Abstract
Two hypotheses about the role of the ventromedial prefrontal cortex (vmPFC) in narrative comprehension inferences, global semantic coherence versus socio-emotional perspective, were tested. Seven patients with vmPFC lesions and seven demographically matched healthy comparison participants read short narratives. Using the consistency paradigm, narratives required participants to make either an emotional or visuo-spatial inference, in which a target sentence provided consistent or inconsistent information with a previous emotional state of a character or a visuo-spatial location of an object. Healthy comparison participants made the inferences both for spatial and emotional stories, as shown by longer reading times for inconsistent critical sentences. For patients with vmPFC lesions, inconsistent sentences were read slower in the spatial stories, but not in the emotional ones. This pattern of results is compatible with the hypothesis that vmPFC contributes to narrative comprehension by supporting inferences about socio-emotional aspects of verbally described situations.
Keywords: Narrative comprehension, Text coherence, Text inferences, Spatial inferences, Emotional inferences, Ventromedial Prefrontal Cortex
1. Introduction
Narratives form the social fabric of people’s lives and become ever more important in clinical settings and verbal-based therapies for behavioral and psychiatric disturbances. Understanding narratives is a complex task that involves basic processing at phonological, lexical-semantic and syntactic levels. Additionally, inferential processes are specific to the discourse level and are required to connect the parts of the narrative to build a mental representation of the events. Thus, inferences consist of information not explicitly stated that has to be added to establish referential and thematic continuity across sentences in order to grasp the gist of a story (Graesser, Singer, & Trabasso, 1994; Singer, 2007; van Dijk & Kintsch, 1983). Local (Graesser et al., 1994) or bridging inferences (Singer, 2007) are needed to solve the referents of pronouns or nominal constructions, and also to unravel some explicit temporal (e.g., before, after, during, and, then) or causal (e.g., because, so, in order to) connectors. Global inferences are required to build a coherent semantic representation of the text or discourse, including causal, spatial and thematic relations between agents, objects and events (Graesser et al., 1994); this is achieved by elaborative inferences (Singer, 2007).
Traditional behavioral studies of inferences started with offline tasks, such as asking questions; however, this procedure confounds comprehension with reasoning and text recall (Singer, 2007). Alternative experimental approaches were designed to capture inferences as they occur online during comprehension (Haberlandt, 1994). In the experimental paradigm termed consistency tasks (McKoon & Ratcliff, 1992; O’Brien & Albrecht, 1992), subjects read a narrative that involves a protagonist and a situation describing his or her state, spatial location, time frame, and/or causal chain of events. At the end of the narrative, a critical phrase refers to the protagonist in an emotional state, in a location, or performing an action, which may be consistent or inconsistent with his or her previous status. For example, in O’Brien and Albrecht (1992, p. 781) “As Kim stood inside (consistent)/outside (inconsistent) the health club she felt a little sluggish. She decided to go outside and stretch her legs for a little.” Longer reading times for the critical sentence in the inconsistent condition compared to the consistent condition suggest that readers infer the narrative dimension (i.e., spatial location in the example) even though it is not explicitly stated at the moment. The consistency effect is robust and has been established for the visuo-spatial dimension (De Vega, 1995; Glenberg, Meyer, & Lindem, 1987; Irrazabal & Burin, 2006; O’Brien & Albrecth, 1992; Therriault & Rinck, 2006), emotional or personal traits of the protagonist (Albrecht & O’Brien, 1993; Gernsbacher, Goldsmith & Robertson, 1992; Gygax, Garnham & Oakhill, 2004; De Vega, León & Díaz, 1996; Molinari, Burin, Saux et al., 2009; Therriault & Rinck, 2006), temporal inferences (Rinck, Hahnel & Becker, 2001; Therriault & Rinck, 2006), and other dimensions that lead to causal relations between characters and actions (see Singer, 2007).
Neuroimaging research of diverse higher-language comprehension tasks such as reading connected sentences vs. unconnected sentences or words (Robertson, Gernsbacher, Guidotti, Robertson, Irwin, Mock, & Campana, 2000), reading text with and without title (St. George, Kutas, Martinez, & Sereno, 1999), and comprehension of fables (Nichelli, Grafman, Pietrini et al., 2005), suggests that an extended language network involving frontal (dorsolateral, middle, and ventrolateral), temporal (anterior, middle, inferior), temporo-parietal, and posterior cingulate areas of both hemispheres is involved in language comprehension at the discourse level (Ferstl, Neumann, Bogler, & von Cramon, 2008). In particular, studies with online tasks presenting two or several sentences needing cohesive inferences across them, highlight the role of medial prefrontal regions in detection of coherence (or the lack of it) (Ferstl, Rinck & von Cramon, 2005; Hasson, Nusbaum, & Small, 2007; Kuperberg, Lakshmanan, Caplan, & Holcomb, 2006; Mason & Just, 2004; Virtue, Haberman, Clancy, Parrish, & Jung-Beeman, 2006; Virtue, Parrish & Jung-Beeman, 2008; Xu, Kemeny, Park, Frattali, & Braun, 2005). Studies vary in design, and so do contrasts of active areas; they include varying portions of temporal, dorso and ventrolateral prefrontal, ventromedial prefrontal, and cingulate regions. Mason and Just (2004), Kuperberg et al. (2006), and Virtue et al. (2008) varied the predictability or causal relatedness of the inference and found that bilateral dorsolateral and inferior prefrontal areas showed increased activation as the sentences became less causally related, thus forcing strategic interpretation and taxing working memory. But medial prefrontal cortex was observed to be active in all of the studies mentioned, even when passive listening was examined (including cingulate areas, Hasson et al., 2007), suggesting that its role in extracting coherence is not driven by exogenous task demands, effort, or working memory demands. According to these studies, the medial prefrontal cortex would carry on implicit processes of semantic activation and inhibition in order to extract global coherence; while on the other hand, dorsolateral prefrontal areas would process strategic, elaborative search, and tasks with high working memory load. Synthesizing these results, it appears that the ventral and medial prefrontal brain structures are a key component of text semantic integration and coherence across sentences.
An alternative interpretation of these results posits that the ventral medial prefrontal cortex (vmPFC) might be selectively involved in understanding a socio-emotional perspective akin to the theory of mind (ToM) conceptual framework (e.g., Stone, Baron-Cohen, & Knight, 1998). The overlap between narrative comprehension and ToM neural network has been underlined by many of the authors in the text comprehension literature (Ferstl & Von Cramon, 2002; Ferstl et al., 2008; Mar, 2004; Mason & Just, 2004, 2009). For example, Mason and Just (2009, 2011) have proposed that narrative comprehension includes parallel networks for coherence monitoring (which would be carried by bilateral dorsolateral prefrontal areas) and for text integration (left inferior frontal/left anterior temporal), and a “protagonist perspective” network (bilateral medial frontal/posterior right temporal /parietal), by which the reader applies ToM processes for interpreting the intentions, goals, and actions of characters within a narrative. Thus, emotional inferences would stem from a socio-emotional stance. Several neuroimaging studies with healthy participants (Fletcher, Happe, Frith, Baker, Dolan, Frackowiak, & Frith, 1995; Ferstl & von Cramon, 2002; Mason & Just, 2011; Vogeley, Bussfeld, Newen, Herrmann, Happe, Falkai, Maier, Shah, Fink, & Zilles, 2001) have compared stories with socio-emotional versus physical or logical inferences, but required an explicit social or emotional judgment, and results are mixed. An early study addressing this issue (Fletcher et al., 1995) asked participants to read three types of texts: ToM stories (which required the reader to make inferences about the internal mental states of the characters), physical stories (about physical events, not the mental processes of the characters in the story), and unrelated sentences. Relative to sentences, both story conditions showed increased activation at the temporal poles and left superior temporal gyrus; activation in mid frontal cortex was specifically associated with ToM stories. However, it is not clear whether activation was related to differential difficulty or cognitive demands of the tasks (Tompkins, 2008, who placed the discussion regarding the role of the right hemisphere). On the other hand, Ferstl and von Cramon (2002) presented pairs of coherent or incoherent sentences eliciting inferences based on socio-emotional or logical, inanimate causation, and asked for an explicit consistency judgment. In the logical pairs (e.g. “Sometimes a truck drives by the house./That’s when the dishes start to rattle” vs.”/The car doesn’t start”) the instruction called to judge if there was a logical connection between sentences. In the ToM pairs (e.g., “Mary’s exam was about to begin./Her palms were sweaty” vs. “/Some friends had remembered the birthday”) instructions asked to understand the protagonist’s motivations, feelings, and actions, and judge if both sentences made sense. Although medial prefrontal activation was stronger in ToM stimuli, it was also active in coherence judgments for logical pairs, thus arguing for a domain-general initiation and maintenance of coherence function. Furthermore, the extent to which activation is related to an implicit semantic inference is not known, since in Ferstl and von Cramon (2002) an explicit judgment of coherence was required. In a similar vein, Mason and Just (2011) studied narratives with intentional (mental) versus physical content requiring inferences for coherence. There were differences in regional activation as a function of type of inference: intentional inferences elicited more activation in the right temporo-parietal junction, right inferior, middle and superior frontal gyrus; while physical inferences did so in middle occipital regions. However, drawing an inference of either type activated the “extended language network”: medial and superior frontal areas, bilateral inferior frontal gyri, the left posterior superior temporal gyrus, and the anterior temporal lobes bilaterally. Thus, neuroimaging studies comparing mental versus physical inferences have been scarce and do not adjudicate between accounts of the critical role of medial prefrontal structures as being specific to socio-emotional understanding or performing general semantic coherence processes.
Neuroimaging experiments in healthy subjects have delineated the brain networks activated by narrative comprehension and ToM; but such findings alone cannot identify whether any or all of these regions play a critical role. Complementary studies in patients with focal injuries can help identify which brain structures are key components for normal functioning. In general, subjects with vmPFC lesions present deficits in verbal and non-verbal ToM paradigms (Beadle & Tranel, 2011; Rowe, Bullock, Polkey, & Morris, 2001; Shamay-Tsoory, Tomer, Berger, & Aharon-Peretz, 2003; Stone et al., 1998). However, recent findings reported that subjects with this type of lesion did not have problems in developing and using common ground in social communication (e.g., using labels or verbal play) (Gupta, Tranel & Duff, 2012). On the other hand, these patients typically show deficits in judgment and decision making under uncertainty and risk, as shown in the Iowa Gambling Task (Anderson, Barrash, Bechara, & Tranel, 2006). In addition, as compared to subjects with dorsolateral frontal damage and normal controls, subjects with vmPFC lesions have shown a different, less efficient search procedure in complex, multi-attribute decision making task like a simulated real life choice (Fellows, 2006). The latter would suggest that, in line with the semantic activation and coherence hypothesis, the vmPFC is involved in a more general evaluation of contextual information.
The objective of the present study was to test the role of the vmPFC in text comprehension inferences: does it play a role in global semantic coherence or in the understanding of socio-emotional narratives? We compared the performance of subjects with vmPFC lesions and demographically match healthy comparison participants in making emotional or visuo-spatial inferences under the consistency paradigm. Subjects read short stories until a target sentence provided consistent or inconsistent information with the rest of the narrative. If the correct inference was made, the reader had to assume that the character was at a particular place with associated objects (visuo-spatial inference), or in a particular emotional state (emotional inference). The inconsistent sentence contradicted that implicit assumption, stating that the character was in a different place or emotional state. Longer reading times for the target inconsistent sentence signaled that subjects drew the inference. If the role of the vmPFC in narrative text comprehension consists of general semantic coherence processing, individuals with vmPFC lesions would show deficits in online inferential processing during narrative comprehension, as shown in reading time differences for consistent and consistent sentences, regardless of inferential content. If the vmPFC is involved in understanding socio-emotional perspectives, subjects with that type of lesion will show deficits in online inferential processing during narrative comprehension with emotional content, but not with visuo-spatial content. We included a matched comparison sample to examine the same, intra-group comparison of the consistency effect, which is predicted to hold for this group.
2. Method
2.1. Participants
2.1.1. Ventromedial prefrontal lesion group (vmPFC)
Seven patients with bilateral vmPFC damage (4 female) were recruited from the Patient Registry of the Division of Cognitive Neuroscience at the University of Iowa. Consistent with previous work, we defined the vmPFC as the region encompassing the medial orbital and lower medial sector of the prefrontal lobe, Broadmann areas, 25, 24, 32, 10, 11, 12 (Barrash, Tranel, & Anderson, 2000). Figure 1 shows the overlap of affected regions in all patients. As a group, the participants with vmPFC damage have been well characterized both from a neuropsychological and neuroanatomical perspective (Barrash et al., 2000, and in particular Gupta el al., 2012; Kurczek & Duff, 2013). Although these patients perform in the normal range on standardized measures of intelligence, memory, working memory, language, and even executive functioning (see Table 2) they all display deficits in ”real world competencies” (i.e., judgment, planning, and initiation in social, financial and occupational realms) (Anderson, Barrash, Bechara, & Tranel, 2006). Consistently, they perform poorly in experimental tests designed to measure real-life decision making such as the Iowa Gambling Task or Multiple Errands Test.
Figure 1.
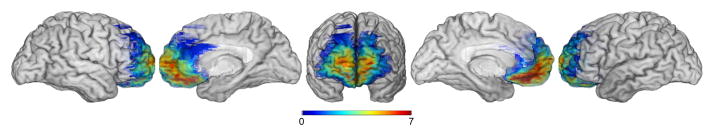
Table 2.
Neuropsychological Profiles for Patients with vmPFC Lesion.
| Subject | FSIQ | WMS GMI | WMS WMI | Digit Span | Arith | Sent Rept | Boston Naming | Token | COWA | WCS # Cat. | BDI | SIF | APP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 318 | 143 | 109 | 124 | 12 | 15 | 14 | 60 | 44 | 54 | 6 | 0 | Yes (3) | 3 |
| 1983 | 104 | 74 | 105 | 7 | 11 | N/A | 58 | 44 | 39 | 6 | 5 | Yes (3) | 3 |
| 2352 | 106 | 109 | 124 | 13 | 10 | N/A | 54 | 44 | 34 | 6 | 1 | Yes (3) | 2 |
| 2391 | 109 | 132 | 102 | 9 | 13 | 11 | 57 | 43 | 59 | 6 | 4 | Yes (2) | 2 |
| 2577 | 84 | 96 | 88 | 7 | 9 | 15 | 55 | 44 | 44 | 0 | 7 | Yes (3) | 3 |
| 3349 | 101 | 103 | 104 | 11 | 11 | 11 | 53 | 44 | 40 | 5 | 4 | Yes (1) | 1 |
| 3350 | 118 | 108 | 118 | 11 | 11 | 14 | 52 | N/A | 40 | 6 | 3 | Yes (1) | 1 |
|
| |||||||||||||
| Average (SD) | 109.3 (18.1) | 104.4 (17.4) | 109.3 (13.3) | 10.0 (2.4) | 11.4 (1.99) | 13.0 (1.9) | 55.6 (2.9) | 43.9 (0.4) | 43.9 (9.3) | 5.0 (2.4) | 3.4 (2.4) | 5.0 (2.4) | |
Note: FSIQ = Full Scale IQ from the Wechsler Adult Intelligence Scale IV; WMS GMI = Wechsler Memory Scale General Memory Index; WMS WMI = Wechsler Memory Scale Working Memory Index; WMS-III, Wechsler memory scale-III; Digit Span, digit span subtest; WAIS-III, age-corrected scaled score, Arith = arithmetic subtest; WAIS-III, age-corrected scaled score; Sent Rept = sentence repetition subtest; Boston Naming = Boston Naming Test; COWA = Controlled Oral Word Association Test; WCS # Cat. = Wisconsin Card Sort Test, Number of Categories Achieved; BDI = Beck Depression Inventory; SIF = Social and Interpersonal Functioning - the extent of post-lesion change or impairment in aspects of social conduct and interpersonal functioning was rated on a three-point scale, with 1 = no change or impairment, 2 = moderate change or impairment, 3 = severe change or impairment; APP = Acquired Personality Problems - refer to whether or not the participant had acquired problems in personality functioning, as derived from data on the Iowa Rating Scales of Personality Change. The numbers in parentheses denote degree of severity, where 1 = mild, 2 = moderate, and 3 = severe.
2.1.2. Healthy comparison participants
Seven individuals, without neurological or psychiatric disturbance also participated. All participants with vmPFC damage were matched pair-wise to these comparison participants respectively, on age 63.4 (SD = 8.1), 63.7 (SD = 7.6; t (10) = 0.068, p = 0.95), sex, and education 13.6 (SD = 2.3), 14.71 (SD = 1.38; t (10) = 1.128, p = 0.29).
2.2. Materials
Stories were adapted from Irrazabal and Burin (2006) and Molinari et al. (2008), based on seminal work by De Vega (1995) and De Vega et al. (1996). Both the original set of stories and the adapted ones have repeatedly shown the consistency effect on spatial and emotional narratives in healthy participants, when the instructions asked for reading to understand, or under different instructions. There were two sets of 16 experimental stories, each set containing spatial or emotional stories. Half of the stories had inconsistencies. Participants were asked to attend to the story and after each one answer a yes-no question. Questions asked about factual, explicit information in the story, to ensure that participants read comprehensively; they did not probe inferences. Tables 3 and 4 show the structure of spatial and emotional stories, respectively, and an example.
Table 3.
Structure of Spatial Stories (Example in the Second Column).
| A sentence introducing a protagonist | Miss Julia lives in a typical English house |
| Two long sentences (4 lines), describing two scenarios placed in-out or up-down and each scenario associated with an object | In the front, the house has a very well-kept garden with beautiful colored roses. In the living-room, there is a collection of clocks, which she inherited from her grandfather |
| The protagonist goes from one scenario to the other (one sentence) | Miss Julia enters into the house, walking with her cane |
| A target sentence, in which the protagonist interacts with an object associated with that scenario (consistent condition), or with an object associated with the other (inconsistent condition). | As usual, she contemplates her clocks with pride. |
| Consistent and inconsistent versions of the sentence have the same number of words, approximately the same (±2) number of syllables, and differ by 1 to 3 words. | As usual, she contemplates her roses with pride. |
| Question | Is the garden in front of the house? |
Table 4.
Structure of Emotional Stories (Example in the Second Column).
| A sentence introducing a protagonist | Andrew was the last one to finish the exam |
| 6–7 lines describing goals, states, actions and / or relations with other characters, without explicit mention of emotions. | He looked through it several times before handing it in. He was certain that he had failed. All the money and effort invested would go down the drain. That exam was almost his last chance to stay in college. He spent the weekend locked at home. He did not meet his friends as usual. He watched a game on TV and did the laundry. |
| A target sentence, stating how the protagonist feels, with an emotional word consistent or inconsistent with previous text. | Andrew felt really miserable. |
| Consistent and inconsistent versions of the sentence have the same number of words, differing only in the emotion label, and have approximately the same (±2) number of syllables. | Andrew felt really enthusiastic. |
| Question | Did Andrew finish the exam? |
Because all participants in the current study were monolingual English speakers, stories were translated from Spanish (Irrazabal & Burin, 2006; Molinari et al., 2008) to English by a Spanish/English bilingual research assistant. A consensus procedure with a second experimenter (the third author) matched as closely as possible grammar and meaning in the translated English stories. Stories were then pilot tested in a small sample (N = 10; 7 female, 3 male, undergraduate and graduate students at the University of Iowa) of healthy, English speaking college-aged adults to check whether the stories produced the same manner and size of effects as the Spanish stories from which they were adapted. Repeated measures ANOVAs on reading time for the critical sentence showed a significant effect for Type of Inference (F (1,9) = 16.11, p = 0.003, partial eta 2 = 0.64), and for Consistency (F (1,9) = 16.6, p = 0.003, partial eta 2 = 0.65). Partial eta 2 sizes indicate a moderate effect size. Inconsistent sentences took longer than Consistent ones, and Spatial stories took longer than Emotional ones. Mean (SD) for reading times, in milliseconds, were: Spatial, Consistent: 2221.33 (592.23); Spatial, Inconsistent 3358.69 (1479.80); Emotional, Consistent: 1595.20 (483.55); Emotional, Inconsistent: 2034.95 (493.43). Accuracy in the comprehension question was high irrespective of condition, and there was no evidence of a difference in accuracy of consistent and inconsistent sentences.
2.3. Procedure
Stories of each type were divided into two sets counterbalanced for consistency (e.g., one set has the consistent and the other one the inconsistent version). These sets were again divided in two, rendering 4 sets of texts to counterbalance their presentation in the experiment. The order of text appearance was randomized within each set. Experimental stimuli administration and response registration (time to read the target sentence and accuracy of responses) were implemented in E-prime v.1 software.
3. Results
To address the experimental hypotheses, response times in reading the critical sentence were analyzed. For each subject in each condition, outlier observations (+/− 1.5 SD) were deleted (13 % of all data points, 11% for controls and 15% for patients), and means (SD) for each condition were calculated. Table 5 shows descriptive statistics for reading times of the critical sentence, for each group in each experimental condition. Reading times were not normally distributed. Figure 2 shows the distribution of reading times in the consistent and inconsistent conditions, in spatial and emotional stories, and in the patient and comparison groups.
Table 5.
Mean (SD) Reading Times (Msec) for the Critical Sentence for each Group, by Type of Inference and Consistency of the Critical Sentence.
| VMPF | Control | |||
|---|---|---|---|---|
|
| ||||
| M (SD) Consist | M (SD) Inconsist | M (SD) Consist | M (SD) Inconsist | |
|
|
||||
| Emotional | 2635.5 (1193.4) | 3177.3 (1766) | 1818.16 (532.6) | 2489.3 (789.8) |
| Spatial | 3839.3 (1541.6) | 4525.82 (1641.1) | 2522.2 (745.45) | 3314.12 (1188) |
Figure 2.
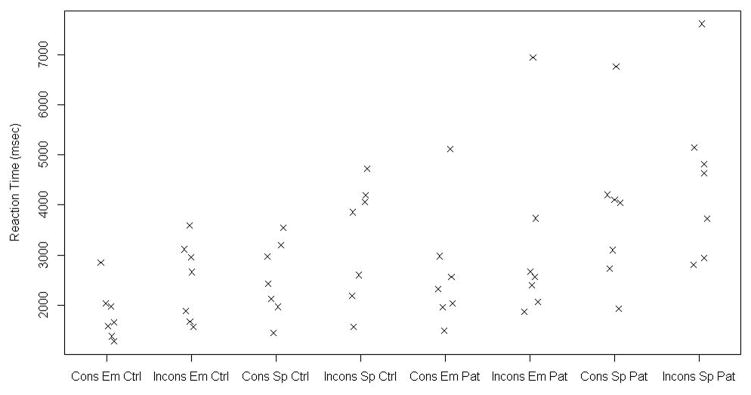
Given the departure from normality, the small sample size (i.e., N=7 in each group), and the dependence between the samples compared, results were examined using the Wilcoxon sign rank test.
Specifically addressing the experimental hypotheses, the difference between Consistent and Inconsistent critical sentences’ reading times were examined. Figure 2 shows that across all groups, tasks and type of stories, reading times for the inconsistent conditions seem longer than the consistent condition. However, non-parametric statistical analyses reveal that for the healthy comparison participants, the median time to read the Inconsistent sentence was significantly longer than the Consistent one, in both the Emotional stories (Wilcoxon Sign Rank test S = 14, p = 0.016) and the Spatial stories (Wilcoxon Sign Rank test S = 14, p = 0.016). For the vmPFC patients group, the median time to read the Inconsistent sentence was significantly longer than the Consistent one in the Spatial stories (Wilcoxon Sign Rank test S = 12, p = 0.047) but there was no significant difference in reading times for the Emotional stories (Wilcoxon Sign Rank test S= 10, p = 0.109).
Regarding differences in reading times for the critical sentence between groups, healthy comparison participants read marginally faster than patients the Consistent stories in both Emotional (Wilcoxon Sign Rank Text S = 11, p = .08) and Spatial (Wilcoxon Sign Rank Text S = 11, p = .08) conditions, but differences were not significant in the Inconsistent Stories (Emotional Wilcoxon Sign Rank Text S = 5, ns; Spatial Wilcoxon Sign Rank Text S = 9, ns).
Mean accuracy in responding to the comprehension question at the end of each story was high among all conditions (between 79% and 92% mean correct answers). Table 6 shows descriptive statistics in accuracy for each condition.
Table 6.
Mean (SD) Percent Correct in Questions for each Group by Type of Inference and Consistency of the Critical Sentence.
| VMPF | Control | |||
|---|---|---|---|---|
|
| ||||
| M (SD) Consist | M (SD) Inconsist | M (SD) Consist | M (SD) Inconsist | |
|
|
||||
| Emotional | 0.92 (0.10) | 0.89 (0.05) | 0.92 (0.10) | 0.86 (0.11) |
| Spatial | 0.86 (0.15) | 0.84 (0.09) | 0.79 (0.12) | 0.83 (0.09) |
Accuracy did not differ as a function of Consistency, for the patients (Emotional inference: Wilcoxon Sign Rank Test S = 3.5, ns; Spatial inference: Wilcoxon Sign Rank Test S = −0.5, ns), or for comparison participants (Emotional inference: Wilcoxon Sign Rank Test S = −5, ns; Spatial inference: Wilcoxon Sign Rank Test S = 3.5, ns). Comparing patients and comparison participants, there was not a significant difference in accuracy (Emotional consistent texts: Wilcoxon Sign Rank Test S = 0, ns; Emotional inconsistent texts: Wilcoxon Sign Rank Text S = 3.5, ns; Spatial consistent texts: Wilcoxon Sign Rank Text S = 4.5, ns; Spatial inconsistent texts: Wilcoxon Sign Rank Text S = 1, ns).
4. Discussion and Conclusions
The present study addressed the role of vmPFC in high-level language comprehension. Participants in the comparison group made the inferences both for spatial and emotional stories, as shown by longer reading times for inconsistent critical sentences when compared to consistent ones. Thus, we can conclude that the task captured inferential activity, in line with the consistency paradigm (Albrecht & O’Brien, 1993; De Vega, 1995; De Vega, et al., 1996; Molinari et al., 2009; O’Brien & Albrecth, 1992). Adding to the validity of the task, accuracy in responding to the control reading question was high, both for patients and comparison participants, and did not vary significantly either by group or by consistency condition. This pattern suggests that all participants complied equally with the instruction to understand the stories.
Patients with vmPFC lesions exhibited a trend towards responding more slowly in the consistent stories, both emotional and spatial. This result would be expected according to the neuropsychological literature, which shows that in general patients have slower processing and motor responses (Hicks & Birren, 1970; MacFlynn, Montgomery, Fenton, & Rutherford, 1984).
Regarding the consistency effect for patients with vmPFC lesions, we found that the critical sentence was read significantly slower in the inconsistent condition than in the consistent condition in the spatial stories, but not in the emotional ones. Thus, there was less reliable evidence of sensitivity to emotional inconsistencies than to spatial ones. However, the overall pattern of reading time distribution shown in Figure 2, shows similarities for patients and comparison participants, and for emotional and spatial-inference stories.
Therefore, in general, this pattern of results shows that damage to medial prefrontal regions does not lead to overall impairment in inferential processing when reading text. The fact that spatial stories elicited the consistency effect in patients, and that some patients seem to draw emotional inferences as well, would go against the hypothesis that the vmPFC plays a critical role in general semantic coherence processing. It is interesting to note that these patients were also able to maintain cohesion and coherence in spoken discourse when they generated narratives, picture descriptions, and story re-tellings (Kurczek & Duff, 2012).
As noted in the introduction, previous studies also showed activation in dorsal prefrontal, superior and middle temporal, and inferior parietal areas (Hasson et al., 2007; Kuperberg et al., 2006; Virtue et al., 2006; Xu et al., 2005). Those studies diverge in the type of inferences, not only socio-emotional, but also physical or practical matters, such as a wrinkled shirt before ironing vs. going to work (Virtue et al., 2006), safe or clean chemicals before chemical burns (Hasson et al., 2007). Thus, one possibility is that differences between studies rely on the type of inference. Nevertheless, neuroimaging studies that compared stories with socio-emotional versus physical or logical inferences also observed temporal and prefrontal activation when performing inferences (Ferstl & von Cramon, 2002, Fletcher et al., 1995; Mason & Just, 2011). Therefore, it is possible that the inferences needed to understand a story are supported by a pool of neurocognitive processes instead of a specialized capacity located in medial prefrontal areas. Mason and Just (2009, 2011) proposed that narrative comprehension relied on parallel networks for coherence monitoring (dorsolateral prefrontal areas) and for text integration (inferior frontal/anterior temporal); while Ferstl et al.’s (2008) meta-analysis of text comprehension included various temporal and frontal regions.
However, results have also shown that emotional inferences were more problematic than spatial ones for these patients. In this regard, they add to neuroimaging studies comparing emotional vs. physical or spatial inferences (Ferstl & von Cramon, 2002, Fletcher et al., 1995; Mason & Just, 2011; Vogeley et al., 2001). These studies had observed medial and ventral frontal activation in the socio-emotional stories when compared to physical or causal ones. Also, the study by Xu et al. (2005) employed narratives based on moral fables, which included goals, outcomes and reactions of the characters (thus, a socio-emotional stance), and found specific activations in the medial prefrontal and cingulate cortices, and in limbic regions, including the amygdala, linking it to emotional responses to the protagonist or other characters (in addition to the frontal and temporal areas observed in other studies). In general, this lesion approach converges with previous activation research, in that medial and ventral prefrontal areas contribute to the comprehension of stories with socio-emotional content. They make a case for a “protagonist perspective” network in which interpreting narrative socio-emotional aspects could recruit neurocognitive processes associated with emotional understanding and ToM (Mason and Just, 2009, 2011). From this perspective (Mason and Just, 2009, 2011), a “protagonist simulation” is recruited when the inference is based on the intention or emotional state of a character in the story. Mason and Just (2011) speculated that the midfrontal component, the “protagonist monitor”, tracks the progress of the characters in the narrative. In our stories, tracking is needed in both spatial (where is the protagonist?) and emotional (how does the protagonist feel?) stories, but patients have more problems with the emotional stories. In light of this, the “protagonist monitor” should be more specific for socio-emotional states.
On the other hand, given that the distribution of the individual reading times of patients in the emotional inconsistent sentences partially overlap with those corresponding to spatial ones, or to the healthy controls, a strong embodiment hypothesis, by which text and language comprehension equals to generating a simulation of the same perceptions, actions and experiences in real life (e.g. Zwaan, 2004) would not be supported.
Previous studies revealed deficits of affective but not cognitive ToM in patients with vmPFC damage (Leopold, Krueger, Dal Monte, Pardini, Pulaski, Solomon, & Grafman, 2011). In the same vein, the same patients tested in this study did not show impairments in coherence in oral discourse (Kurzek & Duff, 2012), nor had problems in establishing and maintenance of common ground, e.g. inferring shared knowledge between conversational partners in actual dialogue (Gupta et al., 2012). It could be possible that damage to vmPFC brings deficits to affective, but not cognitive, aspects of ToM. On the other hand, it could also be the case that ToM relies on an extensive network, just as the case for text comprehension, so that the neural basis of affective responses might contribute, but not be a key factor, for ToM.
In this study, patients with vmPFC lesions were also shown to construct a coherent representation from different sentences or pieces of information, integrating places, objects and actions; thus, they did not have a general problem with multi-attribute situations, in contrast with results suggested in judgment and decision making paradigms (Bechara et al., 2000; Fellows, 2006). However, tasks vary in many ways. For example, the consistency paradigm employed here measured an implicit inference. In contrast, decision making tasks require explicit judgments (Bechara et al., 2000; Fellows, 2006). Experimental tasks bridging both paradigms would be needed to advance this question.
A final limitation of this study is the small size of the sample. These findings should be probed in larger samples of subjects with discrete lesions of the vmPFC, and could also be compared with those observed in individuals with other focal brain lesions. Also, other types of inferences should be explored.
In conclusion, results obtained in this experiment argue that the vmPFC has a role in supporting online inferences about socio-emotional descriptions or scenarios in narrative comprehension. They also suggest that these brain areas are not involved in inferential processing towards semantic coherence in general.
Supplementary Material
Table 1.
Demographic Information for Patients with vmPFC Lesion
| Subject | Sex | Hand | Age | Education | Etiology |
|---|---|---|---|---|---|
| 318 | M | R | 69 | 14 | Meningioma resection |
| 1983 | F | R | 46 | 13 | ACoA aneurysm |
| 2352 | F | R | 60 | 14 | SaH; ACoA aneurysm |
| 2391 | F | R | 63 | 13 | Meningioma resection |
| 2577 | M | R | 69 | 12 | SaH; ACoA aneurysm |
| 3349 | F | L | 66 | 13 | Meningioma resection |
| 3350 | M | R | 57 | 18 | Meningioma resection |
Note: Hand = handedness; Education= Years of formal education; AcoA = anterior communicating artery; SaH = sub-arachnoid hemorrhage
Acknowledgments
Financial support for this project was partially given by CONICET (Consejo Nacional de Investigaciones Cientificas y Tecnicas de Argentina) Research Grant PIP 11220090100036CO and a U.S.A., Department of State, Fulbright Visiting Scholarship awarded to the first author and NINDS P01 NS19632 to DT.
References
- Albrecht JE, O’Brien EJ. Updating a mental model: Maintaining both local and global coherence. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1993;19:1061–1070. [Google Scholar]
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real world complex behavior following childhood- or adult-onset lesions in ventromedial prefrontal cortex. Journal of the International Neuropsychological Society. 2006;12:224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Developmental Neuropsychology. 2000;18:355–381. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Beadle J, Tranel D. Social neuroscience: a neuropsychological perspective. In: Cacioppo J, Decety J, editors. Topics in social neuroscience. New York: Oxford University Press; 2011. [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Beeman M, Bowden E, Gernsbacher M. Right and left hemisphere cooperation for drawing predictive and coherence inferences during normal story comprehension. Brain and Language. 2000;71:310–336. doi: 10.1006/brln.1999.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vega M. Backward updating of mental models during continuous reading of narratives. Journal of Experimental Psychology: Learning, Memory and Cognition. 1995;21:373–386. doi: 10.1037//0278-7393.21.2.373. [DOI] [PubMed] [Google Scholar]
- De Vega M, León I, Diaz JM. The representation of changing emotions in reading comprehension. Cognition and Emotion. 1996;10:303–321. [Google Scholar]
- Fellows LK. Deciding how to decide: Ventromedial frontal lobe damage affects information acquisition in multi-attribute decision making. Brain. 2006;129:944–952. doi: 10.1093/brain/awl017. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, von Cramon DY. What does the fronto-median cortex contribute to language processing: Coherence or Theory of Mind? NeuroImage. 2002;17:1599–1612. doi: 10.1006/nimg.2002.1247. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, Guthke T, von Cramon DY. Text comprehension after brain injury: Left prefrontal lesions affect inference processes. Neuropsychology. 2002;16:292–308. doi: 10.1037//0894-4105.16.3.292. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, Neumann J, Bogler C, von Cramon DY. The extended language network: A meta-analysis of neuroimaging studies on text comprehension. Human Brain Mapping. 2008;29:581–593. doi: 10.1002/hbm.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferstl EC, Rinck M, von Cramon DY. Emotional and temporal aspects of situation model processing during text comprehension: An event-related fMRI study. Journal of Cognitive Neuroscience. 2005;17:724–739. doi: 10.1162/0898929053747658. [DOI] [PubMed] [Google Scholar]
- Fletcher P, Happe F, Frith U, Baker S, Dolan R, Frackowiak R, Frith C. Other minds in the brain: A functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Gernsbacher M, Goldsmith H, Robertson R. Do readers mentally represent characters’ emotional states? Cognition and Emotion. 1992;6:89–111. doi: 10.1080/02699939208411061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenberg A, Meyer M, Lindem K. Mental models contribute to foregrounding during text comprehension. Journal of Memory and Language. 1987;26:69–83. [Google Scholar]
- Graesser AC, Singer M, Trabasso T. Constructing inferences during narrative text comprehension. Psychological Review. 1994;101:371–395. doi: 10.1037/0033-295x.101.3.371. [DOI] [PubMed] [Google Scholar]
- Gupta R, Tranel D, Duff M. Ventromedial prefrontal cortex damage does not impair the development and use of common ground in social interaction: Implications for cognitive theory of mind. Neuropsychologia. 2012;50:145–152. doi: 10.1016/j.neuropsychologia.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygax P, Garnham A, Oakhill J. Understanding emotions in text: Readers do not represent specific emotions. Language and Cognitive Processes. 2004;19:613–638. [Google Scholar]
- Haberlandt K. Methods in reading research. In: Gernsbacher MA, editor. Handbook of Psycholinguistics. 1. New York: Academic Press; 1994. pp. 1–31. [Google Scholar]
- Hasson U, Nusbaum HC, Small SL. Brain networks subserving the extraction of sentence information and its encoding to memory. Cerebral Cortex. 2007;17:2899–2913. doi: 10.1093/cercor/bhm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks L, Birren J. Aging, brain damage, and psychomotor slowing. Psychological Bulletin. 1970;74(6):377–396. doi: 10.1037/h0033064. [DOI] [PubMed] [Google Scholar]
- Irrazabal N, Burin D. Visuo-spatial working memory during strategic text comprehension. Poster session presented at the 16th Annual Meeting of the Society for Text and Discourse; Minneapolis, MN. 2006. Jul, [Google Scholar]
- Kuperberg G, Lakshmanan B, Caplan D, Holcomb PJ. Making sense of discourse: an fMRI study of causal inferencing across sentences. NeuroImage. 2006;33:343–361. doi: 10.1016/j.neuroimage.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Kurczek J, Duff M. Intact discourse cohesion and coherence following bilateral ventromedial prefrontal cortex. Brain and Language. 2012;123:222–227. doi: 10.1016/j.bandl.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A, Krueger F, Dal Monte O, Pardini M, Pulaski SJ, Solomon J, Grafman J. Damage to the left ventromedial prefrontal cortex impacts affective theory of mind. Social, Cognitive and Affective Neuroscience. 2011 doi: 10.1093/scan/nsr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFlynn G, Montgomery E, Fenton G, Rutherford W. Measurement of reaction time following minor head injury. Journal of Neurology, and Psychiatry. 1984;47:1326–1331. doi: 10.1136/jnnp.47.12.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Morris RG. The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain. 1999;122:1839–50. doi: 10.1093/brain/122.10.1839. [DOI] [PubMed] [Google Scholar]
- Mar RA. The neuropsychology of narrative: story comprehension, story production and their interrelation. Neuropsychologia. 2004;42:1414–1434. doi: 10.1016/j.neuropsychologia.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Mason RA, Just MA. How the brain processes causal inferences in text: A theoretical account of generation and integration component processes utilizing both cerebral hemispheres. Psychological Science. 2004;15:1–7. doi: 10.1111/j.0963-7214.2004.01501001.x. [DOI] [PubMed] [Google Scholar]
- Mason RA, Just MA. The role of the theory-of-mind cortical network in the comprehension of narratives. Language and Linguistics Compass. 2009;3:157–174. doi: 10.1111/j.1749-818X.2008.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RA, Just MA. Differentiable cortical networks for inferences concerning people’s intentions versus physical causality. Human Brain Mapping. 2011;32:313–329. doi: 10.1002/hbm.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKoon G, Ratcliff R. Inference during reading. Psychological Review. 1992;99:440–466. doi: 10.1037/0033-295x.99.3.440. [DOI] [PubMed] [Google Scholar]
- Molinari C, Burin D, Saux G, Barreyro JP, Irrazabal N, Bechis MS, Duarte A, Ramenzoni V. Fictional characters’ emotional state representation: What is its degree of specificity? Psicothema. 2009;21:9–14. [PubMed] [Google Scholar]
- Nichelli P, Grafman J, Pietrini P, Clark K, Lee KY, Miletich R. Where the brain appreciates the moral of a story. Neuroreport. 1995;6:2309–2313. doi: 10.1097/00001756-199511270-00010. [DOI] [PubMed] [Google Scholar]
- O’Brien EJ, Albrecht JE. Comprehension strategies in the development of a mental model. Journal of Experimental Psychology: Learning, Memory and Cognition. 1992;18:777–785. doi: 10.1037//0278-7393.18.4.777. [DOI] [PubMed] [Google Scholar]
- Rinck M, Hähnel A, Becker G. Using temporal information to construct, update, and retrieve situation models of narratives. Journal of Experimental Psychology: Learning, Memory and Cognition. 2001;27:67–80. [PubMed] [Google Scholar]
- Robertson DA, Gernsbacher MA, Guidotti SJ, Robertson RR, Irwin W, Mock BJ, Campana E. Functional neuroanatomy of the cognitive process of mapping during discourse comprehension. Psychological Science. 2000;11:255–60. doi: 10.1111/1467-9280.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe AD, Bullock PR, Polkey CE, Morris RG. “Theory of mind” impairments and the relationship to executive functioning following frontal lobe excisions. Brain. 2001;124:600–616. doi: 10.1093/brain/124.3.600. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: The role of the right ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2003;15:324–337. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Singer M. Inference processing in discourse comprehension. In: Gaskell MG, Altmann G, editors. The Oxford handbook of psycholinguistics. Oxford, UK: Oxford University Press; 2007. pp. 343–359. [Google Scholar]
- St George MM, Kutas A Martinez, Sereno MI. Semantic integration in reading: Engagement of the right hemisphere during discourse processing. Brain. 1999;122:1317–1325. doi: 10.1093/brain/122.7.1317. [DOI] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. Journal of Cognitive Neuroscience. 1998;10:640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Therriault DJ, Rinck M. Multidimensional situation models. In: Schmalhofer F, Perfetti CA, editors. Higher level language processes in the brain: Inference and comprehension processes. Mahwah, NJ: Erlbaum; 2006. pp. 311–327. [Google Scholar]
- Tompkins CA. Theoretical considerations for understanding “understanding” by adults with right hemisphere brain damage. Perspectives on Neurophysiology and Neurogenic Speech and Language Disorders. 2008;18:45–54. doi: 10.1044/nnsld18.2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk TA, Kintsch W. Strategies of discourse comprehension. New York: Academic Press; 1983. [Google Scholar]
- Virtue S, Clancy Z, Parrish T, Jung-Beeman M. Inferences during story comprehension: Cortical recruitment affected by predictability of events and working-memory capacity. Journal of Cognitive Neuroscience. 2008;20:2274–2284. doi: 10.1162/jocn.2008.20160. [DOI] [PubMed] [Google Scholar]
- Virtue S, Haberman J, Clancy Z, Parrish T, Jung-Beeman M. Neural activity of inferences during comprehension. Brain Research. 2006;1084:104–114. doi: 10.1016/j.brainres.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happe F, Falkai P, Maier W, Shah NJ, Fink GR, Zilles K. Mind reading: Neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14:170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Xu J, Kemeny S, Park G, Frattali C, Braun A. Language in context: emergent features of word, sentence, and narrative comprehension. NeuroImage. 2005;25:1002–1015. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Zwaan RA. The immersed experiencer: toward an embodied theory of language comprehension. In: Ross BH, editor. The Psychology of Learning and Motivation. Vol. 44. New York: Academic Press; 2004. pp. 35–62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


