Abstract
Ventilator-induced lung injury (VILI) occurs when the lung parenchyma and vasculature are exposed to repetitive and excessive mechanical stress via mechanical ventilation utilized as supportive care for the adult respiratory distress syndrome (ARDS). VILI induces gene expression and systemic release of inflammatory mediators that contribute to the multi-organ dysfunction and morbidity and mortality of ARDS. HMGB1, an intracellular transcription factor with cytokine properties, is a late mediator in sepsis and ARDS pathobiology, however, the role of HMGB1 in VILI remains poorly described. We now report HMGB1 expression in human lung microvessel endothelial cells (EC) exposed to excessive, equibiaxial mechanical stress, an in vitro correlate of VILI. We determined that high amplitude cyclic stretch (18% CS) increased HMGB1 expression (2-4 fold) via a signaling pathway with critical involvement of the transcription factor, STAT3. Concomitant exposure to 18% CS and oxidative stress (H2O2) augmented HMGB1 expression (~13 fold increase) whereas lipopolysaccharide (LPS) challenge increased HMGB1 expression in static EC, but not in 18% CS-challenged EC. In contrast, physiologic, low amplitude cyclic stretch (5% CS) attenuated both oxidative H2O2- and LPS-induced increases in HMGB1 expression, suggesting that physiologic mechanical stress is protective. These results indicate that HMGB1 gene expression is markedly responsive to VILI-mediated mechanical stress, an effect that is augmented by oxidative stress. We speculate that VILI-induced HMGB1 expression acts locally to increase vascular permeability and alveolar flooding, thereby exacerbating systemic inflammatory responses and increasing the likelihood of multi-organ dysfunction.
Keywords: Ventilator-induced lung injury, ARDS, endothelial cells, HMGB1, gene expression, transcription factor, STAT3, oxidant injury, lipopolysaccharide, mechanical stress
Introduction
The adult respiratory distress syndrome (ARDS) is an inflammatory process characterized by the loss of alveolar-capillary integrity with resultant high-permeability and non-hydrostatic pulmonary edema (Ware and Matthay, 2000). Both pulmonary endothelium (Dudek and Garcia, 2001) and epithelium (Bhattacharya and Matthay, 2013) are central in maintaining alveolar-capillary barrier function. The pulmonary endothelium plays a critical role in maintaining a cellular barrier between the vascular space and the pulmonary interstitium, with biochemical or mechanical barrier-disrupting agents producing increased lung fluid flux and alveolar flooding, a hallmark of ALI (Dudek and Garcia, 2001). Mechanical ventilation can exacerbate lung injury via both excessive alveolar stretch (volutrauma) (Amato et al., 1998; Lionetti et al., 2005) and repetitive stretch of non-aerated lung units, a process known as atelectrauma (Jacob and Gaver, 2012; Yalcin et al., 2009). Ventilator-induced lung injury (VILI) results from excessive mechanical stress during mechanical ventilation (Dos Santos and Slutsky, 2000) which produces alveolar and vascular damage leading to multiple organ dysfunction (ARDSNET2000; dos Santos and Slutsky, 2000), a syndrome involving systemic effects of locally produced pro-inflammatory cytokines (Dreyfuss and Saumon, 1998). Cyclic stretch (CS) serves as an in vitro model of the repetitive mechanical stretch placed on pulmonary endothelium and parenchyma throughout the respiratory cycle. Pathologic, high amplitude CS leads to endothelial cell (EC) changes including rearrangement of the actin cytoskeleton, increased paracellular gap formation, and decreased EC barrier function measured by trans-endothelial cell electrical resistance (TER) (Birukov et al., 2003).
Reliable biomarkers and novel targets for ARDS, VILI and sepsis are limited. However, several cytokines have been suggested as potential biomarkers (Barnett and Ware, 2011; Cross and Matthay, 2011; Pierrakos and Vincent, 2010). High-mobility group box 1 (HMGB1) was initially known as a nuclear transcription factor with subsequent identification as a cytokine in a murine model of endotoxin-mediated lethality (Wang et al., 1999). HMGB1 also induces secretion of other pro-inflammatory cytokines, including TNFα, IL-8, and monocyte chemotactic protein 1 (MCP1) (Fiuza et al., 2003). Animal studies implicate HMGB1 in the pathogenesis of ARDS with increased HMGB1 serum and bronchoalveolar lavage fluid (BAL) levels in mice experiencing LPS-induced ARDS (Ueno et al., 2004). Direct intratracheal instillation of HMGB1 produces hallmark pulmonary changes of murine ARDS (Abraham et al., 2000). Furthermore, antibodies targeting HMGB1 ameliorate LPS-induced ARDS in mice (Abraham et al., 2000). In previous in vitro studies addressing the role of HMGB1 in ARDS, we described HMGB1-dependent lung EC actin cytoskeletal rearrangement, paracellular gap formation, and barrier disruption measured by TER (Wolfson et al., 2011).
While the linkage between HMGB1 and LPS-induced murine ARDS has been studied, information addressing the role of HMGB1 in VILI is much more limited. HMGB1 levels were increased in BAL fluid and in lung macrophages and neutrophils in rabbits exposed to high tidal volume ventilation (Ogawa et al., 2006) and in BAL from ventilated patients without pre-existing lung disease (van Zoelen et al., 2008). Further, anti-HMGB1 antibodies attenuated murine VILI (Ogawa et al., 2006). While animal models implicate an association between HMGB1 and pathologic lung stretch, there have not been studies to determine the source of HMGB1 in this setting.
In the present study, we exposed human lung microvessel EC to cyclic stretch to mimic the repetitive and excessive mechanical stress imparted by mechanical ventilation, and examined HMGB1 expression. We found that EC exposure to high amplitude cyclic stretch (18% CS) increases HMGB1 expression, an effect dependent on the transcription factor STAT3. In addition, we identified an additive increase in HMGB1 expression with exposure to oxidative stress. In contrast, physiologic, low amplitude cyclic stretch (5% CS) attenuated both oxidative- and LPS-induced increases in HMGB1 expression, suggesting that physiologic CS is protective in our model. These results indicate that HMGB1 gene expression is markedly responsive to the repeated mechanical stress observed in VILI, an effect that is augmented by oxidative stress. We speculate that VILI-induced HMGB1 expression acts locally to increase vascular permeability and alveolar flooding, thereby exacerbating systemic inflammatory responses and increasing the likelihood of multi-organ dysfunction.
Materials and Methods
Reagents
TRIzol® Reagent was from Invitrogen (Carlsbad, California). Ethanol, chloroform, isopropanol, and lipopolysaccharide (E.coli 0127:B8) were from Sigma-Aldrich (St. Louis, Missouri). Hydrogen peroxide was obtained from Fisher (Hanover Park, Illinois). Reverse transcription and real-time PCR kits and probes were from Applied Biosystems (Carlsbad, California). SiRNA was purchased from Thermo Scientific (Lafayette, Colorado). Silencing RNA transfection reagent, siPORT™ Amine, was purchased from Ambion (Austin, TX). Primary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) (rabbit polyclonal antibodies: STAT2 antibody catalog sc-476; STAT3 antibody catalog sc-482; STAT5 antibody sc-835) and Cell Signaling Technology (Danvers, MA) (β-actin-HRP, Catalog # 12620). Anti-mouse and anti-rabbit secondary antibodies conjugated to horseradish peroxidase were from GE Health Sciences (Chalfont St. Giles, UK). Enhanced chemiluminescence (ECL), and Supersignal West Dura were from Pierce Biotechnology (Rockford, IL).
Cell culture
All experiments used primary human lung microvessel endothelial cells (HLMVEC). HLMVEC were from Lonza Group, Ltd (Switzerland) and were grown in manufacturer’s recommended Endothelial Growth Medium-2 (EGM-2) consisting of defined growth factors and supplemented up to 10% FBS. Media was changed to 5% FBS the day prior to all experiments. Cells were not starved before experiments in order to avoid additional stress prior to cyclic stretch. Cells were grown at 37°C in 5% CO2 incubator and used from passage 5-6. EC were plated at appropriate densities 5-7 days prior to experiments and grown to confluence prior to use. Modifications related to RNA interference are detailed below.
Cyclic stretch
EC were plated on Collagen I coated Bioflex® culture plates from Flexcell International (Hillsborough, North Carolina) and grown to confluence as described above. EC were then subjected to equibiaxial cyclic stretch at either 5% (low amplitude) or 18% stretch (high amplitude) using the Flexcell® FX-4000 Tension System for various durations as we have previously described (Birukov et al 2003). Stretch amplitudes, which indicate linear elongation, were selected based on published work in endothelial cells ((Birukov et al., 2003; Birukova et al., 2006; Okada et al., 1998) and alveolar epithelial cells ((Cohen et al., 2008; Tschumperlin and Margulies, 1998; Tschumperlin and Margulies, 1999; Tschumperlin et al., 2000). Unstretched control EC (static) were evaluated in parallel with cyclic stretched EC in the same incubator.
Cell stimulation
For certain experiments, EC were stimulated with either 100μM H2O2 or 100ng/ml E. coli 0127:B8 lipopolysaccharide (LPS). H2O2 or LPS was added to the culture media immediately prior to initiation of CS, and remained in the media for the 4 hour duration of CS utilized for these experiments.
RNA isolation
EC were washed twice with ice cold PBS, treated with TRIzol® (0.5ml per well), removed from the collagen-coated surface using a cell scraper and briefly sonicated using a VirSonic 60 sonicator on setting 4. The solution was then transferred to phase-lock microfuge tubes from Eppendorf (Hauppauge, New York), and 0.1ml chloroform added. Tubes were inverted 20 times by hand, allowed to stand at room temperature for 3 minutes, and then spun at maximum speed (16,000 × g) at 4°C for 15 minutes. The supernatants were then recovered and transferred to new tubes, and 0.5ml isopropanol was added to each sample. Tubes were inverted 20 times by hand, allowed to stand at room temperature for 10 minutes, and then spun at maximum speed (16,000 × g) at 4°C for 10 minutes. Next, the supernatant was aspirated and discarded, taking care not to disturb the RNA pellet. The RNA pellet was then washed with 1ml of 75% ethanol, assisted by vortexing briefly. Samples were then spun at 7,500g at 4°C for 5 minutes. Ethanol was then aspirated and discarded and RNA pellets allowed to dry at room temperature for up to 1 hour. Last, 20μl nuclease free water was added to each RNA pellet, samples were mixed by vortexing, and RNA fully dissolved by incubating at 55°C for 10 minutes. RNA was quantified using NanoDrop (Wilmington, Delaware), and samples were stored at −80°C.
Reverse Transcription
Conversion of RNA to cDNA was accomplished using a cDNA reverse transcription kit from Applied Biosystems (Carlsbad, California). 400ng RNA were used in each 20μl reaction, which included TaqMan reverse transcription buffer (final concentration 1×), dNTP Mixture (500 μM per dNTP), magnesium chloride (5.5 mM), random hexamers (2.5 μM), RNase inhibitor (0.4 U/μl), and MultiScribe™ Reverse Transcriptase (1.25 U/μl) per the manufacturer’s instructions. The reverse transcription reaction was run in a 96 well plate using a Bio-Rad MyCycler thermal cycler (Hercules, California) as follows: 25°C for 10 minutes, 48°C for 30 minutes, 95°C for 5 minutes, and then hold at 4°C.
Real-time PCR
Real-time PCR (RT-PCR) was accomplished using the TaqMan® Universal PCR Master Mix from Applied Biosystems (Carlsbad, California). TaqMan® probes for HMGB1, β-actin, and TBP were purchased from Applied Biosystems. Each reaction was prepared using cDNA template, the appropriate probe, the Universal PCR Master Mix, and water per the manufacturer’s instructions. Reactions were loaded in a 384 well plate and analyzed using the ΔΔCt cycle method on a Bio-Rad CFX384 Real Time System in concert with a Bio-Rad C1000 Thermal Cycler. Data was analyzed using Bio-Rad CFX Manager™ Software (version 3.0).
RNA interference
ON-TARGETplus small interfering RNA (siRNA) against STAT2 (catalog L-012064-00-0005), STAT3 (catalog L-003544-00-0005), and STAT5 (STAT5A catalog L-005169-00-0005, STAT5B catalog L-010539-00-0005) were each obtained as a pool of four siRNA duplexes from Thermo Scientific (Lafayette, CO). ON-TARGETplus siRNA (siCONTROL#2) (catalog D-001810-01-05) targeting the non-human protein, luciferase, was used as a negative control siRNA with minimal off-target silencing. The silencing protocol was optimized to allow transfection of cells shortly after plating in passage 5. Approximately 8-18 hours after cell plating on Collagen I coated Bioflex® culture plates (Flexcell International) at approximately 70% confluence, siRNAs (calculated for final concentration of 100 nM siRNA) were premixed with transfection reagent (4 μl/ml siPORT™ Amine) for 5 min and then diluted with basal media. After 16-24 hrs of incubation, the siRNA/ siPORT™ Amine containing media was removed and replaced with 5% FBS serum media. At 48hr post-transfection, cells were used for CS experiments.
Western Blots
Cells were washed with cold Endothelial Basal Medium (EBM) once and extracted with 0.3% SDS in 10mM Tris lysis buffer (300 μl/D60) containing protease and phosphatase cocktail inhibitors. DNA was sheared with a 26-gauge syringe. Each sample was boiled for 5 min, and diluted with 5× sample buffer (0.56 M Tris pH7.0, 10% SDS, 25% β-ME, 25% sucrose, 0.025% bromophenol blue). Sample proteins were separated with a 4-20% gradient SDS-PAGE gel using the Mini-Protean III (Bio-Rad, Hercules, CA). Proteins were transferred onto Immobilion-P PVDF membrane (Millipore, Bedford, MA), immunoblotted with primary antibodies (1:200-1:1000, 4°C, overnight) followed by secondary antibodies conjugated to HRP (1:5000, room temperature, 30 min), and detected with enhanced chemiluminescence (Pierce ECL or SuperSignal West Dura, Pierce Biotechnology, Rockford, IL) on Biomax MR film (Kodak, Rochester, NY).
Statistical analysis
Values are shown as the mean +/− standard error. Data were analyzed using the Wilcoxon Rank Sum test for paired data and the two-way non-repeated analysis of variance (ANOVA) for unpaired data. Significance in all cases was defined at p < 0.05.
Results
High amplitude cyclic stretch (18% CS) increases endothelial cell (EC) HMGB1 expression
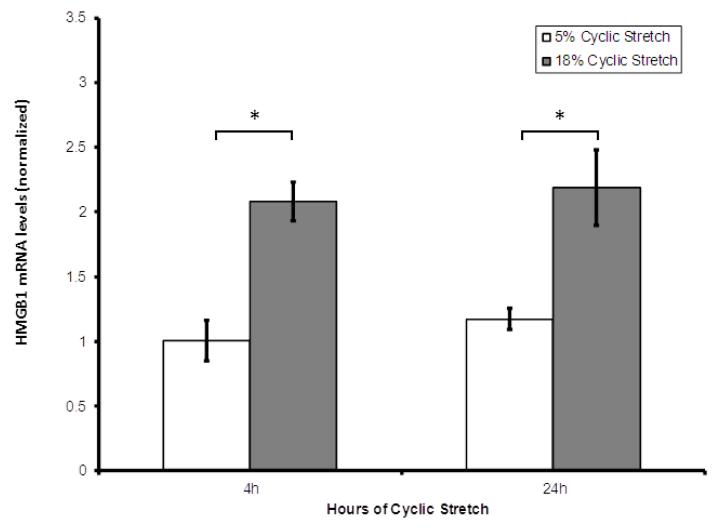
Human lung EC were cultured for 4 or 24 hours under static, low amplitude (5%), or high amplitude (18%) CS conditions. Analysis of HMGB1 expression by RT-PCR revealed significant increases in HMGB1 expression in EC subjected to high amplitude 18% CS compared low amplitude 5% CS after 4 hours and 24 hours (Figure 1).
Figure 1. High amplitude mechanical stress upregulates HMGB1 mRNA expression in HLMVEC.
Real-time quantitative polymerase chain reaction shows HMGB1 mRNA expression in HLMVEC treated with 18% cyclic stretch is significantly greater than in EC treated with 5% cyclic stretch at 4 hours and 24 hours of cyclic stretch. HMGB1 expression in the setting of CS was normalized both to HMGB1 expression in EC grown under static conditions and to the housekeeping gene β-actin. (* indicates p < 0.005, paired data analyzed using Wilcoxon Rank Sum Test).
Oxidant stress increases HMGB1 expression in lung endothelium
We sought to identify stimuli that would augment HMGB1 expression in EC with concomitant CS exposure and chose an oxidant challenge, given that H2O2 increases HMGB1 expression in cardiomyocytes (Yu et al., 2012) and in alveolar epithelial A549 cells (Chiou et al., 2013). EC were subjected to oxidant stress with H2O2 (Stephens et al., 2010; Vepa et al., 1999) concomitant with either 5% or 18% CS for 4 hours and compared to static EC exposed to H2O2 alone. H2O2 challenge significantly increased HMGB1 expression in all conditions (static, 5% and 18% CS) (Figure 2). In static EC, H2O2 increased HMGB1 expression (9-fold) compared to unstimulated EC. In 5% and 18% CS-exposed EC, H2O2 increased HMGB1 expression approximately 3-fold compared to unstimulated, stretched EC, with maximal increase in EC challenged with both 18% CS and H2O2 (13-fold increase compared to static, unstimulated EC). We compared HMGB1 expression between static EC and 5% CS EC, and noted that 5% CS may be protective against increased HMGB1 expression induced by H2O2. Low amplitude 5% CS attenuated the H2O2–induced increase in HMGB1 expression seen in static EC challenged with H2O2.
Figure 2. High amplitude 18% CS is additive with oxidative stress in HMGB1 upregulation in HLMVEC.
Real-time quantitative polymerase chain reaction of HMGB1 using β-actin as a housekeeping gene. 100μM H2O2 increases HMGB1 mRNA expression in HLMVEC at 4 hours in all conditions (static, 5% cyclic stretch, 18% cyclic stretch) with ~13-fold increase in EC challenged with both H2O2 and 18% CS. 5% CS significantly attenuates the H2O2-induced increase in HMGB1 expression seen in static EC, as analyzed by the Wilcoxon Rank Sum Test (p<0.005). HMGB1 mRNA expression is normalized to HMGB1 expression in unstimulated, static EC. Two way ANOVA shows that H2O2 challenge significantly increases HMGB1 expression (p<0.05) in all conditions (static, 5% CS, and 18% CS), that 18% CS significantly increases HMGB1 expression compared to static conditions (p<0.05), and shows a significant interaction (p<0.05) between 18% CS and H2O2 in increasing HMGB1 expression (* indicates p < 0.05).
Lipopolysaccharide increases HMGB1 in lung EC
Lipopolysaccharide (LPS) increases HMGB1 levels in a mouse model of sepsis (Wang et al., 1999). We investigated the effect of LPS challenge on HMGB1 expression in EC treated with concomitant CS for 4 hours. In contrast to H2O2 challenge, the LPS–induced increase in HMGB1 expression was restricted to static EC, and was not observed in EC concomitantly exposed to either low or high amplitude CS (Figure 3). However, similar to H2O2 challenge, low amplitude 5% CS significantly attenuated the ~6-fold LPS-induced increase in HMGB1 expression in static EC. Thus, low amplitude 5% CS protects EC from both oxidant- and LPS-induced increases in HMGB1 expression in lung EC.
Figure 3. LPS-induced HMGB1 gene expression is attenuated by low-amplitude 5% CS.
Real-time quantitative polymerase chain reaction of HMGB1 using β-actin as a housekeeping gene. Wilcoxon Rank Sum Test shows that 100ng/ml LPS (E. coli 0127:B8) increases HMGB1 expression in static EC, and that 5% CS significantly attenuates the LPS-induced increase in HMGB1 expression seen in static EC. HMGB1 expression is normalized to expression in unstimulated, static EC. Two way ANOVA confirms the significance of these effects (* indicates p < 0.05).
STAT3 is critical to high amplitude 18% CS-induced HMGB1 expression in lung EC
The transcription factor, STAT3, is important in ARDS (Severgnini et al., 2004) and a STAT3 binding site exists within the HMGB1 promoter, 872 base pairs removed from the transcription start site (Lum and Lee, 2001) (http://www.sabiosciences.com/chipqpcrsearch). We silenced STAT3 in EC using siRNAs, and compared HMGB1 expression after exposure to either 5% or 18% CS for 4 hours (Figure 4A). Silencing RNAs targeting STAT3 substantially reduced STAT3 protein expression in EC, whereas STAT2 and STAT5 expression were unaffected (Figure 4B). STAT3 silencing failed to alter HMGB1 expression in EC treated with low amplitude 5% CS, however, in marked contrast, reductions in STAT3 expression completely abolished the increased HMGB1 expression induced by high-amplitude 18% CS (Figure 4A) indicating that STAT3 is required for the mechanotransduction of high amplitude 18% CS that results in increased HMGB1 expression in EC.
Figure 4. Reduced STAT3 expression abolishes HMGB1 upregulation mediated by excessive mechanical stress.
(A) Real-time quantitative polymerase chain reaction of HMGB1 using TBP as a housekeeping gene. EC treated with control siRNA (siControl) show the predicted increase in HMGB1 expression after 4 hours of 18% CS. However, this increase is abolished in EC treated with siRNA targeting STAT3 (siSTAT3). The significance of this interaction was confirmed with two-way ANOVA (* indicates p < 0.05). (B) Western blot imaging confirms decreased expression of STAT3 in EC treated with corresponding siRNA. Silencing is specific, as siRNA targeting STAT3 does not cause decreased expression of either STAT2 or STAT5.
Discussion
Understanding the cellular events that increase vascular permeability in VILI is critical to the ultimate development of therapeutic strategies targeting this pathologic process. Our data show for the first time that excessive mechanical stretch (18% CS) significantly increases expression of HMGB1, a molecule implicated in sepsis (Wang et al., 1999) and in lung vascular barrier disruption (Wolfson et al., 2011), whereas low amplitude 5% CS fails to increase HMGB1 expression in lung microvessel ECs. These results are consistent with prior studies showing that pathologic, high amplitude CS increased expression of signaling and contractile proteins in pulmonary EC, including Rho GTPase, MLC, MLCK, ZIP kinase, PAR-1, caldesmon, and Hsp27 (Birukova et al., 2008). We now add HMGB1 to the roster of genes whose expression is increased by excessive mechanical stress in EC. Both pathologic CS and HMGB1 are known to cause rearrangement of EC actin cytoskeleton, thereby leading to loss of EC barrier function and vascular leak that is central to ARDS pathophysiology (Birukov et al., 2003; Wolfson et al., 2011). It is interesting to note that pathologic CS is known to upregulate pro-inflammatory cytokines in endothelial cells, including IL-6, IL-8, and MCP-1 ((Iwaki et al., 2009; Okada et al., 1998). HMGB1 also increases IL-8 and MCP-1 expression in endothelial cells (Fiuza et al., 2003). Future investigations will address whether HMGB1 is necessary for pathologic CS to increase pro-inflammatory cytokine production.
High partial pressures of oxygen have long been known to be toxic to the lung (Aggarwal et al., 2010; Davis et al., 1983; Deneke and Fanburg, 1980; Deneke and Fanburg, 1982; Fisher and Beers, 2008) and contribute to the severity of ARDS and VILI. We found that oxidant injury (H2O2) directly increases HMGB1 expression in EC suggesting a novel role for HMGB1 in pulmonary EC oxidant injury. Interestingly, oxidant-augmented HMGB1 expression was differentially altered by 5% and 18% CS conditions with high amplitude 18% CS exposure producing additively increased expression (Figure 2). These results are consistent with prior studies showing that EC preconditioned with high amplitude 18% CS exhibited an augmented response to thrombin, a known barrier-disrupting agent (Birukov et al., 2003). In contrast to oxidative challenge, LPS stimulation augmented HMGB1 expression in static EC, but failed to increase HMGB1 expression in EC treated with CS. Rather, exposure to 5% CS conditions served to reduce LPS-mediated increases in HMGB1 expression. The mechanism underlying these differential findings is unclear, but may be related to differential sensitivities of the HMGB1 promoter to oxidant- and NFκB-related transcription factors and merits further investigation.
We identified significant effects of exposure to low amplitude 5% CS on HMGB1 expression in EC exposed to concomitant H2O2 or LPS challenge. Low amplitude 5% CS significantly ameliorated the increase in HMGB1 expression seen with H2O2 or LPS challenge alone, and this effect was more marked in EC challenged with LPS (Figures 2, 3). This is congruent with studies showing that EC preconditioning with low amplitude 5% CS is protective against subsequent challenge with the barrier-disrupting agent, thrombin. EC preconditioned with low amplitude 5% CS and then challenged with thrombin had less marked declines in trans-endothelial electrical resistance (TER) and a more rapid recovery to baseline TER compared to EC preconditioned with high amplitude 18% CS (Birukov et al., 2003). Physiologic, low amplitude 5% CS also induces recovery from thrombin-induced paracellular gap formation and rapid peripheral redistribution of focal adhesions and cortactin (Birukova et al., 2006). Interrogation of the effect of physiologic CS is an interesting phenomenon which merits further investigation focused on the effects of H2O2 and LPS.
EC are routinely exposed to both shear stress and mechanical stretch from either pulsatile blood flow/blood pressure and in the case of pulmonary EC, changes in lung volumes. EC sense mechanical stress and transmit these signals to increase expression of a host of transcription factors, including HIF-1α, HIF-2α, NF-κB, and zyxin, subsequently leading to changes in expression of greater than 30 different genes affecting cell proliferation, inflammation, and extracellular matrix metabolism (Anwar et al., 2012). We sought to investigate potential transcription factors that mediate increased HMGB1 expression in the setting of high amplitude 18% CS. A detailed study by Lum and Lee (2001) revealed that the HMGB1 promoter is controlled by an upstream silencer element and also by an enhancer element in the first intron, and identified multiple potential transcription factor-binding sites in the promoter. An on-line query yielded a lengthy list of transcription factor-binding sites proximate to the HMGB1 transcription start site predicted by SABiosciences’ Text Mining Application in concert with the UCSC Genome Browser (http://www.sabiosciences.com/chipqpcrsearch.php?species_id=0&nfactor=n&ninfo=n&ngene=n&B2=Search&src=genecard&factor=Over+200+TF&gene=HMGB1) and identified a binding site for STAT3 that was located 872 base pairs from the transcription start site.
STAT transcription factors are activated by various extracellular signals, and ultimately bind to DNA to regulate gene expression (Darnell, 1997; Levy and Darnell, 2002; Severgnini et al., 2004). Furthermore, STATs play a central role in immune and inflammatory responses (Severgnini et al., 2004), and have a known role in the development of ALI. In a mouse model of LPS-induced ALI, both STAT1 and STAT3 were found to be tyrosine phosphorylated and activated (measured by DNA binding) in the lung after intraperitoneal LPS exposure (Severgnini et al., 2004). STAT3 is also activated in an acid aspiration murine ARDS model (direct injury) and an acute pancreatitis murine model (indirect injury), suggesting that STAT3 activation may contribute to ARDS pathobiology (Severgnini et al., 2004). In an IgG immune complex-induced mouse model of ARDS, decreased STAT3 expression resulted in decreased indicators of lung injury and inflammation (Tang et al., 2011). We hypothesized that STAT3 may mediate increased HMGB1 expression in the lung in an in vitro model of VILI and identified STAT3 to be essential for high amplitude 18% CS-induced increases in HMGB1 expression (Figure 4A). As we have previously shown that HMGB1 is important in the pulmonary EC barrier disruption that is the hallmark of ARDS (Wolfson et al., 2011), these results support STAT3 as an essential contributor to VILI and a potential therapeutic target. Future work will define the role of STAT3 in LPS and H2O2-mediated increases in HMGB1 expression.
In summary, we found that high amplitude 18% CS, an in vitro model of pathologic lung vascular stretch that occurs in VILI, results in increased HMGB1 expression in lung microvessel EC. STAT3, a transcription factor known to play a role in the development of ARDS, is necessary for increased HMGB1 expression in response to excessive mechanical stress. HMGB1 expression was augmented by oxidant stress in EC in both static and CS conditions. Furthermore, physiologic, low amplitude 5% CS protected EC from increased HMGB1 expression induced by either oxidant injury or LPS. These novel results provide evidence for a role for HMGB1 in the development of VILI. Further investigation into the control of HMGB1 gene expression and STAT3 in the setting of VILI is needed to allow ultimate translation of these findings.
Highlights.
High amplitude cyclic stretch increases HMGB1 expression in lung endothelial cells
STAT3 is critical to high amplitude cyclic stretch-induced HMGB1 expression
Oxidative stress augments HMGB1 expression
Low amplitude cyclic stretch attenuates oxidative- and LPS-induced HMGB1 expression
Acknowledgements
This work was supported by NHLBI K08-HL093359, R01-HL91889, P01-HL98050, R01-HL73994 and PO1-HL58064. The authors acknowledge and thank Lakshmi Natarajan for maintenance and supply of high quality endothelial cell culture stocks.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ARDSNET Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Abraham E, et al. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–4. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- Aggarwal NR, et al. Moderate oxygen augments lipopolysaccharide-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L371–81. doi: 10.1152/ajplung.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato MB, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–54. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- Anwar MA, et al. The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. J Vasc Res. 2012;49:463–78. doi: 10.1159/000339151. [DOI] [PubMed] [Google Scholar]
- Barnett N, Ware LB. Biomarkers in acute lung injury--marking forward progress. Crit Care Clin. 2011;27:661–83. doi: 10.1016/j.ccc.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- Birukov KG, et al. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol. 2003;285:L785–97. doi: 10.1152/ajplung.00336.2002. [DOI] [PubMed] [Google Scholar]
- Birukova AA, et al. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol. 2006;168:1749–61. doi: 10.2353/ajpath.2006.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, et al. Long-term cyclic stretch controls pulmonary endothelial permeability at translational and post-translational levels. Exp Cell Res. 2008;314:3466–77. doi: 10.1016/j.yexcr.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SY, et al. Moderate hypothermia attenuates oxidative stress injuries in alveolar epithelial A549 cells. Exp Lung Res. 2013 doi: 10.3109/01902148.2013.792881. [DOI] [PubMed] [Google Scholar]
- Cohen TS, et al. Frequency and peak stretch magnitude affect alveolar epithelial permeability. Eur Respir J. 2008;32:854–61. doi: 10.1183/09031936.00141007. [DOI] [PubMed] [Google Scholar]
- Cross LJ, Matthay MA. Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit Care Clin. 2011;27:355–77. doi: 10.1016/j.ccc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE. STATs and gene regulation. Science. 1997;277:1630–5. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Davis WB, et al. Pulmonary oxygen toxicity. Early reversible changes in human alveolar structures induced by hyperoxia. N Engl J Med. 1983;309:878–83. doi: 10.1056/NEJM198310133091502. [DOI] [PubMed] [Google Scholar]
- Deneke SM, Fanburg BL. Normobaric oxygen toxicity of the lung. N Engl J Med. 1980;303:76–86. doi: 10.1056/NEJM198007103030204. [DOI] [PubMed] [Google Scholar]
- Deneke SM, Fanburg BL. Oxygen toxicity of the lung: an update. Br J Anaesth. 1982;54:737–49. doi: 10.1093/bja/54.7.737. [DOI] [PubMed] [Google Scholar]
- Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol. 2000;89:1645–55. doi: 10.1152/jappl.2000.89.4.1645. [DOI] [PubMed] [Google Scholar]
- dos Santos CC, Slutsky AS. Mechanotransduction, ventilator-induced lung injury and multiple organ dysfunction syndrome. Intensive Care Med. 2000;26:638–42. doi: 10.1007/s001340051217. [DOI] [PubMed] [Google Scholar]
- Dreyfuss D, Saumon G. From ventilator-induced lung injury to multiple organ dysfunction? Intensive Care Med. 1998;24:102–4. doi: 10.1007/s001340050529. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Fisher AB, Beers MF. Hyperoxia and acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L1066. doi: 10.1152/ajplung.90486.2008. author reply L1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiuza C, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–60. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- Iwaki M, et al. Mechanical stretch enhances IL-8 production in pulmonary microvascular endothelial cells. Biochem Biophys Res Commun. 2009;389:531–6. doi: 10.1016/j.bbrc.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob AM, Gaver DP. Atelectrauma disrupts pulmonary epithelial barrier integrity and alters the distribution of tight junction proteins ZO-1 and claudin 4. J Appl Physiol. 2012;113:1377–87. doi: 10.1152/japplphysiol.01432.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Darnell JE. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–62. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- Lionetti V, et al. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care. 2005;11:82–6. doi: 10.1097/00075198-200502000-00013. [DOI] [PubMed] [Google Scholar]
- Lum HK, Lee KL. The human HMGB1 promoter is modulated by a silencer and an enhancer-containing intron. Biochim Biophys Acta. 2001;1520:79–84. doi: 10.1016/s0167-4781(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Ogawa EN, et al. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med. 2006;174:400–7. doi: 10.1164/rccm.200605-699OC. [DOI] [PubMed] [Google Scholar]
- Okada M, et al. Cyclic stretch upregulates production of interleukin-8 and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in human endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:894–901. doi: 10.1161/01.atv.18.6.894. [DOI] [PubMed] [Google Scholar]
- Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severgnini M, et al. Activation of the STAT pathway in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1282–92. doi: 10.1152/ajplung.00349.2003. [DOI] [PubMed] [Google Scholar]
- Stephens RS, et al. cGMP increases antioxidant function and attenuates oxidant cell death in mouse lung microvascular endothelial cells by a protein kinase G-dependent mechanism. Am J Physiol Lung Cell Mol Physiol. 2010;299:L323–33. doi: 10.1152/ajplung.00442.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, et al. An essential role for Stat3 in regulating IgG immune complex-induced pulmonary inflammation. FASEB J. 2011;25:4292–300. doi: 10.1096/fj.11-187955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschumperlin DJ, Margulies SS. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am J Physiol. 1998;275:L1173–83. doi: 10.1152/ajplung.1998.275.6.L1173. [DOI] [PubMed] [Google Scholar]
- Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol. 1999;86:2026–33. doi: 10.1152/jappl.1999.86.6.2026. [DOI] [PubMed] [Google Scholar]
- Tschumperlin DJ, et al. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med. 2000;162:357–62. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- Ueno H, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310–6. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- van Zoelen MA, et al. Pulmonary levels of high-mobility group box 1 during mechanical ventilation and ventilator-associated pneumonia. Shock. 2008;29:441–5. doi: 10.1097/SHK.0b013e318157eddd. [DOI] [PubMed] [Google Scholar]
- Vepa S, et al. Hydrogen peroxide stimulates tyrosine phosphorylation of focal adhesion kinase in vascular endothelial cells. Am J Physiol. 1999;277:L150–8. doi: 10.1152/ajplung.1999.277.1.L150. [DOI] [PubMed] [Google Scholar]
- Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Wolfson RK, et al. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc Res. 2011;81:189–97. doi: 10.1016/j.mvr.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin HC, et al. Influence of cytoskeletal structure and mechanics on epithelial cell injury during cyclic airway reopening. Am J Physiol Lung Cell Mol Physiol. 2009;297:L881–91. doi: 10.1152/ajplung.90562.2008. [DOI] [PubMed] [Google Scholar]
- Yu Y, et al. Heat shock transcription factor 1 inhibits H2O2-induced cardiomyocyte death through suppression of high-mobility group box 1. Mol Cell Biochem. 2012;364:263–9. doi: 10.1007/s11010-012-1226-x. [DOI] [PubMed] [Google Scholar]