming growth factor-beta. J Neurosci. 2012;32:1197–1206. doi: 10.1523/JNEUROSCI.5648-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P. Intraventricular hemorrhage in premature infants: mecha- nism of disease. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, Lou N, Ungvari Z, Goldman SA, Csiszar A, Nedergaard M. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13:477–485. doi: 10.1038/nm1558. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509(7501):507–11. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Berg JN, Gallione CJ, Stenzel TT, Johnson DW, Allen WP, Schwartz CE, Jackson CE, Porteous ME, Marchuk DA. The activin receptor-like kinase 1 gene: genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2. Am J Hum Genet. 1997;61:60–67. doi: 10.1086/513903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergametti F, Denier C, Labauge P, Arnoult M, Boetto S, Clanet M, Coubes P, Echenne B, Ibrahim R, Irthum B, Jacquet G, Lonjon M, Moreau JJ, Neau JP Parker F, Tremoulet M, Tournier-Lasserve E Societe Francaise de Neurochirugie. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am J Hum Genet. 2005;76:42–51. doi: 10.1086/426952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulday G, Rudini N, Maddaluno L, Blecon A, Arnould M, Gaudric A, Chapon F, Adams R, Dejana E, Tournier-Lasserve E. Developmental timing of CCM2 loss influences cerebral cavernous malformations in mice. J Exp Med. 2011;208:1835–1847. doi: 10.1084/jem.20110571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Xu H, Hu F, Kocherlakota P, Siegel D, Chander P, Ungvari Z, Csiszar A, Nedergaard M, Ballabh P. Paucity of pericytes in germinal matrix vasculature of premature infants. J Neurosci. 2007;27:12012–12024. doi: 10.1523/JNEUROSCI.3281-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Breier G, Clauss M, Risau W. Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev Dyn. 1995;204:228–239. doi: 10.1002/aja.1002040303. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gaijar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin αvβ8-mediated activation of transforming growth factor-β by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, Li X, Chaudhary A, Xu L, Hilton MB, Logsdon D, Hsiao E, Stein EV, Cuttitta F, Haines DC, Nagashima K, Tessarollo L, St Croix B. GPR124, an orphan G protein–coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc Natl Acad Sci USA. 2011;108:5759–5764. doi: 10.1073/pnas.1017192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P, Williams G, Williams EJ. CAMs and axonal growth: a critical evaluation of the role of calcium and the MAPK cascade. Mol Cell Neurosci. 2000;16:283–295. doi: 10.1006/mcne.2000.0907. [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43:3500–3510. [PubMed] [Google Scholar]
- Gallione CJ, Repetto GM, Legius E, Rustgi AK, Schelley SL, Tejpar S, Mitchell G, Drouin E, Westermann CJ, Marchuk DA. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4) Lancet. 2004;363:852–859. doi: 10.1016/S0140-6736(04)15732-2. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- Gu CH, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Nomura M, Yamagishi S, Harada S, Yamashita J, Yamamoto H. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia. 1997;19:13–26. [PubMed] [Google Scholar]
- Hellström M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- Heuchan AM, Evans N, Henderson Smart DJ, Simpson JM. Perinatal risk factors for major intraventricular haemorrhage in the Australian and New Zealand Neonatal Network, 1995–97. Arch Dis Child Fetal Neonatal Ed 2002. 2002;86:F86–F90. doi: 10.1136/fn.86.2.F86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S, Liu Q, Lee HS, Hossain MG, Lacy-Hulbert A, McCarty JH. The astrocyte-expressed integrin alphavbeta8 governs blood vessel sprouting in the developing retina. Development. 2011;138:5157–5166. doi: 10.1242/dev.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, Guttmacher AE, Jackson CE, Attisano L, Kucherlapati R, Porteous MEM, Marchuk DA. Nature Genetics. 1996;13:189–195. doi: 10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, Kuo CJ. Essential regulation of CNS angiogenesis by the orphan G protein–coupled receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RA, Bishop JM. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A, Wang Y, Yang X. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell. 2011;20:291–302. doi: 10.1016/j.devcel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B–deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A, Chen L, Two AM, Zhang H, Min W, Günel M. Loss of cerebral cavernous malformation 3 (Ccm3) in neuroglia leads to CCM and vascular pathology. Proc Natl Acad Sci U S A. 2011;108(9):3737–42. doi: 10.1073/pnas.1012617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Breant C, Claes F, De Smet F, Thomas JL, Autiero JL, Carmeliet P, Tessier-Lavigne M, Eichmann A. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- Ma S, Kwon HJ, Johng H, Zang K, Huang Z. Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling. PLos Biol. 2013;11(1):e1001469. doi: 10.1371/journal.pbio.1001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Boulday G, Tournier-Lasserve E, Chapon F, Richichi C, Retta SF, Lampugnani MG, Dejana E. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498:492–496. doi: 10.1038/nature12207. [DOI] [PubMed] [Google Scholar]
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrel J, McCormick MK, Pericak-Vance MA, Heutink P, Oostra BA, Haitjema T, Westerman CJJ, Porteous ME, Guttmacher AE, Letarte M, Marchuk DA. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nature Genetics. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, Shani M, Dvorak HF, Wolburg H, Bader BL, Dvorak AM, Hynes RO. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol Cell Biol. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya IM, Umans L, Maas E, Pereira PN, Beets K, Francis A, Sents W, Robertson EJ, Mummery CL, Huylebroeck D, Zwijsen A. Stalk cell phenotype depends on integration of Notch and Smad1/5 signaling cascades. Dev Cell. 2012;22(3):501–514. doi: 10.1016/j.devcel.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Jiang X, Sheldon RA, Fox CK, Hamrick SE, Vexler ZS, Ferriero DM. Regulation of hypoxia- inducible factor 1alpha and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiol Dis. 2003;14:524–534. doi: 10.1016/j.nbd.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Ng YS, Rohan R, Sunday ME, Demello DE, D’Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn. 2001;220:112–21. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1093>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pen A, Moreno MJ, Durocher Y, Deb-Rinker P, Stanimirovic DB. Glioblastoma-secreted factors induce IGFBP7 and angiogenesis by modulating Smad-2-dependent TGF-beta signaling. Oncogene. 2008;27:6834–6844. doi: 10.1038/onc.2008.287. [DOI] [PubMed] [Google Scholar]
- Phng LK, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I, Ondr JK, Rao S, Lang RA, Thurston G, Gerhardt H. Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev Cell. 2009;16:70–82. doi: 10.1016/j.devcel.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci. 2005;25:9940–9948. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu B, Zhang D, Wang C, Tao J, Tie X, Qiao Y, Xu K, Wang Y, Wu A. IL-10 and TGF-beta2 are overexpressed in tumor spheres cultured from human gliomas. Mol Biol Rep. 2011;38:3585–3591. doi: 10.1007/s11033-010-0469-4. [DOI] [PubMed] [Google Scholar]
- Raab S, Beck H, Gaumann A, Yuce A, Gerber HP, Plate K, Hammes HP, Ferrara N, Breier G. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost. 2004;91:595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- Risau W. Development of the vascular system of organs and tissues. In: Schaper W, Schaper J, editors. Collateral circulation. Kluwer Academic Publishers; 1993. pp. 17–28. [Google Scholar]
- Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1996;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Powner MB, Gandhi P, Clarkin C, Gutmann DH, Johnson RS, Ferrara N, Fruttiger M. Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS One. 2010;5:e11863. doi: 10.1371/journal.pone.0011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail–chick transplantation chimeras. Dev Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs D, DeProto J, Nie K, Englund C, Mahmud I, Hevner R, Molnar Z. Neurovascular congruence during cerebral cortical development. Cereb Cortex. 2009;19(Suppl 1):i32–i41. doi: 10.1093/cercor/bhp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- Tam SJ, Watts RJ. Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu Rev Neuroscience. 2010;33:379–408. doi: 10.1146/annurev-neuro-060909-152829. [DOI] [PubMed] [Google Scholar]
- Tchaicha JH, Reyes SB, Shin J, Hossain MG, Lang FF, McCarty JH. Glioblastoma angiogenesis and tumor cell invasiveness are differentially regulated by β8 integrin. Cancer Res. 2011;71:6371–6381. doi: 10.1158/0008-5472.CAN-11-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Tomita S, Ueno M, Sakamoto M, Kitahama Y, Ueki M, Maekawa N, Sakamoto H, Gassmann M, Kageyama R, Ueda N, Gonzalez FJ, Takahama Y. Defective brain development in mice lacking the Hif-1 alpha gene in neural cells. Mol Cell Biol. 2003;19:6739–6749. doi: 10.1128/MCB.23.19.6739-6749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan A, Long JE, Crandall JE, Rubenstein JL, Bhide PG. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat Neurosci. 2008;11:429–439. doi: 10.1038/nn2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics. 2005;116:221–241. doi: 10.1542/peds.2005-0191. [DOI] [PubMed] [Google Scholar]
- Weidenfeller C, Svendsen CN, Shusta EV. Differentiating embryonic neural progenitor cells induce blood-brain barrier properties. J Neurochem. 2007;101:555–565. doi: 10.1111/j.1471-4159.2006.04394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SX, Chen JH, Jiang XF, Wang QL, Chen ZQ, Zhao W, Feng YH, Xin R, Shi JQ, Bian XW. Activation of chemokine receptor CXCR4 in malignant glioma cells promotes the production of vascular endothelial growth factor. Biochem Biophys Res Commun. 2005;335:523–528. doi: 10.1016/j.bbrc.2005.07.113. [DOI] [PubMed] [Google Scholar]
- Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Chen ZL, Norris E, Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nature Comm. 2014;5:3413. doi: 10.1038/ncomms4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. β8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]