Abstract
Purpose
Atypical teratoid/rhabdoid tumor (AT/RT) of the central nervous system is a rare cancer primarily affecting children younger than age five. Because patients are young and receive intensive chemotherapy, there is concern regarding late radiation toxicity, particularly as survival rates improve. Therefore, there is interest in using proton therapy to treat these tumors. This study was undertaken to investigate outcomes and acute toxicities associated with proton therapy for AT/RT.
Materials and Methods
The records of 31 patients with AT/RT treated with proton radiation from October 2008 to August 2013 were reviewed. Demographics, treatment characteristics and outcomes were recorded and analyzed.
Results
Median age at diagnosis was 19 months (range, 4 – 55 months), with median age at radiation start of 24 months (range, 6 – 62 months). Seventeen received local radiation with median dose of 50.4 GyRBE (range, 9 – 54). Fourteen received craniospinal radiation; half received 24 GyRBE or less and half received 30.6 GyRBE or higher. For patients receiving craniospinal radiation, the median tumor dose was 54 GyRBE (range, 43.2 – 55.8). Twenty-seven (87%) completed the planned radiation. With median follow-up of 24 months for all patients (range, 3 – 53 months), median progression-free survival was 20.8 months and median overall survival was 34.3 months. Five patients (16%) developed clinical findings and imaging changes in the brainstem one to four months after radiation consistent with radiation reaction; all resolved with steroids or bevacizumab.
Conclusions
This is the largest report of children with AT/RT treated with proton therapy. Preliminary survival outcomes in this young pediatric population are encouraging compared to historic results, but further study is warranted.
Keywords: AT/RT, rhabdoid tumor, brain, pediatric, proton, radiation
Introduction
Atypical teratoid/rhabdoid tumor (AT/RT) is a highly lethal tumor of the central nervous system (CNS) that primarily affects children less than five years of age. Despite aggressive multi-modality intervention, median survival is historically 6 – 11 months [1,2].
Because AT/RT is an uncommon malignancy, the optimal approach to treatment has not been identified. Surgery and chemotherapy are mainstays of therapy; the role of radiation remains ill-defined because many patients are younger than 3 years old and very susceptible to radiation toxicities. Initial approaches in this disease emphasized delayed radiation to minimize neurotoxicity [3]. Emerging evidence supports early radiation, even in very young patients, to increase the likelihood of disease control and long-term survival [4-7].
Given the increasing awareness of the role of radiation in AT/RT and the young age of patients, there is considerable interest in using proton radiation. Proton therapy decreases low dose radiation exposure to uninvolved brain as well as to structures anterior to the craniospinal axis compared with standard photon-based radiotherapy [8-10]. Therefore, the use of proton therapy may allow for therapeutic doses of radiation to the target volumes with greater sparing of adjacent normal tissue compared to photon therapy [11-15]. In the short term, these features of proton therapy may increase tolerance of concurrent and adjuvant chemotherapy by decreasing hematologic and gastrointestinal side effects [16]. In the long term, protons may decrease the neurocognitive, endocrine, vascular, and developmental sequelae of treatment, as well as the risk of radiation-induced second malignancies [17,18]. This is particularly important because AT/RT typically affects very young children who are more likely to have devastating late effects from therapy [19-21].
The aim of this study was to evaluate a single institutional experience in the use of proton radiation for the treatment of pediatric AT/RT of the CNS.
Methods
Almost all patients treated with protons at MD Anderson Cancer Center are enrolled on a prospective in-house registry protocol to follow the normal tissue toxicity and outcomes of patients. The registry protocol was approved by the institutional IRB and informed consent was obtained at the time of enrollment. Thirty-one patients with AT/RT of the CNS with at least six months of potential follow-up treated at MD Anderson Cancer Center from October 2008 to August 2013 were identified from the registry of 700 patients. Their medical records were retrospectively reviewed for clinical data, treatment details, and outcomes.
Patients underwent initial diagnosis and treatment at 23 different institutions. Surgical pathology for 26 of 31 patients (84%) was reviewed at MD Anderson Cancer Center or Texas Children's Hospital prior to radiotherapy. Patients underwent lumbar puncture, MRI of the brain, and MRI of the spine at diagnosis and prior to the initiation of radiotherapy. The extent of resection was defined as a gross total resection (GTR) or subtotal resection (STR) based on analysis of post-operative imaging and the intraoperative determination of the neurosurgeon [4]. Metastatic disease was staged according to the modified Chang criteria [22]. Briefly, M0 is no evidence of microscopic or gross metastatic disease, M1 is microscopic tumor cells present in cerebrospinal fluid, M2 is gross disease in the subarachnoid space of the cerebellum or cerebrum or in the lateral or third ventricles, M3 is gross disease in the subarachnoid space of the spine, and M4 is metastatic disease present outside the neuroaxis.
Chemotherapy was delivered according to a variety of protocols (Supplementary Material). All protocols but SJMB03 included patients less than 3 years of age and had a period of induction chemotherapy after surgery and before radiation.
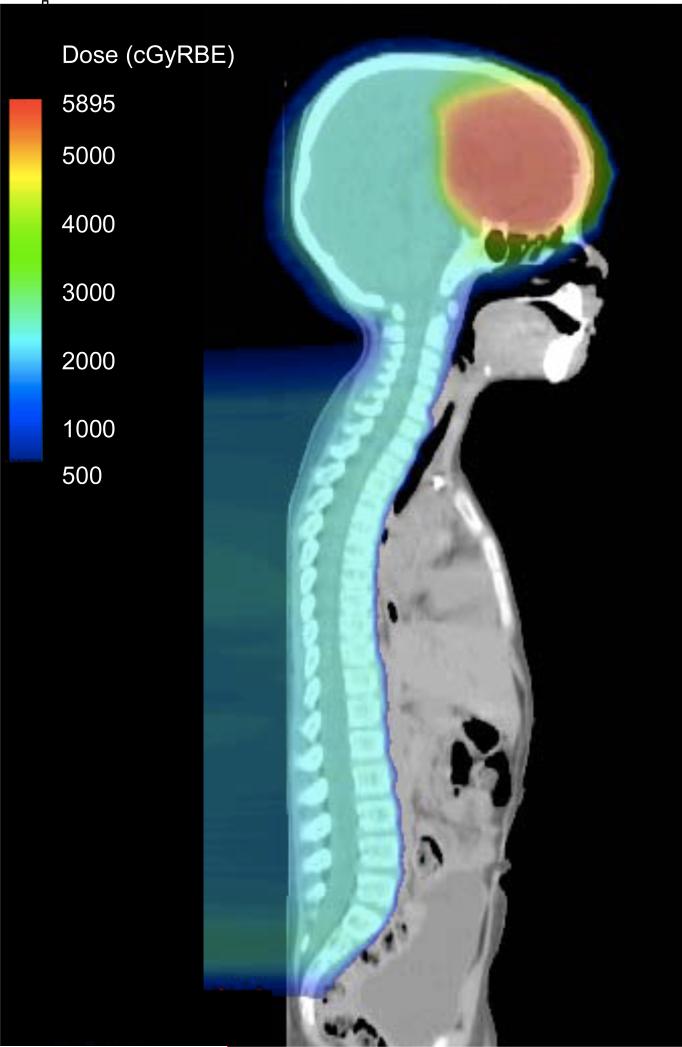
All patients were treated with passive scatter proton therapy. For craniospinal irradiation (CSI), the brain was treated with opposed oblique fields and the spine was treated with two posterior-anterior spinal fields [23]. Junctions between fields were 1 cm apart and were shifted every 4 – 5 fractions. Because of the young age of the patients in this study, the entire vertebral body was covered for all patients. Figure 1 shows a representative CSI plan.
Figure 1.
Sagittal view of craniospinal radiation plan for Patient #29. His primary tumor in the right frontal lobe was completely resected and he did not have metastatic disease. He was treated per SJMB 03 and received CSI to 23.4 GyRBE followed by a boost to the resection cavity to a total dose of 55.8 GyRBE. Dose is shown in colorwash in cGyRBE (centi-GyRBE).
CSI was followed by a focal boost to the tumor bed. Boost plans and focal radiation alone was delivered with passive scatter proton therapy using two or three beams. Gross tumor volume (GTV) included the surgical cavity and any gross residual disease identified from imaging or the neurosurgeon's assessment. Clinical tumor volume (CTV) was a 1 cm anatomically-constrained volumetric expansion of the GTV. To ensure coverage of the CTV, fields were designed to encompass the CTV plus uncertainty margins [23]. Distal and proximal margins included factors to account for uncertainties in the CT number and stopping power as well as a range uncertainty of 0.3 cm. The lateral margin incorporated internal motion, setup uncertainty, and penumbral width. When the brainstem was part of the treatment volume, no more than one beam was aimed directly at the brainstem and the distal margin of that beam was placed past the brainstem so that its end of range was outside the brainstem.
All patients required anesthesia for simulation and radiation treatment. All patients were treated with 1.8 GyRBE per fraction, except Patient 21 who received CSI at 1.6 GyRBE per fraction because she was treated according to the EU-RHAB protocol [24] (Supplementary Material). Because patients were treated according to several different protocols, a variety of total doses and volumes were used as detailed in Table 1. The protocols allowed for decreased radiation volumes or doses for patients less than 3 years of age. A relative biological effectiveness (RBE) of 1.1 was used in accordance with ICRU 78 [25].Acute radiation toxicities were scored according to the RTOG Acute Radiation Morbidity Scoring Criteria [26]. Neurologic toxicities in patients with imaging changes and/or clinical toxicity after radiation were scored according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
Table 1.
Patient characteristics, local therapy, and outcome
| Pt # | Age at diagnosis (m) | Gender | Protocol | Stage | Surgery | Age at RT (m) | Site of PD before RT | Primary site RT (GyRBE) | CSI (GyRBE) | All planned RT given | Site of PD after RT | Vital status | OS from diagnosis (m) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | F | DFCI02-294 | M1 | GTR | 6 | 9 | No | Dead | 3 | |||
| 2 | 6 | M | DFCI02-294 | M2 | GTR | 9 | 32.4 | No | brain | Dead | 5 | ||
| 3 | 7 | M | DFCI02-294 | M4* | STR | 10 | spine | 45 | Yes | Dead | 5 | ||
| 4 | 8 | F | ACNS0333 | M0 | Biopsy | 19 | brain | 21.6 | No | brain, spine | Dead | 14 | |
| 5 | 8 | M | ACNS0333 | M0 | GTR | 11 | 50.4 | Yes | spine | Alive | 27 | ||
| 6 | 8 | M | DFCI02-294 | M0 | STR | 12 | 54 | Yes | brain | Dead | 13 | ||
| 7 | 12 | F | ACNS0333 | M0 | STR | 15 | 50.4 | Yes | Alive | 24 | |||
| 8 | 13 | F | EU-RHAB | M0 | GTR | 20 | 52.2 | Yes | Alive | 28 | |||
| 9 | 14 | F | ACNS0333 | M3 | GTR | 20 | 45 | 23.4 | Yes | Alive | 17 | ||
| 10 | 15 | F | EU-RHAB | M0 | GTR | 21 | 54 | Yes | Alive | 7 | |||
| 11 | 16 | M | DFCI02-294 | M2 | STR | 31 | brain | 50.4 | 30.6 | Yes | Dead | 30 | |
| 12 | 16 | F | ACNS0333 | M0 | STR | 19 | 50.4 | Yes | Dead | 25 | |||
| 13 | 17 | F | ACNS0333 | M0 | GTR | 19 | 50.4 | Yes | Alive | 48 | |||
| 14 | 18 | M | DFCI02-294 | M0 | GTR | 20 | 54 | Yes | Alive | 48 | |||
| 15 | 18 | F | None | M2 | STR | 21 | 50.4 | Yes | brain | Dead | 5 | ||
| 16 | 19 | M | ACNS0333 | M0 | GTR | 21 | 50.4 | Yes | Alive | 37 | |||
| 17 | 20 | M | None | M3 | STR | 24 | 45 | Yes | CSF | Dead | 27 | ||
| 18 | 25 | F | DFCI02-294 | M0 | STR | 27 | brain | 52.2 | Yes | Alive | 16 | ||
| 19 | 25 | F | EU-RHAB | M2 | STR | 33 | 54 | Yes | Alive | 39 | |||
| 20 | 35 | M | SJMB03 | M3 | Biopsy | 36 | 54 | 36 | Yes | Alive | 11 | ||
| 21 | 35 | F | EU-RHAB | M3 | STR | 45 | 51 | 24 | Yes | Alive | 13 | ||
| 22 | 36 | F | ACNS0333 | M1 | GTR | 42 | 54 | 36 | Yes | Alive | 24 | ||
| 23 | 41 | F | SJMB03 | M0 | GTR | 44 | 54 | 23.4 | Yes | Dead | 21 | ||
| 24 | 41 | M | DFCI02-294 | M3 | STR | 44 | 54 | 36 | Yes | Alive | 53 | ||
| 25 | 43 | M | SJMB03 | M0 | GTR | 45 | 55.8 | 23.4 | Yes | Dead | 7 | ||
| 26 | 46 | F | None | M1 | Biopsy | 49 | 54 | 30.6 | Yes | brain, spine | Dead | 22 | |
| 27 | 48 | F | None | M0 | GTR | 49 | 54 | 30.6 | Yes | Alive | 41 | ||
| 28 | 51 | F | ACNS0333 | M3 | STR | 57 | brain, spine | 43.2 | 36 | No | Alive | 22 | |
| 29 | 55 | M | SJMB03 | M0 | GTR | 58 | 55.8 | 23.4 | Yes | brain | Dead | 35 | |
| 30 | 55 | M | ACNS0333 | M2 | STR | 62 | 54 | 23.4 | Yes | Alive | 40 | ||
| 31 | 55 | F | SJMB03 | M0 | GTR | 57 | 55.8 | 23.4 | Yes | Alive | 26 |
Patient had positive CSF, brain metastases, spine metastases, and kidney lesion.
Pt, Patient; m, months; RT, radiation therapy; PD, progressive disease; CSI, craniospinal irradiation; GyRBE, cobalt-Gray equivalent; F, female; M, male; DFCI, Dana-Farber Cancer Institute; GFR, gross total resection; STR, subtotal resection; RT, radiation therapy; OS, overall survival
Progression-free survival (PFS) from diagnosis was defined as time from diagnosis to progression or death from any cause. Overall survival (OS) from diagnosis was defined as time from diagnosis to death. PFS from radiation end was defined as time from the last day of radiation to progression or death from any cause. OS from radiation end was defined as time from the last day of radiation to death. For living patients without known progression of disease, PFS was censored at time of last follow-up. For patients not known to have died, OS was censored at time of last follow-up. Kaplan-Meier survival plots were calculated using S-PLUS© 8.0 for Windows (Copyright 1988, 2007 Insightful Corp., Seattle, Washington).
Results
Patient characteristics
The characteristics of 31 patients treated with proton radiation are shown in Table 1. Thirteen boys and 18 girls were treated, and the median age at diagnosis was 19 months (range, 4 – 55 months). Sixteen patients (52%) had disease confined to the primary site in the brain. Three had stage M1 disease, five had stage M2 disease, and six had stage M3 disease. One patient had synchronous disease in the kidney.
Complete resection of the primary disease was attempted when feasible (Table 1). Fifteen had gross total resection (48%), 13 had subtotal resection (42%), and three underwent biopsy alone (10%). Two patients (6%) underwent second look surgery prior to radiation.
Most patients were treated according to a clinical protocol, including DFCI 02-294 [4] (n = 8), ACNS0333 (n = 10), EU-RHAB [24] (n = 4), and SJMB03 (n = 5). Consequently, most patients (26/31, 84%) received chemotherapy prior to radiation and eleven patients received chemotherapy concurrently with radiation (35%). Seventeen patients were documented to have received chemotherapy after radiation.
Median time from surgery to radiation was three months (range, 1 – 15 months) and median age at radiation was 24 months (range, 6 – 62 months). Four patients did not complete the planned radiation course due to toxicity (n = 2) or disease progression during radiation (n = 2). Seventeen patients, all less than 36 months old at diagnosis, received local radiation with a median dose of 50.4 GyRBE (range, 9 – 54). Fourteen received CSI; half received 24 GyRBE or less and half received 30.6 GyRBE or higher. For patients treated with CSI, the median tumor bed dose was 54 GyRBE (range, 43.2 – 55.8).
All patients at least 36 months of age at diagnosis received CSI. Eleven of these 12 patients had either metastatic disease or M0 disease and were treated per SJMB03, which includes CSI for patients with M0 disease. Doses for these patients were guided by the various treatment protocols (Supplementary Material). Additionally, two patients less than 36 months of age were treated with CSI at the discretion of the referring and treating physicians for disseminated disease.
Outcomes
Median follow-up for all patients was 24 months from diagnosis (range, 3 to 53 months). Median follow-up for patients alive at last follow up (n = 18) was 26 months (range, 7 to 53 months).
Five patients (16%) had progressive disease between surgery and radiation. Sites and timing of progression are detailed in Table 1. Patient #3 had widely metastatic disease and received only focal radiation to the brain; he died one month after finishing radiation. Patient #4 progressed during radiation and did not complete the planned treatment; she continued to progress and died three months after radiation was stopped. The remaining three patients (#11, 18, and 28) were not known to have further progression during or after radiation. For these five patients, the median time between surgery and radiation was 192 days (range, 57 to 456 days) compared to 82.5 days (range, 29 to 307 days) for the 26 patients who did not progress prior to radiation (p = 0.10).
Eight patients had documented progression within the neuroaxis during or after radiation (Table 1), seven outside of the high radiation dose volume and one in the margin. Five patients developed progression during radiation or within two months of completion of radiation, and the remaining three recurred 12 or more months after the end of radiation. Three patients received additional chemotherapy and radiation, one received chemotherapy, one received cediranib on the PBTC 020 protocol, and two received supportive care. The treatment of one patient with a recurrence was unknown. Seven of eight patients with recurrences subsequently died.
Thirteen patients (42%) were known to have died (Table 1). For all patients, the median PFS from diagnosis was 20.8 months (Figure 2A). The 2-year PFS from diagnosis was 47.6% (95% CI, 32.2% - 70.5%). The median OS from diagnosis was 34.3 months (Figure 2B). The 2-year OS from diagnosis was 68.3% (95% CI, 52.9% - 88.1%).
Figure 2.
A) Progression-free survival from diagnosis, B) overall survival from diagnosis, C) progression-free survival from end of radiation and D) overall survival from end of radiation.
The 2-year PFS from the end of radiation was 45.9% (95% CI, 29.4% - 71.4%; Figure 2C). The 2-year OS from the end of radiation was 52.9% (95% CI, 36.0% - 77.8%; Figure 2D).
Toxicities
For the 27 patients who completed radiation, they generally tolerated radiation well. Most patients developed the expected grade 1 or 2 skin toxicities of erythema and alopecia. Grade 3 – 5 acute toxicities are detailed in Table 2.
Table 2.
Toxicities during radiation.
| Pt # | Primary site RT (GyRBE) | CSI (GyRBE) | Concurrent chemo | Toxicity during RT* |
|---|---|---|---|---|
| 1 | 9 | VCR, triple IT | Death due to sepsis (5) | |
| 2 | 32.4 | Etoposide | ||
| 3 | 45 | CYC, etoposide, VCR, triple IT | Neutropenia (3) | |
| 4 | 21.6 | |||
| 5 | 50.4 | |||
| 6 | 54 | CDDP, CYC, etoposide | ||
| 7 | 50.4 | |||
| 8 | 52.2 | |||
| 9 | 45 | 23.4 | ||
| 10 | 54 | Etoposide | ||
| 11 | 50.4 | 30.6 | Bevacizumab, irinotecan | Sepsis (4), emesis (3) |
| 12 | 50.4 | |||
| 13 | 50.4 | |||
| 14 | 54 | CDDP, CYC, etoposide | ||
| 15 | 50.4 | IT depocyt, carbo | ||
| 16 | 50.4 | |||
| 17 | 45 | Carbo, IT MTX | ||
| 18 | 52.2 | |||
| 19 | 54 | |||
| 20 | 54 | 36 | Neutropenia (3) | |
| 21 | 51 | 24 | Etoposide | |
| 22 | 54 | 36 | Pancytopenia (3) | |
| 23 | 54 | 23.4 | ||
| 24 | 54 | 36 | Pancytopenia (4) | |
| 25 | 55.8 | 23.4 | ||
| 26 | 54 | 30.6 | Triple IT, ICE | Pancytopenia (4) |
| 27 | 54 | 30.6 | ||
| 28 | 43.2 | 36 | Thrombocytopenia (4), hypertension (4) | |
| 29 | 55.8 | 23.4 | ||
| 30 | 54 | 23.4 | Vononistat, accutane | Anemia (3) |
| 31 | 55.8 | 23.4 |
Grade 3 – 5 per RTOG Acute Radiation Morbidity Scoring Criteria. Grade of each toxicity shown in parentheses.
Pt, Patient; RT, radiation therapy; GyRBE, cobalt-Gray equivalent; VCR, vincristine; CDDP, cisplatin; CYC, cyclophosphamide; MTX, methotrexate; ICE, ifosfamide, carbo, etoposide; IT, intrathecal; carbo, carboplatin
Two patients did not complete the planned radiotherapy course due to toxicity. Patient #1 was neutropenic during and after induction chemotherapy. She died after four fractions of radiation due to sepsis from a Pseudomonas diaper rash. Patient #28 was thrombocytopenic throughout induction chemotherapy and developed severe hypertension with an acute intracranial bleed during radiation. She subsequently recovered and was alive with no evidence of recurrence at last follow-up.
Within four months of completing radiation, five patients developed imaging and clinical findings in the brainstem and adjacent structures that were interpreted as radiation reaction or necrosis (Table 3). All were treated according to ACNS0333 and received cisplatin, etoposide, cyclophosphamide, methotrexate, and vincristine prior to radiation but no chemotherapy concurrently with radiation. Review of the radiation plans for these patients confirmed that the distal margins of the treatment beams were outside of the brainstem. Four of the five patients (#5, 7, 12, 13) received local radiation followed by consolidation high dose chemotherapy with carboplatin and thiotepa and developed radiation reaction during or within one month after consolidation chemotherapy. One of the five patients (#22) received consolidation chemotherapy with carboplatin and thiotepa prior to CSI and developed radiation reaction one month after CSI.
Table 3.
Patients with radiographic and clinical evidence of radiation reaction
| Pt # | Age at RT (m) | Surgery | Chemo before RT | Chemo with RT | Location of tumor bed | Location of imaging changes | Time to imaging changes after RT end (m) | Clinical signs concerning for RT reaction* | Brainstem dose (GyRBE) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Max | |||||||||
| 5 | 11 | GTR | Yes | No | PF | Pons, medulla | 4 | Ataxia (3), hypotonia (3) | 52.0 | 55.5 |
| 7 | 15 | STR | Yes | No | PF | Brainstem, bilateral thalami, cerebellum | 4 | Ataxia (2) | 47.2 | 53.2 |
| 12 | 19 | STR | Yes | No | Left hemisphere | Pons, midbrain, bilateral hemsipheres | 3 | Quadraplegia (3) bulbar palsies (4) | 28.7 | 52.8 |
| 13 | 19 | GTR | Yes | No | Foramen of Monroe | Basal ganglia, midbrain | 4 | Hemiparesis (3) bulbar palsies (4) | 20.1 | 51.3 |
| |22 | 42 | GTR | Yes | No | PF | Pons | 1 | Unknown | 48.5 | 56.4 |
Grade per CTCAE v4.0 shown in parentheses.
Pt, Patient; RT, radiation therapy; PF, posterior fossa; m, months; GTR, gross total resection; STR, subtotal resection
Patients #5, 7, 12, and 22 were treated with steroids, and Patient #13 received steroids and bevacizumab. All five patients subsequently improved over the following months, as illustrated in Figure 3 for Patient #7. Three of the patients (#5, 7, and 13) returned to their baseline with no or mild residual symptoms. Patient #12 improved but had moderate residual symptoms. Patient #22 did not have documented clinical changes but her imaging findings resolved over the following months. Patient #13 had recurrence of similar imaging and clinical findings four months later and again improved following bevacizumab.
Figure 3.
A) Radiation plan of Patient #7, with representative isodose lines. The prescription dose was 50.4 GyRBE, shown in blue. B) Axial T1 with contrast MRI, one month after completion of radiation. C) Axial T1 with contrast MRI, four months after completion of radiation, showing patchy enhancement in brainstem and cerebellum corresponding to her radiation field. D) Axial T1 with contrast MRI, 18 months after completion of radiation, with resolution of enhancement.
Discussion
Historically, practitioners have been hesitant to offer radiation to AT/RT patients less than three years of age because of the long-term impact on neurocognitive development and the risk of second malignancies. However, several recent analyses have suggested that radiation confers a meaningful improvement in survival in AT/RT [27-29]. The use of protons may lessen the long-term risks of radiation associated with this survival benefit. We found that proton-based radiation for AT/RT can be delivered with acceptable short-term toxicity.
For all patients on this study, the 2 year PFS was 47.6% and the 2 year OS was 68.3%. These outcomes are comparable to other modern series in this population. Chi, et al. reported a 2 year PFS of 53% and OS of 70% from DFCI 02-0294 [4]. In their registry of 42 AT/RT patients, Hilden, et al. reported a median survival of 16.75 months and a median event-free survival (EFS) of 10 months; for the 13 patients who received radiotherapy, the median survival and EFS were 48 months [27]. Review of the St. Jude's experience yielded a 2 year EFS and OS of 90% for 10 patients treated with both chemotherapy and radiation compared to a 1 year EFS of 0% and a 1 year OS of 42% for the 21 patients treated with chemotherapy [6].
Our study reflects the poor prognosis associated with progression. Of twelve patients with progression, nine subsequently died. This is similar to the results from DFCI 02-0294; of eight patients with progressive disease on that study, seven ultimately died [4].
Notably, patients with metastatic disease did not uniformly have the worst outcomes. Of 12 patients alive at least 24 months after diagnosis, eight had stage M0 disease and four had metastatic disease at presentation. This is consistent with DFCI 02-0294 [4], which found two patients with metastatic disease at presentation who survived at least 1.5 and 2.5 years.
For the patients who completed the planned course of radiation, the acute toxicities were manageable. This is consistent with other investigations into the use of proton radiation to treat AT/RT. de Amorim Bernstein, et al. reported on the successful treatment of ten AT/RT patients with protons at Massachusetts General Hospital [12]. At a median follow up of 27 months, no major radiation related toxicities were reported. Suneja et al. described the experience of treating 48 children with brain tumors, including three AT/RT patients, with proton radiation at the University of Pennsylvania [30]. These investigators found that acute toxicity was acceptable and manageable with supportive care.
Five patients developed clinical signs or imaging changes in the brainstem and adjacent structures 1 – 4 months after radiation that were interpreted as radiation reaction or necrosis. All patients had received chemotherapy per ACNS0333 prior to radiation, and all improved after treatment with steroids or bevacizumab. This pattern is consistent with findings by Sabin, et al., who described brain imaging changes in 8 of 17 pediatric patients who underwent proton radiation after chemotherapy [31]. Similar to the patients in our study, the patients were 3.5 years old or younger, and the median time to the development of imaging changes was 3.9 months after the end of proton therapy. This is earlier than would be expected for patients treated with photon therapy, for which imaging changes consistent with radiation reaction may occur at 6 – 10 months after completion of treatment [31-33]. Also similar to the findings our study, these changes occurred at doses that satisfy typical dose constraints for the brainstem [34]. The authors suggested that surgery and chemotherapy may decrease the tolerance of the brain to radiation. In particular, the brainstem and adjacent structures in very young children may be particularly sensitive to aggressive multimodality therapy. Timing and doses of chemotherapy, geometry of proton beams, or nuances of surgical technique may each contribute to this phenomenon, but a definitive cause has not yet been identified. Given the increasing use of proton therapy to treat pediatric brain tumors, this is an area of active investigation, and more results can be expected in the near term.
Appropriate volumes and radiation doses for the treatment of patients with AT/RT remain undefined. Of 21 patients treated without radiation at St. Jude's, 13 failed only locally and 6 had distant failure (3 distant only, 3 local and distant) [6]. The use of radiation is likely to alter this pattern; in our study, seven of eight recurrences were outside of the high dose radiation volume and one was on the margin of the field. These observations suggest that even if disease within the high dose volume is controlled, the remainder of the neuroaxis is at risk.
The question of local vs. craniospinal radiation is particularly relevant for patients older than 3 years of age with non-metastatic disease at presentation. All five patients in our study with stage M0 disease and at least 35 months of age at diagnosis received craniospinal radiation. Results from ACNS0333 and SJMB03 are awaited to clarify the appropriate radiation volumes for patients with non-metastatic disease
In conclusion, this study is the largest single institution report of using proton radiation to treat pediatric AT/RT of the CNS. Proton radiation for AT/RT is well-tolerated and can be delivered safely, even in very young children. Survival outcomes are encouraging compared to historical series, and ongoing prospective studies will further define the late effects of therapy for this aggressive disease.
Supplementary Material
Summary.
Proton therapy is increasingly used to treat pediatric patients with atypical teratoid/rhabdoid tumor (AT/RT) of the central nervous system, but the outcomes are poorly defined. Records of 31 patients with AT/RT treated with protons were retrospectively reviewed. Median overall survival was 34.3 months. Five patients (16%) developed brainstem changes consistent with radiation reaction. Aggressive multimodality therapy including protons may improve outcomes, but there is potential for radiation reaction, even when routine dose constraints are satisfied.
Acknowledgements
The authors thank Beth de Gracia for assistance with data collection.
This work was supported by the Cancer Center Support Grant (NCI Grant P30 CA016672). This work was presented in part at the 17th Annual Society for Neuro-Oncology Meeting in Washington, DC, November 15 – 18, 2012.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Burger PC, Yu IT, Tihan T, et al. Atypical teratoid/rhabdoid tumor of the central nervous system: A highly malignant tumor of infancy and childhood frequently mistaken for medulloblastoma: A pediatric oncology group study. The American journal of surgical pathology. 1998;22:1083–1092. doi: 10.1097/00000478-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Weiss E, Behring B, Behnke J, et al. Treatment of primary malignant rhabdoid tumor of the brain: Report of three cases and review of the literature. International journal of radiation oncology, biology, physics. 1998;41:1013–1019. doi: 10.1016/s0360-3016(98)00106-0. [DOI] [PubMed] [Google Scholar]
- 3.Gardner SL, Asgharzadeh S, Green A, et al. Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatric blood & cancer. 2008;51:235–240. doi: 10.1002/pbc.21578. [DOI] [PubMed] [Google Scholar]
- 4.Chi SN, Zimmerman MA, Yao X, et al. Intensive multimodality treatment for children with newly diagnosed cns atypical teratoid rhabdoid tumor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:385–389. doi: 10.1200/JCO.2008.18.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squire SE, Chan MD, Marcus KJ. Atypical teratoid/rhabdoid tumor: The controversy behind radiation therapy. Journal of neuro-oncology. 2007;81:97–111. doi: 10.1007/s11060-006-9196-z. [DOI] [PubMed] [Google Scholar]
- 6.Tekautz TM, Fuller CE, Blaney S, et al. Atypical teratoid/rhabdoid tumors (atrt): Improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:1491–1499. doi: 10.1200/JCO.2005.05.187. [DOI] [PubMed] [Google Scholar]
- 7.Packer RJ, Biegel JA, Blaney S, et al. Atypical teratoid/rhabdoid tumor of the central nervous system: Report on workshop. Journal of pediatric hematology/oncology. 2002;24:337–342. doi: 10.1097/00043426-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Howell RM, Giebeler A, Koontz-Raisig W, et al. Comparison of therapeutic dosimetric data from passively scattered proton and photon craniospinal irradiations for medulloblastoma. Radiation oncology. 2012;7:116. doi: 10.1186/1748-717X-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar RJ, Zhai H, Both S, et al. Breast cancer screening for childhood cancer survivors after craniospinal irradiation with protons versus x-rays: A dosimetric analysis and review of the literature. Journal of pediatric hematology/oncology. 2013;35:462–467. doi: 10.1097/MPH.0b013e31829bcdf8. [DOI] [PubMed] [Google Scholar]
- 10.Yoon M, Shin DH, Kim J, et al. Craniospinal irradiation techniques: A dosimetric comparison of proton beams with standard and advanced photon radiotherapy. International journal of radiation oncology, biology, physics. 2011;81:637–646. doi: 10.1016/j.ijrobp.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Boehling NS, Grosshans DR, Bluett JB, et al. Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. International journal of radiation oncology, biology, physics. 2012;82:643–652. doi: 10.1016/j.ijrobp.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 12.De Amorim Bernstein K, Sethi R, Trofimov A, et al. Early clinical outcomes using proton radiation for children with central nervous system atypical teratoid rhabdoid tumors. International journal of radiation oncology, biology, physics. 2013;86:114–120. doi: 10.1016/j.ijrobp.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Hattangadi JA, Rombi B, Yock TI, et al. Proton radiotherapy for high-risk pediatric neuroblastoma: Early outcomes and dose comparison. International journal of radiation oncology, biology, physics. 2012;83:1015–1022. doi: 10.1016/j.ijrobp.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald SM, Trofimov A, Safai S, et al. Proton radiotherapy for pediatric central nervous system germ cell tumors: Early clinical outcomes. International journal of radiation oncology, biology, physics. 2011;79:121–129. doi: 10.1016/j.ijrobp.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 15.Merchant TE, Hua CH, Shukla H, et al. Proton versus photon radiotherapy for common pediatric brain tumors: Comparison of models of dose characteristics and their relationship to cognitive function. Pediatric blood & cancer. 2008;51:110–117. doi: 10.1002/pbc.21530. [DOI] [PubMed] [Google Scholar]
- 16.Song S, Park HJ, Yoon JH, et al. Proton beam therapy reduces the incidence of acute haematological and gastrointestinal toxicities associated with craniospinal irradiation in pediatric brain tumors. Acta oncologica. 2014:1–7. doi: 10.3109/0284186X.2014.887225. [DOI] [PubMed] [Google Scholar]
- 17.Greenberger BA, Pulsifer MB, Ebb DH, et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. International journal of radiation oncology, biology, physics. 2014;89:1060–1068. doi: 10.1016/j.ijrobp.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 18.Sethi RV, Shih HA, Yeap BY, et al. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer. 2014;120:126–133. doi: 10.1002/cncr.28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merchant TE, Conklin HM, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. The lancet oncology. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 21.Yock TI, Caruso PA. Risk of second cancers after photon and proton radiotherapy: A review of the data. Health physics. 2012;103:577–585. doi: 10.1097/HP.0b013e3182609ba4. [DOI] [PubMed] [Google Scholar]
- 22.Chang CH, Housepian EM, Herbert C., Jr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93:1351–1359. doi: 10.1148/93.6.1351. [DOI] [PubMed] [Google Scholar]
- 23.Giebeler A, Newhauser WD, Amos RA, et al. Standardized treatment planning methodology for passively scattered proton craniospinal irradiation. Radiat Oncol. 2013;8:32. doi: 10.1186/1748-717X-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benesch M, Bartelheim K, Fleischhack G, et al. High-dose chemotherapy (hdct) with auto-sct in children with atypical teratoid/rhabdoid tumors (at/rt): A report from the european rhabdoid registry (eu-rhab). Bone marrow transplantation. 2014;49:370–375. doi: 10.1038/bmt.2013.208. [DOI] [PubMed] [Google Scholar]
- 25.Icru 78 . Prescribing, recording, and reporting proton-beam therapy. International Commission on Radiation Units and Measurements, Inc.; Bethesda, MD: 2007. [Google Scholar]
- 26.RTOG Acute radiation morbidity scoring criteria. 2014 [Google Scholar]
- 27.Hilden JM, Meerbaum S, Burger P, et al. Central nervous system atypical teratoid/rhabdoid tumor: Results of therapy in children enrolled in a registry. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:2877–2884. doi: 10.1200/JCO.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 28.Athale UH, Duckworth J, Odame I, et al. Childhood atypical teratoid rhabdoid tumor of the central nervous system: A meta-analysis of observational studies. Journal of pediatric hematology/oncology. 2009;31:651–663. doi: 10.1097/MPH.0b013e3181b258a9. [DOI] [PubMed] [Google Scholar]
- 29.Chen YW, Wong TT, Ho DM, et al. Impact of radiotherapy for pediatric cns atypical teratoid/rhabdoid tumor (single institute experience). International journal of radiation oncology, biology, physics. 2006;64:1038–1043. doi: 10.1016/j.ijrobp.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Suneja G, Poorvu PD, Hill-Kayser C, et al. Acute toxicity of proton beam radiation for pediatric central nervous system malignancies. Pediatric blood & cancer. 2013;60:1431–1436. doi: 10.1002/pbc.24554. [DOI] [PubMed] [Google Scholar]
- 31.Sabin ND, Merchant TE, Harreld JH, et al. Imaging changes in very young children with brain tumors treated with proton therapy and chemotherapy. AJNR American journal of neuroradiology. 2013;34:446–450. doi: 10.3174/ajnr.A3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fouladi M, Chintagumpala M, Laningham FH, et al. White matter lesions detected by magnetic resonance imaging after radiotherapy and high-dose chemotherapy in children with medulloblastoma or primitive neuroectodermal tumor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:4551–4560. doi: 10.1200/JCO.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 33.Helton KJ, Edwards M, Steen RG, et al. Neuroimaging-detected late transient treatment-induced lesions in pediatric patients with brain tumors. Journal of neurosurgery. 2005;102:179–186. doi: 10.3171/jns.2005.102.2.0179. [DOI] [PubMed] [Google Scholar]
- 34.Mayo C, Yorke E, Merchant TE. Radiation associated brainstem injury. International journal of radiation oncology, biology, physics. 2010;76:S36–41. doi: 10.1016/j.ijrobp.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.