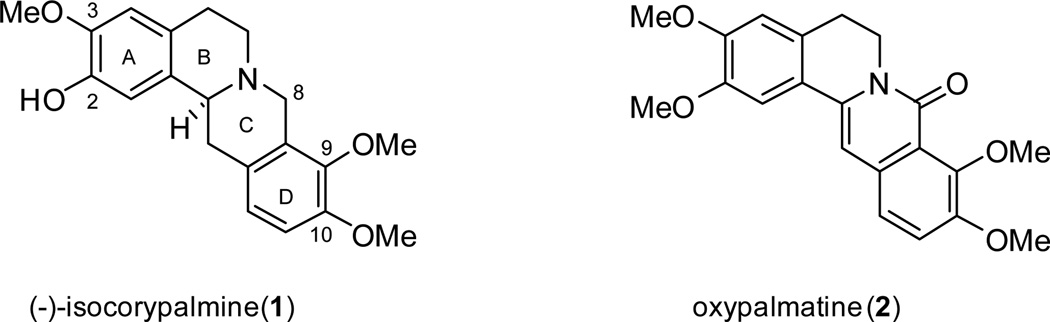Abstract
A new route which is germane to the synthesis of 9,10-oxygenated tetrahydroprotoberberines and 8-oxoprotoberberines is described. The route features the use of a diester (14) generated from reaction of dimethylmalonate with an aryl halide in the presence of n-butyllithium. The amide 17 prepared in subsequent steps is a versatile precursor for the synthesis of tetrahydroprotoberberine and 8-oxoprotoberberine scaffolds using standard high-yielding reactions. In this manner, (±)-isocorypalmine and oxypalmatine have been synthesized in 23% and 22 % yields respectively.
Keywords: THPB, Tetrahydroprotoberberine, Isocorypalmine, Oxypalmatine, 8-oxoprotoberberine
1. Introduction
The tetracyclic tetrahydroprotoberberine (THPB) alkaloids are a sub-group of isoquinoline alkaloids that have displayed interesting biological activities - for example as antimicrobial,1,2 antitumor,3,4 antifungal5 and anti-cholinesterase agents.6,7 There have also been several reports on THPBs as central nervous system receptor (particularly dopamine receptor) ligands with potential as antipsychotic agents.8–10 The core tetracyclic nucleus of naturally occurring THPBs is usually decorated with hydroxyl or alkoxyl substituents in the aromatic A and D rings.
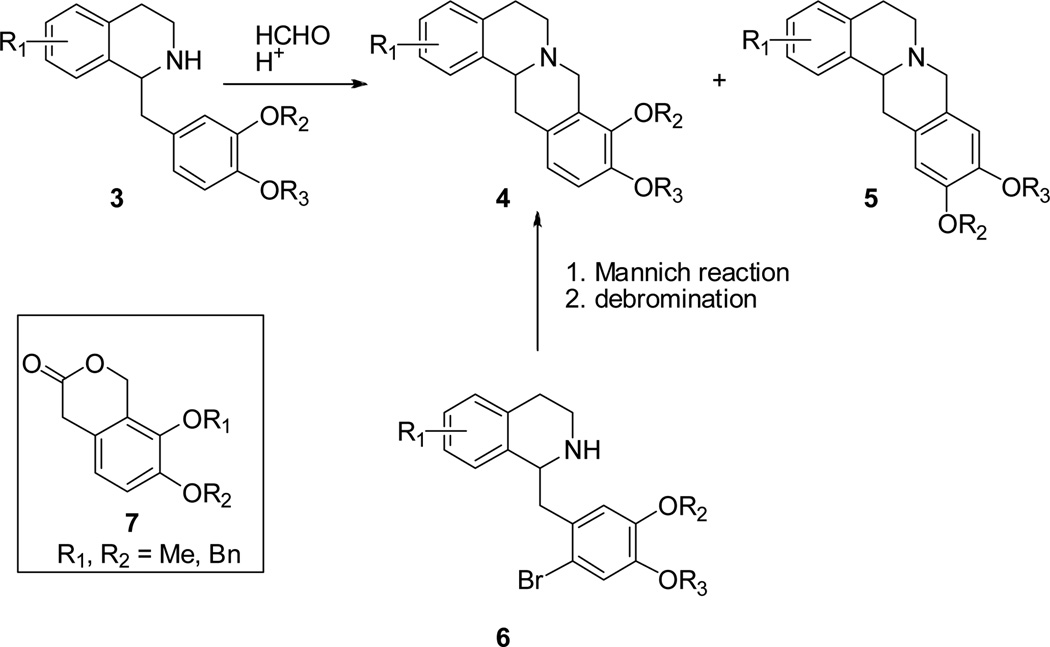
The THPB (−)-isocorypalmine (ICP, 1) is a natural product that has been isolated from a number of Corydalis species.11,3 (−)-ICP was recently reported to reduce the rewarding effects of cocaine in mice via acting on dopamine receptors.12 As such, (−)-ICP is a promising molecule towards the development of anti-cocaine therapeutics. Several methods have been developed for the synthesis of the THPB core. Among them, closure of ring C via a Mannich reaction on a benzyltetrahydroisoquinoline (BTHIQ) substrate (3; Figure 2) has been most utilized owing to the relative ease with which the BTHIQ precursors can be prepared. However, the Mannich method is non-ideal for the synthesis of 9,10-oxygenated THPBs, since Mannich reaction on the required BTHIQ substrate yields both the 9,10- (4) and 10,11-oxygenated (5) THPBs (often giving the latter either predominantly or exclusively).13,14
Figure 2.
Synthesis of 9, 10-oxygenated THPBs
Use of bromine as a blocking group (at the incipient C12 position of the THPB nucleus; 6) has been used to circumvent this problem. However, this strategy lengthens the synthesis, is often low yielding and may be less desirable for the synthesis of more structurally rich THPBs.14–16
Enantioselective synthesis of THPBs has been accomplished via reduction of intermediate BTHIQs with Noyori catalysts.10,17,18 Sun et al recently reported an enantioselective synthesis of several 9,10-oxygenated THPBs (including 1) via key lactone precursors typified by 7. Here, condensation of a phenthylamine with 7, gave an amide.10 Further synthetic manipulations eventually transformed the aromatic ring of 7 into ring D of the THPB core. However, the synthesis overall is lengthy (>10 steps) and preparation of lactones such as 7 is known to be problematic, particularly on large scale.19,20
These troublesome issues with synthesis of 9,10-oxygenated THPBs prompted an investigation into alternative routes to prepare molecules with this structural feature. In this paper, we report our efforts in this regard as applied to the synthesis of (±)-ICP. The route described may also be modified to prepare oxidized 8-oxoprotoberberine congeners - exemplified herein in our synthesis of oxypalmatine (2).
2. Results and Discussion
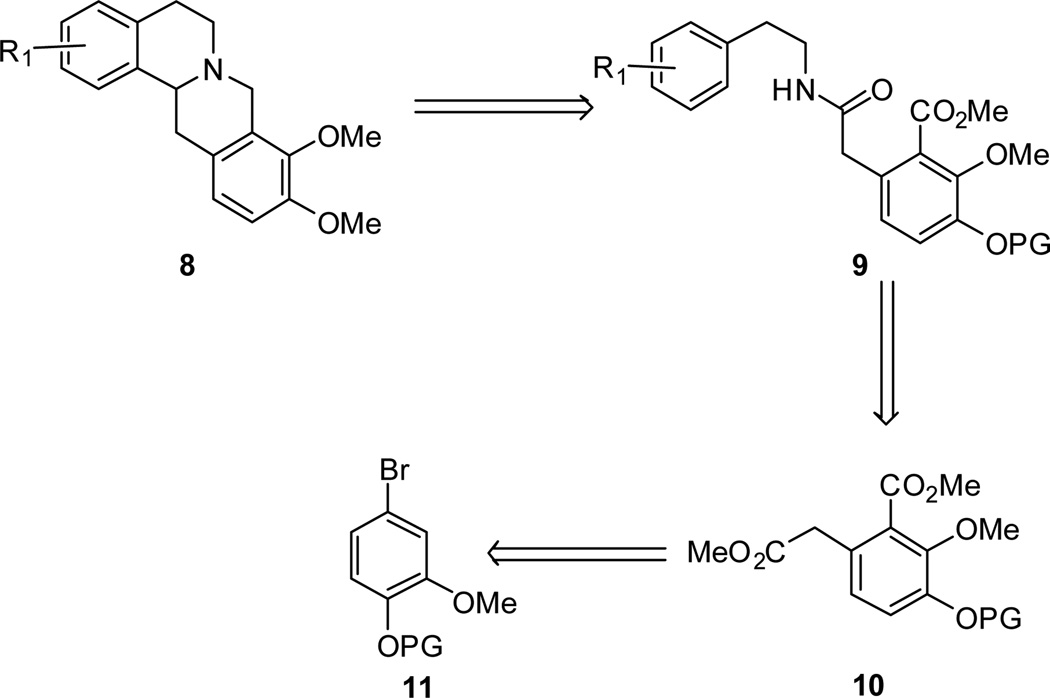
We envisaged that the 9,10-methoxylated THPB core (8) could be prepared via the amide intermediate 9 (Figure 3). This intermediate in turn is derivable from the diester 10 (via amide coupling with the corresponding aliphatic acid). Compound 10 we reasoned, would serve as an easily reachable synthetic surrogate for the isochroman 7. The diester 10 would be prepared from aryl bromide 11.
Figure 3.
Retrosynthetic analysis to THPB core
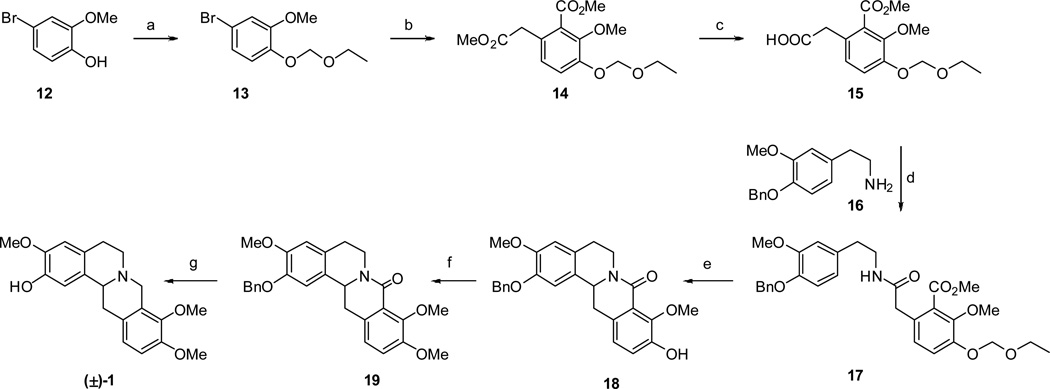
This general approach was applied to the synthesis of (±)-ICP as depicted in Scheme 1. Commercially available bromo cresol 12 was protected as the ethoxymethyl ether by reaction with chloromethoxyethane affording 13. (We considered that the ethoxymethyl ether group could be selectively removed later so that various C10 alkoxy analogues in addition to ICP itself could be prepared). Thereafter reaction of 13 with n-butyllithium in the presence of dimethylmalonate gave diester 14.21,22 This reaction presumably proceeds via nucleophilic attack of malonate anion on a benzyne intermediate generated from dehydrobromination of 13.23 Selective hydrolysis of the aliphatic ester group was accomplished with K2CO3 in refluxing H2O:MeOH to give acid 15. This acid was coupled to readily available amine 1624 with carbonyldiimidazole (CDI) to provide key amide precursor 17.
Scheme 1. Synthesis of (±)-Isocorypalmine (1).
Reagents and conditions: (a) DIPEA, (chloromethoxy)ethane, DCM, 98%; (b) DIPA, n-BuLi, DMM, THF, −78 °C, 54%; (c) K2CO3, 1:1 H2O/MeOH, reflux, 95%; (d) CDI, 16, THF, 78%; (e) i) POCl3, CH3CN, reflux; ii) NaBH4, MeOH, 79% over 2 steps; (f) CH3I, K2CO3, DMF, 91%; (g) i) LAH, THF, reflux; ii) conc. HCl, MeOH, reflux; 81% over 2 steps.
Bischler-Napieralski cyclization on amide 17 proved to be successful with POCl3 as dehydrating agent and acetonitrile as solvent. This cyclization was fortuitously accompanied by simultaneous removal of the ethoxymethyl protecting group. Other dehydrating conditions (neat POCl3, P2O5 or PCl5) and solvents (dichloromethane or toluene) were tried but gave inferior yields of imine product. Bischler-Napieralski cyclization was followed by immediate reduction (due to instability of the intermediate imines of this type) with NaBH4 to afford the 8-oxotetrahydroprotoberberine 18. The C10 phenol of 18 was methylated to give 19. Reduction of the 8-oxo group of 19 with LAH provided an intermediate tertiary amine which was subsequently heated with concentrated HCl, thus effecting debenzylation to give (±)-ICP.
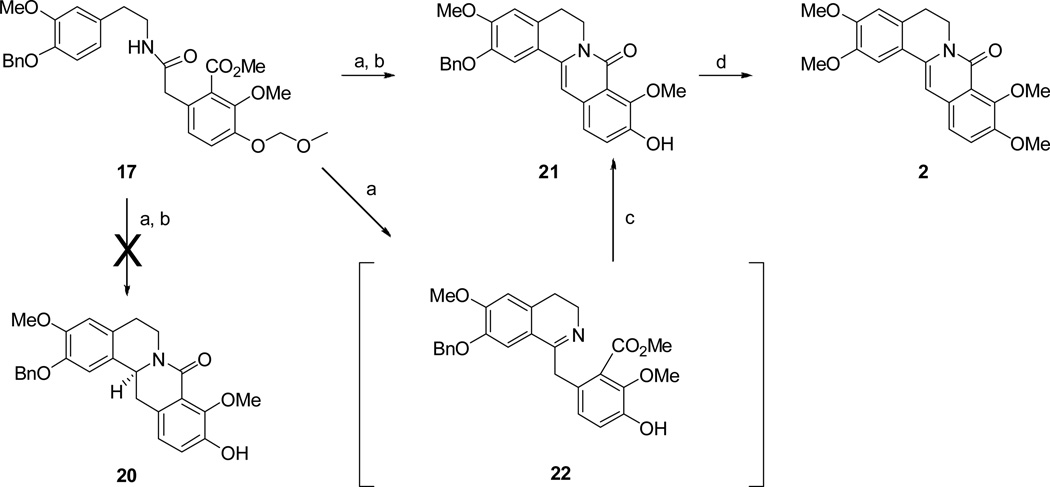
The success of this route propelled us to investigate an analogous asymmetric route to (−)-ICP. We presumed that this could be easily accomplished by replacing NaBH4 as the reducing agent in Scheme 1 (17 to 18) with Noyori's asymmetric hydrogenation method.25 The chiral 8-oxotetrahydroprotoberberine thus formed (i.e., analogous to 18) would then be transformed to (−)-ICP by using the identical sequence of reagents that was used in Scheme 1. Thus, Bischler-Napieralski cylization on amide 17 was performed as described in Scheme 1 (Scheme 2).
Scheme 2. Synthesis of oxypalmatine (2).
Reagents and conditions: (a) POCl3, CH3CN, reflux; (b) (Ru[(R,R)-Tsdpen](η6-p-cymene), DMF, HCOOH/TEA, rt, 53%over 2 step ; c) Et3N, DCM, 2h, 70% over 2 steps; (d) i) conc. HCl, MeOH, reflux; ii) CH3I, K2CO3, DMF, 79%, over 2 steps;
The imine formed was then subjected to Noyori's asymmetric hydrogenation conditions. To our surprise this did not yield the desired chiral 8-oxotetrahydroprotoberberine 20. Instead the 8-oxoprotoberberine 21 was produced. One possible cause for failure of the reduction is due to formation (and subsequent isomerization) of an acyl iminium ion formed from reaction of the imine nitrogen (from Bischler-Napieralski reaction) with the aryl ester group. Another possibility is that when the imine derived from 17 (ie compound 22) is subjected to Noyori reduction conditions, isomerization of the imine to an enamine precedes the cyclization of ring C. Treatment of the 22 with triethylamine (the base used in the Noyori reduction) gives 21. Presumably, the isomerization/cyclization steps occur at a faster rate than Noyori reduction.
Through this attempt to achieve an enantioselective synthesis of (−)-ICP, it was recognized that naturally occurring 8-oxoprotoberberines could be synthesized by variations of this method. Previous syntheses of the 8-oxoprotoberberine skeleton have required several synthetic steps and/or suffer from low-yielding steps.26–30 Thus to demonstrate the pliability of the route, compound 21 was transformed to the natural product oxypalmatine (2, Scheme 2) by removal of the C2 benzyl group followed by Williamson ether methylation. Insofar as synthesis of oxypalmatine is concerned, this method is comparable to published methods in both yield and number of steps (7 steps and 22% overall yield for the present synthesis; 9 steps 10% yield for the previous synthesis).31
3. Conclusions
Herein, a new synthesis of (±)-ICP is delineated which features the use of the easily obtainable diester intermediate 14. (±)-ICP was prepared in 9 steps from readily available precursors in 23% overall yield. The route is attractive due to its efficiency and practical ease; no chromatographic purification was necessary for a number of steps. The yield obtained is comparable to a reported synthesis of (±)-ICP.32 However, enantioselective preparation of (−)-ICP by modifications to this route remains to be optimized. The key amide intermediate (17) that is used for (±)-ICP synthesis can also be used to prepare a 9,10-oxygenated-8-oxoprotoberberine core, and we adapted this method to prepare oxypalmatine (2). Hence, the route examined here is a versatile one for the preparation of 9,10-oxygenated THPBs as well as their 8-oxoprotoberberine analogs.
4. Experimental section
General methods
All moisture-sensitive and oxygen-sensitive reactions were carried out in flame-dried glassware under a nitrogen atmosphere. Solvents and all other reagents were purchased at the highest commercial quality from Aldrich and Fisher Scientific USA and used without further purification, unless otherwise stated. Anhydrous sodium sulfate was used as drying agent for work-up of reactions. HRESIMS spectra were obtained using an Agilent 6520 QTOF instrument. 1H NMR and 13C NMR spectra were recorded using Bruker DPX-500 spectrometer (operating at 500 MHz for 1H; 125 MHz, for 13C) using CDCl3 as solvent unless stated otherwise. Tetramethylsilane (δ 0.00 ppm) served as an internal standard in 1H NMR and CDCl3 (δ 77.0 ppm) in 13C NMR unless stated otherwise. Chemical shift (δ 0.00 ppm) values are reported in parts per million and coupling constants in Hertz (Hz). Splitting patterns are described as singlet (s), doublet (d), triplet (t), and multiplet (m). Reactions were monitored by TLC with Whatman Flexible TLC silica gel G/UV 254 precoated plates (0.25 mm). TLC plates were visualized by UV (254 nm) and by staining in an iodine chamber. Flash column chromatography was performed with silica gel 60 (EMD Chemicals, 230–400 mesh, 0.063 mm particle size).
4-Bromo-1-(ethoxymethoxy)-2-methoxybenzene (13)
Compound 12 (8.0 g, 39 mmol) and diisopropylethyl amine (13.7 mL, 78.8 mmol) were stirred in DCM (100 mL) at 0 °C for 15 min. Ethoxycholoromethyl ether (5.48 mL, 59.1 mmol) was added dropwise to the solution at 0 °C. The solution was allowed to stir at rt for 1 h. The reaction mixture was washed with 0.1N HCl (120 mL). The organic layers were combined, dried over sodium sulfate and evaporated to dryness to give 13 (10.1 g, 98%) as a brownish oil. This product was used in the subsequent reaction without any further purification: 1H NMR (CDCl3, 500 MHz) δ 7.06-7.00 (m, 3H), 5.26 (s, 2H), 3.86 (s,3H), 3.76 (q, J = 7 Hz, 2H), 1.22 (t, J = 7 Hz, 3H); 13C NMR (CDCl3, 125 MHz) δ 150.5, 145.9, 123.6, 117.7, 115.2, 114.4, 94.2, 64.5, 56.1, 15.1; HRMS (ESI) m/z calcd. for C10H13BrO3 ([M+Na]+), 282.9940, found 282.9942.
Methyl 3-(ethoxymethoxy)-2-methoxy-6-(2'-methoxy-2'-oxoethyl) benzoate (14)
Diisopropylamine (11.5 mL, 84.3 mmol) was stirred in dry THF (100 mL) at −78 °C in an oven-dried round bottom flask for 15 min. n-Butyllithium (2.5 M in hexane, 100 mL) was added and the solution was allowed to stir at −78 °C for 30 min. Dimethylmalonate (18.9 mL, 164 mmol) in 60 mL dry THF was added. The solution was stirred at −78 °C for 1 h and a solution of 13 (10.0 g, 38.3 mmol) in 50 mL dry THF was added. After 45 min, the reaction mixture was quenched with a saturated solution of ammonium chloride. THF was evaporated and the crude mixture was extracted with dichloromethane (4 × 100 mL). The solvent was reduced in vacuo and the crude product was purified via flash chromatography on silica gel (10% acetone/hexanes) to afford compound 14 (6.5 g, 54%) as a yellowish oil: 1H NMR (CDCl3, 500 MHz) δ 7.20 (d, J = 9 Hz, 1H), 6.96 (d, J = 9 Hz, 1H), 5.26 (s, 2H), 3.90 (s, 3H), 3.89 (s, 3H), 3.75 (q, J = 7 Hz, 2H), 3.68 (s, 3H), 3.62 (s, 2H), 1.23 (t, J = 7 Hz, 3H); 13C NMR (CDCl3, 125 MHz) δ 171.5, 167.6, 149.8, 147.6, 129.0, 126.5, 125.6, 118.0, 93.8, 64.6, 61.7, 52.2, 52.1, 38.4, 15.1; HRMS (ESI) m/z calcd. for C15H20O7 ([M+Na]+), 315.1101, found 315.1104.
2-[4'-(Ethoxymethoxy)-3'-methoxy-2'-(methoxycarbonyl)phenyl]acetic acid (15)
Compound 14 (5.8 g, 18 mmol) and potassium carbonate (5.1 g, 37 mmol) were refluxed in a 1:1 water/methanol mixture (150 mL) for 1 h. The methanol was evaporated and the crude mixture was acidified with 0.1N HCl. The solution was extracted with ethyl acetate (3 × 100 mL) and dried over sodium sulfate. Filtration and evaporation of the ethyl acetate extract afforded 15 (5.2 g, 95%) as a yellowish oil. This was used in the next step without purification: 1H NMR (CDCl3, 500 MHz) δ 7.21 (dd, J = 8, 3 Hz, 1H), 6.97 (dd, J = 8, 3 Hz, 1H), 5.26 (d, J = 2 Hz, 2H), 3.90 (d, J = 4 Hz, 3H), 3.88 (d, J = 2 Hz, 3H), 3.76 (q, J = 2 Hz, 2H), 3.64 (s, 2H), 1.27-1.21 (m, 3H); 13C NMR (CDCl3, 125 MHz) δ 171.1, 168.8, 150.0, 147.9, 128.3, 126.6, 125.4, 118.5, 93.7, 64.6, 61.7, 52.7, 38.9, 15.1; HRMS (ESI) m/z calcd. for C14H18O7 ([M+Na]+), 321.0945, found 321.0948.
Methyl 6-{2'-[(4"-(benzyloxy)-3"-methoxyphenethyl)amino]-2'-oxoethyl}-3-(ethoxymethoxy)-2-methoxybenzoate (17)
Compound 15 (5.0 g, 17 mmol) was dissolved in 60 mL THF and stirred at 0 °C for 15 min. 1,1'-Carbonyldiimidazole (2.7 g, 17 mmol) was added portion-wise to the solution at 0 °C and the mixture allowed to stir at rt for 1 h. A solution of 16 (4.3 g, 17 mmol) in 60 mL THF was added to the above reaction mixture at 0 °C and allowed to stir overnight at rt. THF was evaporated and the residue was dissolved in 150 mL dichloromethane and washed with sodium bicarbonate and 0.1N HCl consecutively. The organic layer was dried, filtered and concentrated in vacuo to afford 17 (7.0 g, 78%) as a brown oil : 1H NMR (CDCl3, 500 MHz) δ 7.43 (d, J = 7 Hz, 2H), 7.37 (t, J = 8 Hz, 2H), 7.29 (t, J = 7 Hz, 1H), 7.18 (d, J = 9, 1H), 6.98 (d, J = 9 Hz, 1H), 6.74 (d, J = 8 Hz, 1H), 6.68 (d, J = 2 Hz, 1H), 6.50 (dd, J = 8, 2 Hz, 1H), 6.16 (t, J = 5 Hz, 1H), 5.25 (s, 2H), 5.11 (s, 2H), 3.88 (s, 6H), 3.84 (s, 3H), 3.75 (q, J = 7 Hz, 2H), 3.40-3.39 (m, 3H), 2.67 (t, J = 7 Hz, 2H), 1.23 (t, J = 7 Hz, 3H); 13C NMR (CDCl3, 125 MHz) δ 170.3, 168.4, 149.7, 149.6, 147.3, 146.7, 137.4, 132.1, 128.8, 128.5, 128.5, 127.8, 127.3, 127.3, 126.8, 126.1, 120.6, 118.5, 114.2, 112.4, 93.8, 71.1, 64.7, 61.6, 55.9, 52.6, 41.2, 40.9, 35.2, 15.1; HRMS (ESI) m/z calcd. for C30H35NO8 ([M+Na]+), 560.2255, found 560.2260.
2-(Benzyloxy)-10-hydroxy-3,9-dimethoxy-5,6,13,13atetrahydro-8H-isoquinolino[3,2-a]isoquinolin-8-one (18)
Compound 17 (5.0 g, 9.3 mmol) and POCl3 (6.95 mL, 74.4 mmol) were refluxed in 60 mL acetonitrile for 3 h. The solvent was evaporated and the residue was dissolved in 120 mL DCM. The resulting solution was washed with sodium bicarbonate and then dried over sodium sulfate. The organic solvent was removed in vacuo and methanol (100 mL) was added. Sodium borohydride (1.4 g, 37 mmol) was added at 0 °C and the mixture allowed to stir for 6 h. The reaction was quenched with water and methanol was evaporated. The residue was extracted with DCM (3 × 80 mL). The organic layer was dried, filtered and evaporated to dryness to afford 18 (3.2 g, 79%). It was used in the next step without further purification: 1H NMR (CDCl3, 500 MHz) δ 7.44 (d, J = 7 Hz, 2H), 7.37 (t, J = 8 Hz, 2H), 7.29 (t, J = 7 Hz, 1H), 7.06 (d, J = 10 Hz, 1H), 6.84 (d, J = 10 Hz, 1H), 6.71 (s, 1H), 6.68 (s, 1H), 6.01 (s, 1H), 5.15 (s, 2H), 4.99 (d, J = 10 Hz, 1H), 4.68 (dd, J = 16, 4, 1H), 4.00 (s, 3H), 3.90 (s, 3H), 2.93-2.70 (m, 5H); 13C NMR (CDCl3, 125 MHz) δ 162.6, 149.0, 148.8, 147.3, 146.9, 137.0, 130.7, 128.6, 128.6, 128.1, 127.9, 127.5, 127.4, 127.4, 122.8, 121.5, 118.1, 112.6, 111.9, 71.6, 62.4, 56.1, 54.9, 38.9, 38.3, 29.4; HRMS (ESI) m/z calcd. for C26H25NO5 ([M+H]+), 432.1805, found 432.1809.
2-(Benzyloxy)-3,9,10-trimethoxy-5,8,13,13a-tetrahydro-6H-isoquinolino[3,2-a]isoquinoline (19)
Compound 18 (0.03 g, 0.07 mmol) was dissolved in 5 mL DMF. Potassium carbonate (0.02 g, 0.1 mmol) and methyl iodide (0.013 mL, 0.21 mmol) were added and reaction mixture was stirred at rt for 6 h. DMF was evaporated and 0.1 N HCl (15 mL) was added. The crude mixture was extracted with dichloromethane (3 × 20 mL). The combined organic layer was dried and concentrated to yield 19 (0.027 g, 91%) which was used in the next step without further purification: 1H NMR (CDCl3, 500 MHz) δ 7.46 (t, J = 7 Hz, 2H), 7.38 (t, J = 7 Hz, 2H), 7.31 (t, J = 7 Hz, 1H), 6.83 (d, J = 9 Hz, 1H), 6.78 (d, J = 8 Hz, 1H), 6.75 (s,1H), 6.64 (s, 1H), 5.14 (s, 2H), 4.20 (d, J = 16 Hz, 1H), 3.88 (s, 3H), 3.85 (s, 6H), 3.53-3.46 (m, 2H), 3.20-3.07 (m, 3H), 2.75-2.60 (m, 3H); 13C NMR (CDCl3, 125 MHz) δ 162.7, 152.1, 150.9, 148.0, 147.9, 137.0, 131.7, 128.6, 128.6, 127.9, 127.7, 127.6, 127.3, 127.3, 123.9, 122.0, 118.1, 111.4, 109.1, 71.4, 61.7, 56.2, 55.97, 54.9, 39.3, 38.2, 29.5; HRMS (ESI) m/z calcd. for C27H27NO5 ([M+H]+), 446.1962, found 446.1968.
(±)-Isocorypalmine [(±1)]
Compound 19 (0.02 g, 0.05 mmol) in 5 mL THF was added to a suspension of lithium aluminum hydride (0.005 g, 0.1 mmol) and the reaction mixture allowed to heat at reflux for 1 h. The reaction mixture was allowed to cool to rt and excess of lithium aluminum hydride was quenched with water. THF was evaporated and the crude mixture was extracted with dichloromethane (4 × 10 mL). The organic layer was evaporated and the resulting crude product was refluxed in a mixture of methanol (5 mL) and concentrated hydrochloric acid (3 mL) for 3 h. Methanol was evaporated and the resulting mixture was basified with ammonia solution, and extracted with dichloromethane (3 × 10 mL). The combined organic layer was dried, filtered and concentrated to give the crude product, which was purified by flash column chromatography on silica gel (2% – 5% MeOH/DCM) to give (±)-Isocorypalmine (0.012 g, 81%); mp: 216–221 °C (lit.33 219–222 °C); 1H NMR (CDCl3, 500 MHz) δ 6.87 (d, J = 8 Hz, 1H), 6.83 (s, 1H), 6.78 (d, J = 8 Hz, 1H), 6.60 (s, 1H), 4.24 (d, J = 16 Hz, 1H), 3.88 (s, 3H), 3.85 (s, 6H), 3.55-3.51 (m, 2H), 3.28-3.14 (m, 3H), 2.81 (dd, J = 16, 12 Hz, 1H), 2.68-2.61 (m, 2H); 13C NMR (CDCl3, 125 MHz) δ 150.2, 145.1, 145.0, 143.9, 130.6, 128.7, 127.9, 126.1, 123.9, 111.3, 110.9, 110.6, 60.2, 59.2, 55.9, 55.9, 54.0, 51.6, 36.3, 29.7; HRMS (ESI) m/z calcd. for C20H23NO4 ([M+H]+), 342.1700, found 342.1704.
2-(Benzyloxy)-10-hydroxy-3,9-dimethoxy-5,6-dihydro-8H-isoquinolino[3,2-a]isoquinolin-8-one: (21)
Compound 17 (0.2 g, 0.4 mmol) and POCl3 (0.28 mL, 3.0 mmol) were refluxed in 10 mL acetonitrile for 3 h. The solvent was evaporated and the residue was dissolved in 25 mL DCM. The resulting solution was washed with sodium bicarbonate and then dried over sodium sulfate. The organic solvent was removed in vacuo and the residues were dissolved in 10 mL dry DCM. Triethylamine (1.6 mL, 0.10 mmol) was added to the solution and allowed to stir at rt for 2 h. The reaction mixture was washed with 0.1N HCl and extracted with DCM (3 × 15 mL). The combined organic layer was evaporated and concentrated to yield 21 (0.11 g, 70%) which was used in the next step without further purification: 1H NMR (CDCl3, 500MHz) δ 7.49 (d, J =7 Hz, 2H), 7.40 (t, J = 7 Hz, 2H), 7.35-7.30 (m, 2H), 7.27 (s, 1H), 7.20 (d, J = 9 Hz, 1H), 6.75 (s, 1H), 6.60 (s, 1H), 5.21 (s, 2H), 4.31 (t, J = 6 Hz, 2H), 4.05 (s, 3H), 3.93 (s, 3H), 2.92 (t, J = 6 Hz, 2H); 13C NMR (CDCl3, 125 MHz) δ 159.7, 151.0, 147.7, 147.6, 145.5, 136.9, 135.3, 132.2, 129.0, 128.5, 128.5, 127.9, 127.4, 127.4, 122.9, 122.3, 121.1, 118.1, 111.3, 111.0, 101.4, 71.9, 62.6, 56.0, 39.4, 28.2; HRMS (ESI) m/z calcd. for C26H23NO5 ([M+H]+), 430.1649, found 430.1652.
Oxypalmatine (2)
Compound 21 (0.015 g, 0.033 mmol) was refluxed in a mixture of methanol (10 mL) and concentrated HCl (5 mL) for 3 h. Methanol was evaporated and the resultant mixture was basified with ammonia solution, and extracted with dichloromethane (3 × 10 mL). The combined organic layer was dried and concentrated to give a crude product, which was used in the next step without further purification. Crude residues were dissolved in 5 mL DMF. Potassium carbonate (0.014 g, 0.10 mmol) and methyl iodide (0.013 mL, 0.20 mmol) were added and reaction mixture was stirred at rt for 6 h. DMF was evaporated and 0.1 N HCl (10 mL) was added. The crude mixture was extracted with dichloromethane (3 × 10 mL). The combined organic layer was dried the crude product was purified by flash column chromatography on silica gel (1% – 3% methanol/dichloromethane) to yield oxypalmatine (2) (0.01 g, 79%); mp: 178–185 °C (lit.31 181–183 °C); 1H NMR (CDCl3, 500 MHz) δ 7.33 (d, J =9 Hz, 1H), 7.3 (d, J = 9 Hz, 1H), 7.23 (s, 1H), 6.76 (s, 1H), 6.73 (s, 1H), 4.32 (t, J = 6 Hz, 2H), 4.02 (s, 3H), 3.98 (s, 3H), 3.96 (s, 3H), 3.94 (s, 3H), 2.93 (t, J = 6 Hz, 2H); 13C NMR (CDCl3, 125 MHz) δ 160.3, 151.4, 150.1, 149.6, 148.4, 135.6, 132.4, 128.5, 122.3, 122.1, 119.4, 118.9, 110.5, 107.5, 100.9, 61.6, 56.9, 56.24, 56.0, 39.4, 28.2; HRMS (ESI) m/z calcd. for C21H21NO5 ([M+H]+), 368.1492, found 368.1497.
Supplementary Material
Figure 1.
Structures of (−)-ICP and oxypalmatine
Acknowledgments
This publication was made possible by Grant Numbers 1SC1GM092282 and G12RR003037 from the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or its divisions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
NMR spectra for all new compounds.
References and notes
- 1.Faizi S, Khan RA, Azher S, Khan SA, Tauseef S, Ahmad A. Planta Med. 2003;69:350–355. doi: 10.1055/s-2003-38883. [DOI] [PubMed] [Google Scholar]
- 2.Costa EV, Marques FdA, Pinheiro MLB, Braga RM, Delarmelina C, Duarte MCT, Ruiz ALTG, Ernesto de Carvalho J, Maia BHLNS. J. Braz. Chem. Soc. 2011;22:1111–1117. [Google Scholar]
- 3.Ito C, Itoigawa M, Tokuda H, Kuchide M, Nishino H, Furukawa H. Planta Med. 2001;67:473–475. doi: 10.1055/s-2001-15815. [DOI] [PubMed] [Google Scholar]
- 4.Desgrouas C, Taudon N, Bun S-S, Baghdikian B, Bory S, Parzy D, Ollivier E. J. Ethnopharmacol. 2014;154:537–563. doi: 10.1016/j.jep.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Sahni S, Maurya S, Jha RN, Pandey VB, Singh UP. Mycobiology. 2004;32:160–163. [Google Scholar]
- 6.Xiao H-T, Peng J, Liang Y, Yang J, Bai X, Hao X-Y, Yang F-M, Sun Q-Y. Nat. Prod. Res. 2011;25:1418–1422. doi: 10.1080/14786410802496911. [DOI] [PubMed] [Google Scholar]
- 7.Adsersen A, Kjoelbye A, Dall O, Jaeger AK. J. Ethnopharmacol. 2007;113:179–182. doi: 10.1016/j.jep.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Lapish CC, Ahn K-C, Chambers RA, Ashby DM, Ahn S, Phillips AG. Neuropsychopharmacology. 2014;39:1754–1762. doi: 10.1038/npp.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma B, Yue K, Chen L, Tian X, Ru Q, Gan Y, Wang D, Jin G, Li C. Neurosci. Lett. 2014;559:67–71. doi: 10.1016/j.neulet.2013.10.066. [DOI] [PubMed] [Google Scholar]
- 10.Sun H, Zhu L, Yang H, Qian W, Guo L, Zhou S, Gao B, Li Z, Zhou Y, Jiang H, Chen K, Zhen X, Liu H. Bioorg. Med. Chem. 2013;21:856–868. doi: 10.1016/j.bmc.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Ma Z-Z, Xu W, Jensen NH, Roth BL, Liu-Chen L-Y, Lee DYW. Molecules. 2008;13:2303–2312. doi: 10.3390/molecules13092303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu W, Wang Y, Ma Z, Chiu Y-T, Huang P, Rasakham K, Unterwald E, Lee DYW, Liu-Chen L-Y. Drug Alcohol Depend. 2013;133:693–703. doi: 10.1016/j.drugalcdep.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kametani T, Terui T, Agui H, Fukumoto K. J. Heterocycl. Chem. 1968;5:753–755. [Google Scholar]
- 14.Bhakuni DS, Kumar P. J. Indian Chem. Soc. 1988;65:417–421. [Google Scholar]
- 15.Kametani T, Noguchi I, Saito K, Kaneda S. J. Chem. Soc. C. 1969:2036–2038. doi: 10.1039/j39690002036. [DOI] [PubMed] [Google Scholar]
- 16.Orito K, Matsuzaki T, Suginome H. Org. Prep. Proced. Int. 1989;21:309–314. [Google Scholar]
- 17.Zhai H, Miller J, Sammis G. Bioorg Med Chem Lett. 2012;22:1557–1559. doi: 10.1016/j.bmcl.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Mujahidin D, Doye S. Eur. J. Org. Chem. 2005:2689–2693. [Google Scholar]
- 19.Cushman M, Dekow FW. J. Org. Chem. 1979;44:407–409. [Google Scholar]
- 20.Gao S, Cheng J-J, Ling C-Y, Chu W-J, Yang Y-S. Tetrahedron Lett. 2014;55:4856–4859. [Google Scholar]
- 21.Rathwell DCK, Yang S-H, Tsang KY, Brimble MA. Angew. Chem. Int. Ed. 2009;48:7996–8000. doi: 10.1002/anie.200903316. S7996/1-S7996/22. [DOI] [PubMed] [Google Scholar]
- 22.Sloman DL, Mitasev B, Scully SS, Beutler JA, Porco JA., Jr Angew. Chem. Int. Ed. 2011;50:2511–2515. doi: 10.1002/anie.201007613. S2511/1-S2511/55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shair MD, Yoon T-y, Danishefsky SJ. Angew. Chem. Int. Ed. Engl. 1995;34:1721–1723. [Google Scholar]
- 24.Crestey F, Jensen AA, Borch M, Andreasen JT, Andersen J, Balle T, Kristensen JL. J. Med. Chem. 2013;56:9673–9682. doi: 10.1021/jm4013592. [DOI] [PubMed] [Google Scholar]
- 25.Uematsu N, Fujii A, Hashiguchi S, Ikariya T, Noyori R. J. Am. Chem. Soc. 1996;118:4916–4917. [Google Scholar]
- 26.Le TN, Cho W-J. Chem. Pharm. Bull. 2008;56:1026–1029. doi: 10.1248/cpb.56.1026. [DOI] [PubMed] [Google Scholar]
- 27.Chen A, Zhao K, Zhang H, Gan X, Lei M, Hu L. Monatsh. Chem. 2012;143:825–830. [Google Scholar]
- 28.Suau R, Lopez-Romero JM, Ruiz A, Rico R. Tetrahedron. 2000;56:993–998. [Google Scholar]
- 29.Lee C-S, Yu T-C, Luo J-W, Cheng Y-Y, Chuang C-P. Tetrahedron Lett. 2009;50:4558–4562. [Google Scholar]
- 30.Reimann E, Grasberger F, Polborn K. Monatsh. Chem. 2003;134:991–1014. [Google Scholar]
- 31.Le TN, Cho W-J. Bull. Korean Chem. Soc. 2007;28:763–766. [Google Scholar]
- 32.Govindachari TR, Rajadurai S, Ramadas CV. Chem. Ber. 1959;92:1654–1657. [Google Scholar]
- 33.Tomita M, Furukawa H. Yakugaku Zasshi. 1967;87:881–882. doi: 10.1248/yakushi1947.87.7_881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.