Abstract
Background
The possibility that mu opioid agonists can influence cancer recurrence is a subject of recent interest. Epidemiologic studies suggested that there were differences in cancer recurrence in breast and prostate cancer contingent on anesthetic regimens. In this study, we identify a possible mechanism for these epidemiologic findings based on mu opioid receptor (MOR) regulation of Lewis lung carcinoma (LLC) tumorigenicity in cell and animal models.
Methods
We utilized human lung tissue and human non-small cell lung cancer (NSCLC) cell lines and evaluated MOR expression using immunoblot and immunohistochemical analysis. LLC cells were treated with the peripheral opioid antagonist methylnalnaltrexone (MNTX) or MOR shRNA and evaluated for proliferation, invasion and soft agar colony formation in vitro and primary tumor growth and lung metastasis in C57BL/6 and MOR knockout mice using Visen FMT imaging and immunohistochemical analysis.
Results
We provide several lines of evidence that the MOR may be a potential target for lung cancer, a disease with high mortality and few treatment options. We first observed that there is ~5 to 10 fold increase in MOR expression in lung samples from patients with NSCLC and in several human NSCLC cell lines. The MOR agonists morphine and DAMGO increased in vitro LLC cell growth. Treatment with MNTX or silencing MOR expression inhibited LLC invasion and anchorage-independent growth by 50–80%. Injection of MOR silenced LLC lead to a ~65% reduction in mouse lung metastasis. In addition, MOR knockout mice do not develop significant tumors when injected with LLC as compared to wildtype controls. Finally, continuous infusion of the peripheral opioid antagonist methylnaltrexone attenuates primary LLC tumor growth and reduces lung metastasis.
Conclusions
Taken together, our data suggests a possible direct effect of opiates on lung cancer progression, and provides a plausible explanation for the epidemiologic findings. Our observations further suggest a possible therapeutic role for opioid antagonists.
Introduction
The effect of anesthetics on cancer recurrence has been a subject of recent interest in the anesthesia literature.1–4 While the cause for these epidemiologic findings remains unclear, there is an evolving experimental literature suggesting that opiates affect tumor growth. Both we and Gupta et al. have shown that clinically relevant concentrations of opiates can cause endothelial cell proliferation and migration in vitro, and the selective peripheral mu opioid receptor (MOR) antagonist, methylnaltexone (MNTX), inhibits angiogenesis, a process required for in vivo tumor growth.5–8 In addition, morphine, at clinically relevant concentrations, promotes human breast cancer growth in animal and in vitro models.9 On the other hand, animal experiments using high concentrations of morphine demonstrated inhibition of tumor metastases and reduced survival in animal models of breast cancer.10 Some experimental data suggests that opioids inhibit lung tumor metastasis following laparotomy in rodents.11–13 These results were explained, in part, by morphine’s potential inhibitory effects on natural killer cell activity.12,14–16 Evidence supporting an immunomodulatory and sympathoneural effect on tumor progression emerges from a recent study in rodents.17
Given the conflicting reports on morphine’s effects on lung cancer progression, we undertook a series of in vitro and in vivo experiments to examine the direct effect of the MOR in lung cancer progression using the well-established model of Lewis Lung carcinoma.18,19 We hypothesized that the activation of the MOR during surgery might be a plausible explanation for the differences in recurrence rates noted in the epidemiologic studies. Importantly, our observations of attenuation of tumor growth and metastasis occurred absent exogenous opioids supporting an underlying role of the MOR in lung cancer progression.
Methods
The experiments presented in this manuscript represent a continuum of cellular, tissue and in vivo studies designed to elucidate the functional role of the mu opioid receptor (MOR) in lung cancer. Cellular studies include determining the relative levels of MOR expression in mouse Lewis Lung Carcinoma (LLC) cells, various human non-small cell lung cancer (NSCLC) cells and non-tumorigenic human BEAS-2B cells using immunoblotting techniques. The role of mu opioid agonists (morphine and DAMGO) in LLC cellular proliferation and the MOR (MOR shRNA) in LLC cellular proliferation, invasion and anchorage-independent growth were also assessed using in vitro assays to determine potential mechanisms of in vivo tumor growth and metastasis. Tissue studies include determining the relative MOR immunohistochemical staining intensity from lung cancer and normal patient samples. In vivo studies include determining the role of MOR in LLC mouse primary flank tumor and lung metastasis models using MOR silencing of LLC cells, continuous infusion of the peripheral MOR inhibitor, MNTX and utilization of MOR knockout mice.
Cell Culture and Reagents
Human NSCLC cell lines H522, H1703, H1993, SW1573, H1437, H358, control BEAS-2B and mouse Lewis lung carcinoma (LLC) cells were obtained from ATCC (Walkersville, MD). LLC cells with stable GFP/RFP expression were a generous gift from Dr. Ralph R. Weichselbaum and Dr. Rosie Xing. Normal human bronchial epithelial cells were purchased from Lonza Group (Walkersville, MD). Cells were cultured in RPMI complete medium (Cambrex) at 37°C in a humidified atmosphere of 5% CO2, 95% air, with passages 6–10 used for experimentation. Unless otherwise specified, reagents were obtained from Sigma (St. Louis, MO). N-methylnaltrexone bromide or methylnaltrexone was purchased from Mallinckrodt Specialty Chemicals (Phillipsburg, NJ). Rabbit anti-MOR-1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and GeneTex (Irvine, CA). Reagents for SDS-PAGE electrophoresis were purchased from Bio-Rad (Richmond, CA) and Immobilon-P transfer membrane from Millipore (Millipore Corp., Bedford, MA). Secondary horseradish peroxidase (HRP)-labeled antibodies were purchased from Amersham Biosciences (Piscataway, NJ). For immunoblotting, cellular and tissue homogenates were run on SDS-PAGE in 4–15% polyacrylamide gels, transfer onto Immobilon™ membranes, and developed with specific primary and secondary antibodies. Visualization of immunoreactive bands was achieved using enhanced chemiluminescence (Amersham Biosciences).
Utilization of shRNA
The shRNA was used to inhibit the protein expression of the MOR in LLC cells by interfering with MOR mRNA. The shRNA for mouse MOR was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). LLC were transfected with shRNA using FuGENE HD™ as the transfection reagent (Roche Applied Sciences) according to the protocol provided by Roche. Cells (~40% confluent) were serum-starved for 1 hour followed by incubated with siRNA for 6 hours in serum-free media. Serum-containing media was then added (10% serum final concentration) for 42 h and puromycin selection reagent was added.20 MOR was efficiently silenced in LLC cells (inset of Figure 1-D).
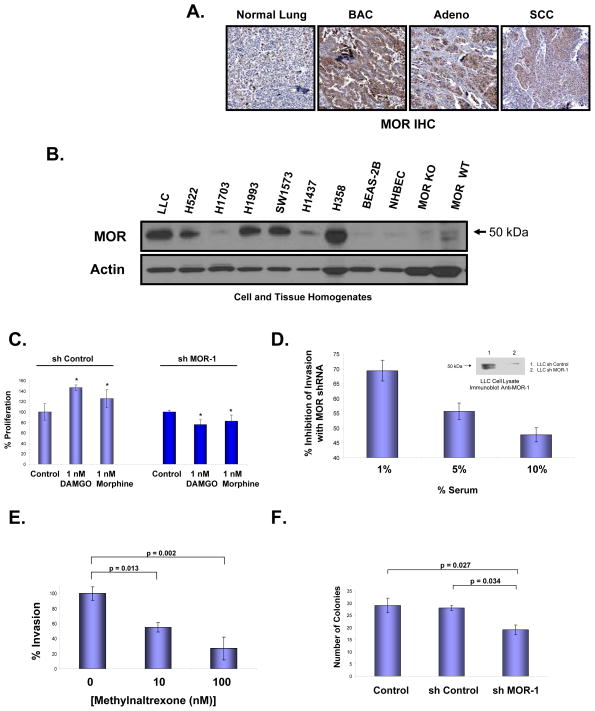
Figure 1. The mu opioid receptor (MOR) is overexpressed in human non-small cell lung cancer (NSCLC) and regulates Lewis lung carcinoma (LLC) oncogenic properties in vitro.
Panel A: Representative immunohistochemical (IHC) analysis of normal lung and lung tumor samples from patients indicate increased expression of MOR in bronchioloalveolar carcinoma (BAC), adenocarcinoma (Adeno) and, to a lesser extent, squamous cell carcinoma (SCC). MOR-specific brown staining intensity from 10 patient samples per group were analyzed with a statistically significant difference (p < 0.05) between normal and BAC, Adeno or SCC. Panel B: MOR immunoblot analysis of cell lysates from human NSCLC adneocarcinoma cell lines (H522, H1703, H1993, H1437), human NSCLC bronchioloalveolar carcinoma cell lines (SW1573, H358), non-cancerous BEAS-2B, normal human bronchial epithelial cells (NHBEC) and tissue homogenates from MOR knockout mouse brain and C57BL/6 mouse brain. There is increased MOR expression in several human NSCLC cell lines. Panel C: Stable control shRNA or MOR-1 shRNA-transfected LLC cells were analyzed for mu opioid agonist-mediated proliferation using a MTS proliferation assay. There is a statistically significant difference (p < 0.05, indicated by an asterisks) between control and DAMGO (1 nM, 24 hous) or morphine (1 nM, 24 hours)-treated LLC cells with n = 3 per condition. Stable silencing (shRNA) of the mu opioid receptor blocks mu opioid agonist-induced LLC proliferation. See the Methods section for experimental details. Panel D: Lewis lung carcinoma (LLC) cells were either transfected with control shRNA or MOR-1 shRNA, selected with puromycin and MOR protein expression analyzed (Panel C-inset). Cells were then analyzed for in vitro invasion with media containing 1, 5 or 10% serum, n = 3 per condition. There is a significant reduction in invasion (p < 0.05) between control shRNA and MOR-1 shRNA transfected cells for all conditions tested. Panel E: The peripheral MOR antagonist, methylnaltrexone (MNTX), inhibits LLC cell invasion. LLC cells were treated with 0, 10 or 100 nM MNTX and invasion assays performed. There is a statistically significant difference (p < 0.05) between groups with n = 3 per condition. Panel F: Stable control shRNA or MOR-1 shRNA-transfected LLC cells were analyzed for anchorage-independent growth using a soft agar colony formation assay. There is a statistically significant difference (p < 0.05) between groups with n = 3 per condition. See the Methods section for experimental details.
LLC Cell Proliferation Assay
For measuring in vitro cell growth, LLC [5 × 103 cells/well pretreated with control or MOR shRNA were incubated with 0.2 ml of serum-free media containing either vehicle (control), 1 nM morphine or 1 nM DAMGO for 24 h at 37°C in 5%CO2/95% air in 96-well culture plates. The in vitro cell proliferation assay was analyzed by measuring increases in cell number using the CellTiter96™ MTS assay (Promega, San Luis Obispo, CA) and read at 492 nm. Each assay was set up in triplicate, repeated at least five times and analyzed statistically by Student’s t test (with statistical significance set at p < 0.05).
LLC Cell Invasion Assay
Twenty-four transwell units with 8 μM pore size coated with Matrigel (QCM ECMatrix Cell Invasion Assay kit (Millipore, Billerica, MA)) were used for monitoring in vitro cell invasion as we have previously described.21 LLC cells (~1 × 104 cells/well) were plated with various treatments (MNTX, control shRNA, MOR shRNA) to the upper chamber and various concentrations of media with serum were added to the lower chamber. Cells were allowed to invade through the Matrigel and pores for 18 hours. Cells from the upper and lower chamber were quantitated using the CellTiter96™ MTS assay (Promega, San Luis Obispo, CA) and read at 492 nm. % invasion was defined as the # of cells in the lower chamber % the number of cells in both the upper and lower chamber. Each assay was set up in triplicate, repeated at least five times and analyzed statistically by Student’s t test (with statistical significance set at p < 0.05). The results of the in vitro invasion assays provide a potential explanation as to how the LLC mouse primary tumor cells can metastasize to the lung by invading through stromal and endothelial barriers.22
Soft Agar Colony Formation Assay
Ninety-six well units (Cytoselect™ 96 well Cell Transformation Assay, Cell Recovery Compatible, Colorimetric (Cell Biolabs, Inc. (San Diego, CA)) were used for monitoring in vitro anchorage-independent tumor cell growth.23,24 LLC cells (~1 × 103 cells/well) were plated with various treatments (control or shRNA) to a base agar matrix layer and monitored for 6 to 8 days. Formation of cell colonies was quantitated using MTT in detergent solution and absorbance was measured at 570 nm in a 96 well microtiter plate reader. Each assay was set up in triplicate, repeated at least five times and analyzed statistically by Student’s t test (with statistical significance set at p < 0.05). The results of the soft agar colony formation assays in this manuscript provide a potential explanation as to how the LLC mouse primary tumor cells can metastasize to the lung by anchorage-independent growth in the bloodstream.
In Vivo LLC Tumor Studies
Following approval of the Institutional Animal Care and Use Committee, three different in vivo experiments were performed. For all three, the cell lines used were LLC and stable GFP/RFP expressing LLC, as described above. All mice were 8 to 12-week-old females obtained from Jackson Laboratories (Bar Harbor, ME). At the end of each experiment, lungs and primary tumors (if present) were collected, fixed in formalin, and embedded in paraffin. All animal procedures were carried out in accordance with the guidelines provided by the IACUC of the University of Chicago. For i.v. injection of LLC (metastatic tumor model), stable GFP/RFP expressing LLC cells were treated with either control or MOR shRNA. For each cell treatment, 1.0 × 105 cells were injected into the jugular vein of wildtype C57BL/6 mice. For the subcutaneous tumor model using MOR knockout mice, 1.0 × 106 LLC cells were injected subcutaneously into the flank of C57BL/6 wildtype or MOR knockout mice. Tumor nodules were measured regularly once they became visible using calipers, and tumor volume VT (mm3) was calculated using the ellipsoid formula A2 × B × π/6, where A represents the smaller diameter.25 For the subcutaneous tumor model using wildtype mice and MNTX, 1.0 × 106 LLC cells were injected subcutaneously into the flank of C57BL/6 wildtype mice. Once tumors reached an average volume of 100 mm3, Alzet osmotic pumps (100 μl, 0.25 μl/hr) containing either MNTX or PBS were implanted subcutaneously on the back. The pumps delivered continuous doses of drug or vehicle over the course of 12 days, during which time the tumors were measured regularly and their volume was calculated.
Quantification of Lung Metastasis
To characterize metastasis from primary tumors growing in mouse hind flank (see above), lungs from wildtype, MOR knockout and MNTX-treated mice were formalin fixed, 5 micron paraffin sections were obtained, hydrated and epitope retrieval performed (DakoCytomation Target Retrieval Solution, pH=6.0, DakoCytomation, Carpinteria, CA). The sections were then histologically evaluated by H & E staining and photographed (100×) using a Leica Axioscope (Bannockburn, IL). Slide images were analyzed using ImageJ (NIH) software. Stained tumors with area greater than 30,000 μm2 were quantified and the pixel variable was normalized with the parameters of the microscope magnification used.
Human Lung Immunohistochemistry
Immunohistochemical analysis was performed on human tissue microarrays (TMA, RayBiotech, Inc., Norcross, GA). For antigen retrieval, sections were heated in Tris-EDTA buffer (pH=9) for 10 min and incubated for 1 hour at room temperature with mouse monoclonal MOR1 antibody (1:100) (GeneTex Inc, Irvine, CA). This was followed by 30 minute incubation with goat anti-rabbit HRP-conjugated IgG (EnVisionTM+, Dako). Slides were developed for five minutes with 3,3′-diaminobenzidene chromogen and counterstained with hematoxylin.
Quantification of MOR Expression in Human Lung Samples
MOR immunohistochemical staining was performed on tissue microarrays (TMA, RayBiotech, Inc., Norcross, GA) containing ten cases of human bronchioloalveolar carcinoma (BAC), adenocarcinoma (Adeno) or squamous cell carcinoma (SCC) lung cancer and adjacent normal tissue. The microscopic images of each slide were scanned and analyzed using Chromavision Automated Cellular Imaging System (ACIS, Clarient, Aliso Viejo, CA) in a digital format that accounts for all individual microscopic fields. Areas of interest were outlined and the intensity was quantified by the software and converted to a score over a set of defined parameters. Brown integrated optical density (IOD) per 10 μ2 was used as a unit of measurement since IOD (intensity multiplied by brown area in squared micrometers).26
Quantification of Lung Metastasis in Mice
To characterized metastasis from primary tumors growing in mouse hind flank (see above), lungs from wildtype, MOR knockout and MNTX-treated mice were formaline fixed. 5 μm paraffin section were subjected to H&E histostaining to evaluate the presence or absence of tumors. Metastasis was defined as a present of small clusters of tumor cells detected by H&E staining.27 The tumors are identifiable and histologically distinct from regular tissue. The sections of entire lungs were photographed (1.25×) using a Leica Axioscope (Bannockburn) and analyzed. Slides images were processed using ImageJ (NIH) software.28 Perfectly circular metastatic lesions that were more than 0.1 mm in radius were defined as macrometastasis, while micrometastasis was defined as a tumor deposit less than 0.1 mm in radius, but large than 0.05 mm. Staining with the radius less than 0.05 mm was too insignificant to quantify. Hence, the method is sensitive enough to identify larger metastasis but fails to account for metastasis less that 0.05 mm due to difficulty in differentiating from the normal tissue.
In Vivo Murine Fluorescent Imaging
To visualize GFP/RFP-labeled LLC growth in mouse lung, the Olympus OV-100 Small Animal Imaging System (Olympus Corp., Tokyo, Japan) was used for imaging to compare tumor development in mice inoculated with MOR-1 silenced as well control LLC tumor cells.25,29 The OV-100 imaging system used in our studies is equipped with a highly sensitive Hamamatsu Electron Multiplying (EM) CCD camera. Two weeks post inoculation with tumor cells; lungs were imaged ex-vivo to evaluate the number of micro-tumors in the lungs. To visualize and quantify primary flank tumor growth changes associated with MOR in real-time, mice were imaged with ViSen FMT. ViSen FMT is optimized for probes incorporating commonly used near-infrared (NIR) fluorochromes in both the 680 nm and 750 nm channels with mouse handling features for high-throughput imaging. We have used Prosense 680 and MMPsense750 NIR probes (ViSen Medicals) to visualize and quantify changes in tumor growth. MOR knockout as well as wild type control mice, inoculated with tumor cells, were injected (i.v.) with probes (2 nM) at 2 wks post tumor cell inoculation and imaged at 24 hrs post probe injection.
Statistical Analysis
Data are expressed as means ± standard deviation. Student’s t test using two tailed analysis of two groups with unequal variance was utilized for data analysis with a p value of less than 0.05 considered statistically significant.
Results
Our initial observations in this study demonstrated increased MOR expression in lung samples from patients with non-small cell lung cancer (NSCLC), a disease with few treatment options that accounts for 85% of all lung cancers30 (Figure 1-A). These data are consistent with PET imaging data in humans demonstrating mu receptors in three major types of lung cancer.31
We next examined several human NSCLC cell lines and observed similar increases in MOR expression (Figure 1-B). To test the functional significance of MOR expression in vitro, we inhibited MOR expression in mouse Lewis lung carcinoma cells (LLC) using shRNA and observed these cells failed to respond to mu opioid agonist (DAMGO or morphine, 1 nM, 24 hours)-mediated increase in proliferation (~35–50%, p < 0.05, Figure 1-C). Further, MOR silenced LLC exhibited less invasive properties (~45–70%)(Figure 1-D). The use of MNTX, at physiologically relevant concentrations (10 nM, p = 0.013; 100 nM, p = 0.002),32 also inhibited LLC invasion (~40–75%) indicating the importance of MOR in this cancer process (Figure 1-E). Attenuating MOR expression in LLC also reduced anchorage-independent growth (~35%), a measure of tumorigenic potential (Figure 1-F). We next hypothesized that MOR shRNA LLC cells would exhibit less invasive properties in vivo.
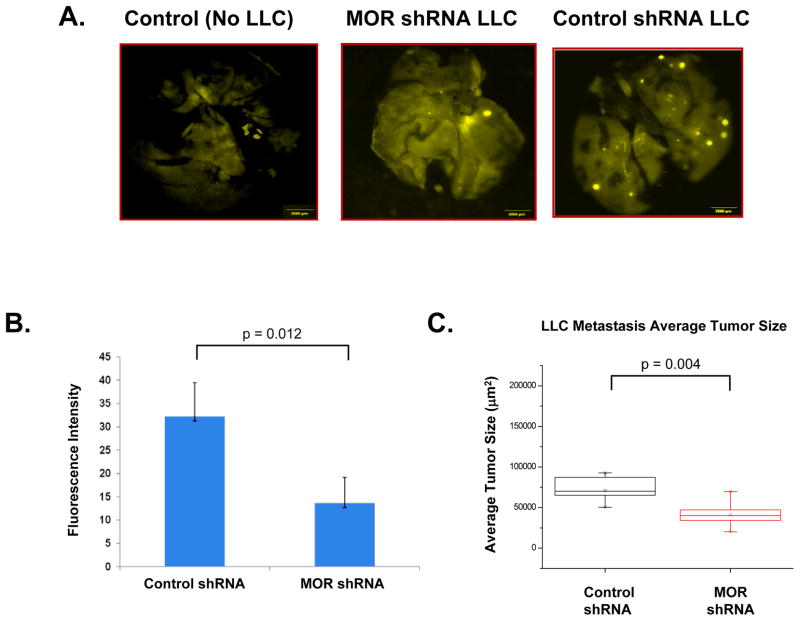
Using a mouse model of lung metastasis with intravenous injection of LLC that express GFP/RFP, we observed reduced lung metastasis size with MOR silencing (Figure 2-A) using lung fluorescence intensity (p = 0.012)(Figure 2-B) and quantitation of H & E stained lung sections (p = 0.004) (Figure 2-C).
Figure 2. Silencing (shRNA) MOR expression in LLC inhibits tumor growth and metastasis.
Panel A: Stable control shRNA or MOR-1 shRNA-transfected LLC cells were generated and either no cells (control), or LLC cells were injected (1×105) intravenously. Mice were imaged at 4 weeks post injection with OV-100. Panel B: Graphical representation of quantitation of fluorescent intensity from experiments described in Panel A (p = 0.012, n = 4 mice per condition). Panel C: Graphical representation of quantitation of H & E stained sections of experiments similar to those described in Panel A. MOR silenced LLC cells exhibited a significant reduction in tumor metastasis volume (p = 0.004, n = 6 mice per condition). See the Methods section for experimental details.
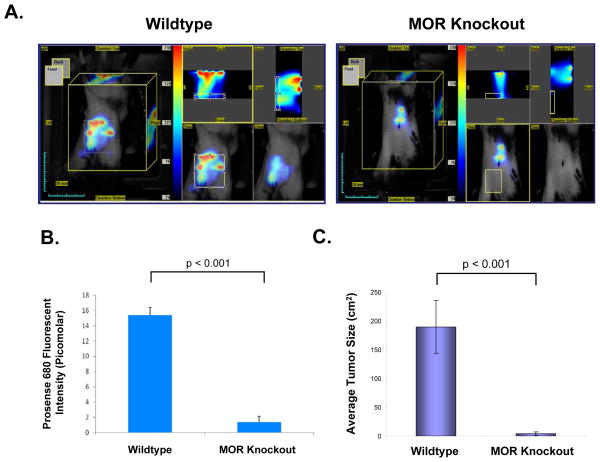
Having previously demonstrated that opiates promote angiogenesis both in vitro and in vivo,5–7 we determined if MOR could have an additional effect on the tumor microenvironment. To test this, LLC cells were injected into the hind flank of wildtype and MOR knockout mice and tumor formation was examined in vivo using Visen FMT imaging of tumor volume at 12 days post visible tumor formation after injection of Prosense68033,34 (activated by cathepsins, known to be up-regulated in cancer35) near infrared probe (Figure 3-A). Quantitation of in vivo fluorescence indicated virtually no tumor formation in the MOR knockout mouse (p < 0.001)(Figure 3-B). These results were independently verified by direct physical measurement using calipers to measure tumor volume (p < 0.001)(Figure 3-C). We maintained MOR knockout mice for up to 12 weeks without significant visible tumor, suggesting that the MOR, even absent exogenous opioids, may play a role in tumor progression.
Figure 3. Inhibition of LLC tumor formation in the mu opioid receptor (MOR) knockout mouse.
C57BL/6 wildtype (WT) and mu opioid receptor knockout mice (MOR−/−) were injected with dual color labeled LLC cells (1×106) subcutaneously in the right flank. Panel A: Visen FMT imaging of tumor volume at 12 days post visible tumor formation in wildtype C57BL/6 mouse (Left Panel) or MOR knockout mouse (Right Panel) after injecting Prosense680 (i.v, 2nM, 24 hours prior imaging) near infrared probe for quantification of tumor volume and lung metastasis. Panel B: Quantitation of Prosense680 fluorescence intensity in wildtype and MOR knockout mice (p < 0.001, n = 5 animals per condition). Panel C: Graphical analysis of LLC tumor volume in wildtype and MOR knockout mice (p < 0.001, n = 5 animals per condition). See the Methods section for experimental details.
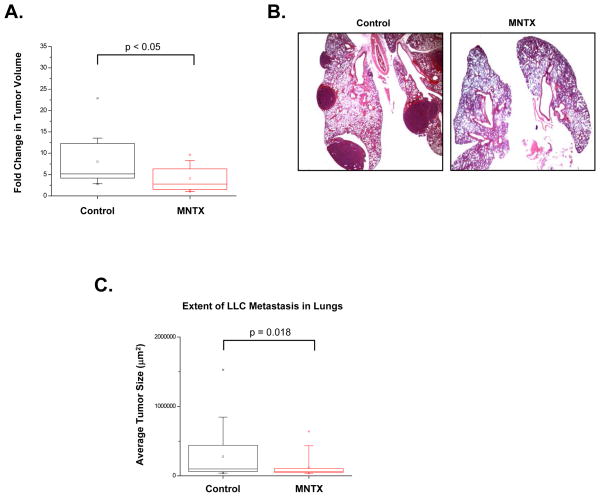
Given our data indicating the importance MOR plays in several cancer related processes, we hypothesized that MNTX could reduce LLC tumor size and metastasis in our animal models. Continuous infusion of MNTX (10 mg/kg/day, Alzet mini pumps) for two weeks after visible primary flank tumor formation in C57BL/6 mice attenuated further tumor growth (p < 0.05)(Figure 4-A). These same mice were evaluated for lung metastasis from the primary flank tumor using H & E staining of excised formalin-fixed lung sections (Figure 4-B). Our data indicate MNTX substantially inhibited lung metastasis, p = 0.018, Figure 4-C) providing further evidence that MOR is a novel regulator of lung cancer progression. Mice treated with naltrexone infusions exhibited a similar effect on tumor growth and metastasis suggesting that this is not a drug specific effect of MNTX (data not presented). These studies were performed using MNTX since opioids are commonly given to cancer patients and MNTX is the only parenterally administrated peripheral opiate antagonist clinically available.
Figure 4. Continuous infusion of MNTX inhibits LLC tumor growth and metastasis.
Panel A: Graph indicating continuous infusion of MNTX (10 mg/kg/day, Alzet mini pumps) for two weeks after visible tumor formation in mice (LLC cells (1×106) injected subcutaneously in the right flank) attenuates further tumor growth (n = 5 mice per condition, p < 0.05). Panel B: The same mice as described in Panel A were evaluated for lung metastasis from the primary flank tumor using H & E staining of excised formalin-fixed lung sections. Panel C: Graphical representation of the extent of lung metastasis with or without MNTX infusion, n = 5 mice per condition, p = 0.018. See the Methods section for experimental details.
Discussion
Our observations demonstrate an effect of the MOR on cancer growth and metastasis. That our observations may have clinical relevance is suggested by recent epidemiologic data demonstrating that anesthetic technique (i.e. use of opiates) may play a role in tumor dissemination and recurrence.1 In a retrospective study of 225 patients who had surgery for prostate cancer, there was a significant difference in the rate of tumor recurrence contingent on the type of anesthesia utilized.3 Patients receiving a combined epidural/general anesthetic had 61% less recurrence (p < 0.0003) than those who received a general anesthetic with opiates for analgesia during their primary surgery. A small retrospective study in breast cancer had similar findings.4 Several recent retrospective analyses also point to a possible effect of anesthetic choice on cancer recurrence although the results are less clear cut. A small Canadian study36 of 99 patients found no difference in recurrence between patients receiving adjunctive epidural anesthesia with opiates and general anesthetics, while another retrospective study37 of 669 patients undergoing colorectal surgery failed to demonstrate an association between epidural use and cancer recurrence, although there appeared to be some benefit in patients older than 64 years. A third study38 from the VA Cooperative Study on patients undergoing colon cancer resection, showed a more complicated effect, demonstrating some benefits of epidural in patients without metastases. Additional support for an effect of anesthetics on cancer progression comes from a study on melanoma. When general anesthesia was used for primary excision, there was a decrease in survival rate (relative risk of 1.46, p < 0.0001) compared to local anesthetic.39 Thus, while retrospective epidemiologic studies suggest a potential difference between epidural and general anesthetics in various malignancies, they do not define a mechanism. Further, these retrospective studies cannot distinguish between a putative beneficial effect of the regional anesthetic or a possible oncogenic effect of the opiate. A large multi-center prospective randomized trial investigating the influence of type of anesthesia on breast cancer recurrence is currently underway.40
A recent review suggests potential immunologic, endocrine, or proangiogenic effects of anesthetics and opioids as explanations for this difference.1 Although the potential mechanisms for an effect of anesthetics on cancer recurrence may be complex and multifactorial,1,41 we focused on exploring the direct effect of opioids on tumor growth and progression. Our findings do not preclude other explanations such as attenuation of the neuroendocrine stress response or immunologic effects. However, a direct action of opioids is more temporally consistent with an effect in the perioperative period than the hypothesis of an effect on immune surveillance.
The current study provides a possible explanation for the epidemiologic findings of an anesthetic effect in prostate and breast cancer. Our most dramatic finding was that we did not observe substantial LLC tumor growth in the MOR knockout mouse (Figure 3). While wildtype mice developed lethal tumors 12 days after injection, none of the MOR knockout mice developed tumors even after 12 weeks. One potential explanation for this finding is that the MOR may be required in angiogenesis, an essential process for in vivo tumor growth. We have previously published that the proangiogenic effects of mu opioid agonists appear to be due to reciprocal transactivation of the VEGF receptor through a mechanism that involves Src activation.5 We further demonstrated that the peripherally acting mu opiate receptor antagonist methylnaltrexone (MNTX), which antagonizes the peripheral effects of opiates while preserving centrally mediated analgesia, inhibits opiate- and VEGF-induced angiogenesis through direct inhibition of MOR and activation of tyrosine phosphatase activity.5–7 MNTX has a synergistic effect with 5-fluorouracil, bevacizumab and mTOR inhibitors on inhibition of angiogenic events through attenuation of different components of a common VEGF-induced signal transduction pathway.6,7 Beyond its effect on the cell membrane, there may also be specific MNTX, but not naltrexone, mediated effects on Src activation since silencing the RPTPμ phosphatase blocks MNTX (but not naltrexone) inhibition of VEGF-induced Src phosphorylation.6
Our results in knockout mice are supported by several lines of in vivo and in vitro evidence. We demonstrate increased MOR expression in lung samples from patients with NSCLC and in several human NSCLC cell lines (Figure 1). Since MOR overexpression is not necessarily a pre-requisite for tumor propagation, our laboratory is currently examining the exact role of MOR overexpression in regard to propagation of tumorigenic properties. Beyond the demonstration of increased MOR expression in tumor samples, treatment of LLC with the MOR agonists morphine and DAMGO increases in vitro cellular growth (Figure 1). Blocking MOR function (MNTX or shRNA) substantially reduces in vitro measures of LLC tumorigenicity such as invasion and anchorage-independent growth (Figure 1). Our in vivo studies demonstrate that intravenous injection of LLC without MOR expression leads to a ~65% reduction in mouse lung metastasis (Figure 2). Finally, continuous infusion of MNTX (10 mg/kg/day) attenuates primary LLC tumor growth and reduces lung metastasis (Figure 4). We have performed preliminary studies on plasma concentrations of MNTX in several of the autopsied animals (data not shown). Plasma concentrations are similar to the doses we achieved in our endothelial barrier function studies42 and are consistent with what may be achieved clinically with parenteral administration of MNTX.
Whether mu opioids facilitate oncogenesis beyond the surgical setting is unclear. One randomized trial of patients receiving either intrathecal opiates or comprehensive (i.e. systemic opiate cancer care) showed a dramatic difference at 180 days (54% vs. 37%) in survival in those patients receiving intrathecal opiates.43 On the other hand, there is no compelling clinical evidence that either opiates or opiate antagonists affect the development of new tumors. We examined this hypothesis in a retrospective study of new tumors from patients receiving methadone maintenance or chronic implanted naltrexone. Although the study was retrospective and small, some 4,000 patient years in all, we did not see a signal suggesting an effect of methadone or naltrexone on the development of new tumors.44 The perioperative period may be special in several regards. First, mu opioid agonists are routinely given parenterally in the perioperative period and often in high doses. Secondly, in cases of tumor surgery, recent data suggests that there may be a release of tumor cells into the circulation.45–48 Finally, there may be direct effects of opioids on barrier function which may allow those tumor cells liberated during surgery to directly breach the endothelial barrier, potentially causing metastasis to distant locations.42 We have previously demonstrated concentration dependent in vivo and in vitro differences in barrier integrity mediated by morphine and other mu opioids.
Beyond their role in anesthetics, our findings of MOR regulation of lung cancer are novel and point to a possible therapeutic role of opioid antagonists. NSCLC has a poor prognosis and considerable heterogeneity. A number of novel therapeutic options have emerged for NSCLC, such as EGFR inhibition49 and VEGF inhibition.50 However, the response to these therapeutics is only short-lived in a majority of patients.30,51 Further, many lung cancer patients take opioids, and this may potentially affect the growth and progression of lung cancer tumors.
Certain caveats apply to our work. First, LLC is amongst the robust tumors in both growth and response to drugs.52 Whether our findings are applicable to other tumors or to humans is not known. While we have not explored a potential role of opiate antagonists in other models of human malignancy, the original study by Gupta was performed in breast cancer xenografts. Additionally, a recent presented study of nude mice inoculated with human squamous head and neck cancer cells demonstrated that chronic naltrexone treatment inhibited growth of these tumors by up to 84% at 3 weeks (p < 0.001). Thus, we cannot exclude a more general role for our observations.53 Additionally, the work presented in this communication deals predominantly with the effects of MOR antagonists. The effect of chronic opioid administration is not specifically assessed. There is considerable evidence that chronic exposure to exogenous opioids changes response in both brain and gut. Whether the MOR expression changes with chronic exposure in tumor cells or endothelial cells is unknown. Finally, our findings do not preclude other effects of opioids, either directly or indirectly. There is an emerging literature on the effects of opioids on the immune system. However, a direct effect such as we define could explain the fact that these effects occur during surgery with brief exposure.
Taken as a whole these results indicate the MOR promotes a mouse model of lung cancer growth and metastasis. In conjunction with our findings that mu opioids alter endothelial barrier integrity and facilitate angiogenesis, our observations (many of which were made without exogenous opioids) suggest a possible explanation for an anesthetic effect on recurrence in patients undergoing cancer surgery. Whether these results can be extended into the therapy of human malignancy remains to be determined. However, our observations do suggest further study of MOR antagonists as a potential therapeutic target is merited.
List of Abbreviations
- Adeno
human lung adenocarcinoma
- BAC
human bronchioloalveolar carcinoma
- DAMGO
[D-Ala2, N-MePhe4, Gly-ol]-enkephalin
- LLC
Lewis Lung Carcinoma
- MNTX
methylnaltrexone
- MOR
mu opioid receptor
- NSCLC
non-small cell lung cancer
- SCC
human lung squamous cell carcinoma
- Src
Rous sarcoma virus proto-oncogene, also called c(cellular)-Src
- VEGF
vascular endothelial growth factor
Footnotes
Competing Financial Interest
Dr. Moss serves as a paid consultant to Progenics Pharmaceuticals, Inc., has a financial interest in methylnaltrexone as a patent holder through the University of Chicago, and receives stock options from Progenics.
References
- 1.Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010;110:1636–1643. doi: 10.1213/ANE.0b013e3181de0ab6. [DOI] [PubMed] [Google Scholar]
- 2.Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109:180–187. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- 3.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss J, Israel RJ. Effects of anesthetics on cancer recurrence. J Clin Oncol. 2009;27:e89. doi: 10.1200/JCO.2009.23.7651. author reply e90. [DOI] [PubMed] [Google Scholar]
- 5.Singleton PA, Lingen MW, Fekete MJ, Garcia JG, Moss J. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: Role of receptor transactivation. Microvasc Res. 2006;72:3–11. doi: 10.1016/j.mvr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Singleton PA, Garcia JG, Moss J. Synergistic effects of methylnaltrexone with 5-fluorouracil and bevacizumab on inhibition of vascular endothelial growth factor-induced angiogenesis. Mol Cancer Ther. 2008;7:1669–1679. doi: 10.1158/1535-7163.MCT-07-2217. [DOI] [PubMed] [Google Scholar]
- 7.Singleton PA, Mambetsariev N, Lennon FE, Mathew B, Siegler JH, Moreno-Vinasco L, Salgia R, Moss J, Garcia JG. Methylnaltrexone Potentiates the Anti-Angiogenic Effects of mTOR Inhibitors. J Angiogenes Res. 2010;2:5. doi: 10.1186/2040-2384-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–4498. [PubMed] [Google Scholar]
- 9.Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–4498. [PubMed] [Google Scholar]
- 10.Tegeder I, Grosch S, Schmidtko A, Haussler A, Schmidt H, Niederberger E, Scholich K, Geisslinger G. G protein-independent G1 cell cycle block and apoptosis with morphine in adenocarcinoma cells: Involvement of p53 phosphorylation. Cancer Research. 2003;63:1846–1852. [PubMed] [Google Scholar]
- 11.Page GG, Ben-Eliyahu S, Yirmiya R, Liebeskind JC. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993;54:21–28. doi: 10.1016/0304-3959(93)90095-7. [DOI] [PubMed] [Google Scholar]
- 12.Page GG, Ben-Eliyahu S, Liebeskind JC. The role of LGL/NK cells in surgery-induced promotion of metastasis and its attenuation by morphine. Brain Behav Immun. 1994;8:241–250. doi: 10.1006/brbi.1994.1022. [DOI] [PubMed] [Google Scholar]
- 13.Page GG, McDonald JS, Ben-Eliyahu S. Pre-operative versus postoperative administration of morphine: impact on the neuroendocrine, behavioural, and metastatic-enhancing effects of surgery. Br J Anaesth. 1998;81:216–223. doi: 10.1093/bja/81.2.216. [DOI] [PubMed] [Google Scholar]
- 14.Singhal PC, Kapasi AA, Reddy K, Franki N, Gibbons N, Ding G. Morphine promotes apoptosis in Jurkat cells. J Leukoc Biol. 1999;66:650–658. doi: 10.1002/jlb.66.4.650. [DOI] [PubMed] [Google Scholar]
- 15.Singhal P, Kapasi A, Reddy K, Franki N. Opiates promote T cell apoptosis through JNK and caspase pathway. Adv Exp Med Biol. 2001;493:127–135. doi: 10.1007/0-306-47611-8_15. [DOI] [PubMed] [Google Scholar]
- 16.Shavit Y, Ben-Eliyahu S, Ziedel A, Beilin B. Effects of fentanyl on natural killer cell activity and on resistance to tumor metastasis in rats. Dose and timing study. Neuroimmunomodulation. 2004;11:255–260. doi: 10.1159/000078444. [DOI] [PubMed] [Google Scholar]
- 17.Cao L, Liu X, Lin E-JE, Wang C, Choi EY, Riban V, Lin B, During MJ. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64. doi: 10.1016/j.cell.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falardeau P, Champagne P, Poyet P, Hariton C, Dupont E. Neovastat a naturally occurring multifunctional antiangiogenic drug in phase III clinical trials. Semin Oncol. 2001;28:620–625. doi: 10.1016/s0093-7754(01)90035-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhou H-J, Zhang J-L, Li A. Dihydroartemisinin improves the efficiency of chemotherapeutics in lung carcinomas in vivo and inhibits murine Lewis lung carcinoma cell line growth in vitro. Cancer Chemother Pharmacol. 2010;66:21–29. doi: 10.1007/s00280-009-1129-z. [DOI] [PubMed] [Google Scholar]
- 20.Rao DD, Senzer N, Cleary MA, Nemunaitis J. Comparative assessment of siRNA and shRNA off target effects: what is slowing clinical development. Cancer Gene Ther. 2009;16:807–809. doi: 10.1038/cgt.2009.53. [DOI] [PubMed] [Google Scholar]
- 21.Faoro L, Singleton PA, Cervantes GM, Lennon FE, Choong NW, Kanteti R, Ferguson BD, Husain AN, Tretiakova MS, Ramnath N, Vokes EE, Salgia R. EphA2 mutation in lung squamous cell carcinoma promotes increased cell survival cell invasion focal adhesions and mTOR activation. J Biol Chem. 2010;285:18575–18585. doi: 10.1074/jbc.M109.075085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Wei LL, Tang C, Slack R, Mueller S, Lippman ME. Overexpression of KAI1 suppresses in vitro invasiveness and in vivo metastasis in breast cancer cells. Cancer Res. 2001;61:5284–5288. [PubMed] [Google Scholar]
- 23.Liao WT, Lin P, Cheng TS, Yu HS, Chang LW. Arsenic promotes centrosome abnormalities and cell colony formation in p53 compromised human lung cells. Toxicol Appl Pharmacol. 2007;225:162–170. doi: 10.1016/j.taap.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi K, Sakamoto M, Yasuda J, Takamura M, Fujita N, Tsuruo T, Todo S, Hirohashi S. Critical involvement of the phosphatidylinositol 3-kinase/Akt pathway in anchorage-independent growth and hematogeneous intrahepatic metastasis of liver cancer. Cancer Res. 2002;62:2971–2975. [PubMed] [Google Scholar]
- 25.Hoffman RM, Yang M. Whole-body imaging with fluorescent proteins. Nat Protoc. 2006;1:1429–1438. doi: 10.1038/nprot.2006.223. [DOI] [PubMed] [Google Scholar]
- 26.Hilbe W, Gächter A, Duba HC, Dirnhofer S, Eisterer W, Schmid T, Mildner A, Bodner J, Wöll E. Comparison of automated cellular imaging system and manual microscopy for immunohistochemically stained cryostat sections of lung cancer specimens applying p53, ki-67 and p120. Oncol Rep. 2003;10:15–20. [PubMed] [Google Scholar]
- 27.Liang Z, Yoon Y, Votaw J, Goodman MM, William L, Shim H. Silencing of CXCR4 blocks cancer metastasis. Cancer Res. 2005;65:967–971. [PMC free article] [PubMed] [Google Scholar]
- 28.Ho B, Pan TJ. The Monascus Metabolite Monacolin K Reduces Tumor Progression and Metastasis of Lewis Lung Carcinoma Cells. Agric Food Chem. 2009;57:8258–8265. doi: 10.1021/jf901619w. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Yang M, Shen J, Gerhold LM, Hoffman RM, Xing HR. The role of the intravascular microenvironment in spontaneous metastasis development. Int J Cancer. 2010;126:2534–2541. doi: 10.1002/ijc.24979. [DOI] [PubMed] [Google Scholar]
- 30.Spiro SG, Tanner NT, Silvestri GA, Janes SM, Lim E, Vansteenkiste JF, Pirker R. Lung cancer: progress in diagnosis, staging and therapy. Respirology. 2010;15:44–50. doi: 10.1111/j.1440-1843.2009.01674.x. [DOI] [PubMed] [Google Scholar]
- 31.Madar I, Bencherif B, Lever J, Heitmiller RF, Yang SC, Brock M, Brahmer J, Ravert H, Dannals R, Frost JJ. Imaging delta- and mu-opioid receptors by PET in lung carcinoma patients. J Nucl Med. 2007;48:207–213. [PubMed] [Google Scholar]
- 32.Moss J, Rosow CE. Development of peripheral opioid antagonists’ new insights into opioid effects. Mayo Clin Proc. 2008;83:1116–1130. doi: 10.4065/83.10.1116. [DOI] [PubMed] [Google Scholar]
- 33.Korideck H, Peterson JD. Noninvasive quantitative tomography of the therapeutic response to dexamethasone in ovalbumin-induced murine asthma. J Pharmacol Exp Ther. 2009;329:882–889. doi: 10.1124/jpet.108.147579. [DOI] [PubMed] [Google Scholar]
- 34.Cortez-Retamozo V, Swirski FK, Waterman P, Yuan H, Figueiredo JL, Newton AP, Upadhyay R, Vinegoni C, Kohler R, Blois J, et al. Real-time assessment of inflammation and treatment response in a mouse model of allergic airway inflammation. J Clin Invest. 2008;118:4058–4066. doi: 10.1172/JCI36335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuester D, Lippert H, Roessner A, Krueger S. The cathepsin family and their role in colorectal cancer. Pathol Res Pract. 2008;204:491–500. doi: 10.1016/j.prp.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Tsui BC, Rashiq S, Schopflocher D, Murtha A, Broemling S, Pillay J, Finucane BT. Epidural anesthesia and cancer recurrence rates after radical prostatectomy. Can J Anaesth. 2010;57:107–112. doi: 10.1007/s12630-009-9214-7. [DOI] [PubMed] [Google Scholar]
- 37.Gottschalk A, Ford JG, Regelin CC, You J, Mascha EJ, Sessler DI, Durieux ME, Nemergut EC. Association between Epidural Analgesia and Cancer Recurrence after Colorectal Cancer Surgery. Anesthesiology. 2010 doi: 10.1097/ALN.0b013e3181de6d0d. [DOI] [PubMed] [Google Scholar]
- 38.Christopherson R, James KE, Tableman M, Marshall P, Johnson FE. Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg. 2008;107:325–332. doi: 10.1213/ane.0b013e3181770f55. [DOI] [PubMed] [Google Scholar]
- 39.Schlagenhauff B, Ellwanger U, Breuninger H, Stroebel W, Rassner G, Garbe C. Prognostic impact of the type of anaesthesia used during the excision of primary cutaneous melanoma. Melanoma Res. 2000;10:165–169. [PubMed] [Google Scholar]
- 40.Sessler DI, Ben-Eliyahu S, Mascha EJ, Parat MO, Buggy DJ. Can regional analgesia reduce the risk of recurrence after breast cancer? Methodology of a multicenter randomized trial. Contemp Clin Trials. 2008;29:517–526. doi: 10.1016/j.cct.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Bovill JG. Surgery for cancer: does anesthesia matter? Anesth Analg. 2010;110:1524–1526. doi: 10.1213/ANE.0b013e3181d8d183. [DOI] [PubMed] [Google Scholar]
- 42.Singleton PA, Moreno-Vinasco L, Sammani S, Wanderling SL, Moss J, Garcia JG. Attenuation of Vascular Permeability by Methylnaltrexone: Role of mOP-R and S1P3 Transactivation. Am J Respir Cell Mol Biol. 2007;37:222–231. doi: 10.1165/rcmb.2006-0327OC. [DOI] [PubMed] [Google Scholar]
- 43.Smith TJ, Staats PS, Deer T, Stearns LJ, Rauck RL, Boortz-Marx RL, Buchser E, Catala E, Bryce DA, Coyne PJ, Pool GE. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain drug-related toxicity and survival. J Clin Oncol. 2002;20:4040–4049. doi: 10.1200/JCO.2002.02.118. [DOI] [PubMed] [Google Scholar]
- 44.Tait RJ, Hulse GK, Moss J. Does methadone maintenance therapy increase the risk of new cancers?. European J Cancer; Proceedings of the European Cancer Conference; Barcelona. September, 2007.2007. p. 166. [Google Scholar]
- 45.Weckermann D, Polzer B, Ragg T, Blana A, Schlimok G, Arnholdt H, Bertz S, Harzmann R, Klein CA. Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J Clin Oncol. 2009;27:1549–1556. doi: 10.1200/JCO.2008.17.0563. [DOI] [PubMed] [Google Scholar]
- 46.Eschwege P, Dumas F, Blanchet P, Le Maire V, Benoit G, Jardin A, Lacour B, Loric S. Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet. 1995;346:1528–1530. doi: 10.1016/s0140-6736(95)92054-4. [DOI] [PubMed] [Google Scholar]
- 47.Wong IH, Lau WY, Leung T, Yeo W, Johnson PJ. Hematogenous dissemination of hepatocytes and tumor cells after surgical resection of hepatocellular carcinoma: a quantitative analysis. Clin Cancer Res. 1999;5:4021–4027. [PubMed] [Google Scholar]
- 48.Denis MG, Lipart C, Leborgne J, LeHur PA, Galmiche JP, Denis M, Ruud E, Truchaud A, Lustenberger P. Detection of disseminated tumor cells in peripheral blood of colorectal cancer patients. Int J Cancer. 1997;74:540–544. doi: 10.1002/(sici)1097-0215(19971021)74:5<540::aid-ijc11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 49.Riely GJ. Second-generation epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. J Thorac Oncol. 2008;3:S146–149. doi: 10.1097/JTO.0b013e318174e96e. [DOI] [PubMed] [Google Scholar]
- 50.Rossi A, Maione P, Ferrara ML, Sacco PC, Schettino C, Bareschino MA, Gridelli C. Angiogenesis inhibitors and vascular disrupting agents in non-small cell lung cancer. Curr Med Chem. 2009;16:3919–3930. doi: 10.2174/092986709789352286. [DOI] [PubMed] [Google Scholar]
- 51.De Greve J, Decoster L, Van Meerbeek J, Vermeij J, Teugels E, Schallier D. Targeted therapies in the treatment of non-small cell lung cancer. Bull Cancer. 2008;95:358–364. doi: 10.1684/bdc.2008.0591. [DOI] [PubMed] [Google Scholar]
- 52.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170:793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLaughlin PJ, Stucki J, Zagon IS. Inhibition of cancer progression by upregulation of the opioid growth factor (OGF) – OGF receptor (OGFr) axis using imquimod or low dose naltrexone. AACR 101st Annual Meeting; April, 2010; Washington, D.C. USA. abstract #697. [Google Scholar]