Abstract
Conventionally developed antiseizure drugs fail to control epileptic seizures in about 30% of patients, and no treatment prevents epilepsy. New etiologically realistic, syndrome-specific epilepsy models are expected to identify better treatments by capturing currently unknown ictogenic and epileptogenic mechanisms that operate in the corresponding patient populations. Additionally, the use of electrocorticography permits better monitoring of epileptogenesis and the full spectrum of acquired seizures, including focal nonconvulsive seizures that are typically difficult to treat in humans. Thus, the combined use of etiologically realistic models and electrocorticography may improve our understanding of the genesis and progression of epilepsy, and facilitate discovery and translation of novel treatments. However, this approach is labor intensive and must be optimized. To this end, we used an etiologically realistic rat model of posttraumatic epilepsy, in which the initiating fluid percussion injury closely replicates contusive closed-head injury in humans, and has been adapted to maximize epileptogenesis and focal non-convulsive seizures. We obtained week-long 5-electrode electrocorticography 1 month post-injury, and used a Monte-Carlo-based non-parametric bootstrap strategy to test the impact of electrode montage design, duration-based seizure definitions, group size and duration of recordings on the assessment of posttraumatic epilepsy, and on statistical power to detect antiseizure and antiepileptogenic treatment effects. We found that use of seizure definition based on clinical criteria rather than event duration, and of recording montages closely sampling the activity of epileptic foci, maximize the power to detect treatment effects. Detection of treatment effects was marginally improved by prolonged recording, and 24 h recording epochs were sufficient to provide 80% power to detect clinically interesting seizure control or prevention of seizures with small groups of animals. We conclude that appropriate electrode montage and clinically relevant seizure definition permit convenient deployment of fluid percussion injury and electrocorticography for epilepsy therapy development.
Keywords: Drug development, Preclinical drug screening, Focal epilepsy, Non-convulsive seizure, Pharmacoresistance, Statistical power analysis
Introduction
Epileptic seizures can be controlled in a majority of patients by chronic administration of one or more of over two dozen anti-seizure drugs (ASD) now on the market (Löscher et al., 2013). However, no non-surgical treatment has ever been found to cure epilepsy, and no treatment has been found to prevent it, or modify its course in those at risk. Over one third of epilepsy patients suffer inadequate seizure control — a proportion that has not changed appreciably despite the introduction of numerous ASDs over the past three decades (Löscher and Schmidt, 2011; Temkin, 2009).
Most of the treatments available today were identified on the basis of their ability to acutely suppress behavioral endpoints of seizure activity evoked by various forms of electrical or chemical stimulation (Löscher et al., 2013; Löscher and Schmidt, 2011, 2012; Smith et al., 2007; White, 1998) and are all thought to act on various neuronal and synaptic mechanisms to nudge the balance between neural inhibition and excitation, thereby preventing the spontaneous abnormal hypersynchronous neuronal activity with associated behavioral changes that define epileptic seizures (Fisher et al., 2005). However, current ASDs share critical shortcomings. First, they do not prevent epilepsy, but control seizures to provide symptomatic relief. While several clinical trials have been conducted to test the antiepileptogenic effect of ASDs based on the hypothesis that “seizures beget seizures” and, thus, that ASDs should modify epileptogenesis, these trials have all failed to identify an effective treatment (Temkin, 2009). Second, these drugs do not cure epilepsy, and must be taken chronically to control sporadic seizures. The resulting global damping of neuronal excitability often produces chronic aversive or debilitating side effects (Gilliam et al., 2004). Third, the expanding ASD pharmacopeia remains incapable of controlling seizures in over a third of epilepsy patients, the vast majority of whom predominantly suffers from non-convulsive focal seizures (Juul-Jensen, 1986; Mattson et al., 1996; Semah et al., 1998). This situation is arguably attributable to drug development strategies that fail to incorporate either the epileptogenic mechanisms that mediate the development of acquired human epilepsies, or the ictogenic mechanisms that cause the epileptic focus to suddenly precipitate spontaneous seizures in patients. Since, with few specific exceptions, the mechanisms of human ictogenesis and epileptogenesis are not yet known, the problem of identifying better epilepsy treatments can be distilled to one of identifying and deploying animal models that prominently incorporate these yet-to-be-identified mechanisms.
To this end, new syndrome-specific models have been developed based on etiologically realistic epileptogenic insults such as viral encephalitis, stroke, febrile seizures, perinatal hypoxia and head trauma (D’Ambrosio et al., 2004; Dubé et al., 2010; Kelly et al., 2001; Rakhade et al., 2011; Williams and Dudek, 2007). These models have the advantages that: 1) they feature chronic spontaneous recurrent seizures (CSRSs), which are the hallmark of epilepsy, 2) they include focal non-convulsive CSRSs, which are known to be difficult to treat with current ASDs, 3) the realism of the initiating injury ensures that mechanisms that operate in the corresponding patient populations are recruited, and 4) the patient populations best suited for clinical tests of treatments discovered using these models are readily identifiable. However, in contrast to evoked motor seizures, which appear predictably and can be reliably and conveniently assessed in a short period of time by simple behavioral observation, spontaneous nonconvulsive seizures generally require prolonged monitoring because they are unpredictable, and also require expert ECoG analysis for accurate detection and evaluation. To further complicate matters, high inter-subject variability in seizure frequency is a consistent feature of all acquired CSRSs models developed to date, which tends to result in larger group sizes and increased labor and cost.
Because the combination of sensitive ECoG monitoring and of etiologically realistic models represents a promising strategy for discovery of novel epilepsy treatments that could translate to readily identifiable patient populations, there is an urgent need to determine the most effective ways to deploy them. Investigators have taken a variety of approaches to the ECoG monitoring and reporting of seizure data in CSRS models. Electrical activity may be recorded with two or more electrodes on the surface of the brain and/or from deep brain structures. Recording montages are most often designed without knowledge of the location(s) of epileptic foci, which are poorly documented in most CSRS models, and investigators often report only seizures that exceed various duration based criteria or that result in Racine scale 4–5 behavioral seizures. These practices are relatively convenient and economical, but bias observation in favor of tonic–clonic and other generalized seizures at the expense of focal non-convulsive seizures (D’Ambrosio and Miller, 2010). In addition, animals may be recorded either continuously or periodically for durations ranging from hours to months, based on each lab’s experience with what might be adequate for the task at hand. Such differences in experimental approach seriously complicate comparison of studies from different laboratories and, critically for therapy development, affect the quality of the assessment of both epileptic syndromes and their treatments. Yet, the impact of these procedural variations on therapy development has never before been systematically investigated.
Here we examine these different experimental approaches in a rostral parasagittal fluid percussion injury (rpFPI) model of posttraumatic epilepsy (PTE). This is an etiologically realistic syndrome specific PTE model based on an experimental brain insult, FPI, that has long been regarded as a realistic model of contusive closed head injury in man (Thompson et al., 2005). We have previously optimized this model with respect to the location and severity of injury to produce rapid and reliable epileptogenesis with low acute mortality (Curia et al., 2011, in press; D’Ambrosio et al., 2009), and have identified the location of the early epileptic focus that develops after injury. This has been mapped with grid and depth electrode recordings (D’Ambrosio et al., 2005, 2009), as well as by focal cooling of the perilesional neocortex (D’Ambrosio et al., 2013). The localization of the neocortical epileptic focus permits use of a recording montage that closely monitors its activity. Detailed characterization of the electrobehavioral seizures generated by this neocortical epileptic focus has permitted the use of realistic seizure detection criteria that are based on clinical practice and are consistent with the ILAE definition of epileptic seizure (D’Ambrosio and Miller, 2010). We also have established statistical methods to efficiently detect effects of investigational ASDs in the presence of non-responders (Eastman et al., 2010, 2011). These studies were based on periodic recordings (24–72 h/week) as a compromise to keep costs down while still sampling much of the variability in seizure frequency. We now use seizure data from rpFPI rats recorded continuously for a full week 1 month after injury, in bootstrapped nonparametric statistical power analyses to estimate a cost-effective duration of recording, as well as the effects of different recording montages and seizure definitions on statistical power to detect antiepileptogenic or disease modifying (AEG/DM) or anti-seizure (AS) effects. The results show how to optimize the deployment of rpFPI for epilepsy therapy development.
Methods
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All experiments were approved by the University of Washington Institutional Animal Care and Use Committee (Animal Welfare Assurance #A3464-0 l). All surgery was performed under halothane anesthesia, and all efforts were made to minimize suffering.
Animals
Outbred male Sprague-Dawley rats (Charles River, Hollister, CA) were administered head injury at 32–36 days of age. Rats were housed 2 to 3 per cage prior to epidural electrode implantation, and individually afterward. Animals were kept in a specific-pathogen-free facility with controlled light (12 h light-dark cycle), temperature and humidity, and ad libitum access to food and water.
Surgical procedures
rpFPI and epidural electrode implantation were performed as detailed previously (D’Ambrosio et al., 2013; Eastman et al., 2010). For rpFPI, 17 animals were anesthetized (4% halothane), intubated and mechanically ventilated (1–1.5% halothane, 30% O2 and air). Rectal temperature was monitored and maintained at 37 °C with a heat pad. A 3 mm burr hole was drilled centered at 2 mm posterior to bregma and 3 mm from the midline over the right convexity. Animals were disconnected from the ventilator and a pressure pulse (8 ms, 3.5 atm) was delivered with the FPI device (Scientific Instruments, University of Washington) and measured by a transducer (Entran EPN-D33-100P-IX, Measurement Specialties, Hampton, VA). Mechanical ventilation was resumed 10 s after injury to standardize posttraumatic apnea and hypoxia, and terminated when spontaneous breathing resumed. All animals survived the injury.
Epidural electrodes were implanted 14–15 days after injury. Briefly, 1 mm diameter stainless-steel screw electrodes were implanted through 0.75 mm diameter guiding craniotomies. The full ECoG montage consisted of five epidural electrodes (Fig. 1A): a reference electrode placed midline in the frontal bone and two electrodes per parietal bone, placed at coordinates bregma 0 mm and −6.5 mm, 4.5–5 mm from the midline. Three anchoring screws (one frontal and two occipital) were implanted to help secure the headset. All electrodes were connected through insulated wire to gold-plated pins in a plastic pedestal (PlasticsOne inc., Roanoke, VA). Parts of the craniotomy not covered with thick connective tissue were covered with biocompatible silicone (Kwik-Cast., WPI, Sarasota, FL). The entire assembly was then cemented onto the skull with measured amount of dental acrylic (Jet, Lang Dental Manufacturing Co., Wheeling, IL) to standardize the headsets, and further secured with VetBond (World Precision Instruments, Sarasota, FL) adhesive. Because unintended damage to the neocortex induces focal astroglial reactivity and is often associated with focally abnormal ECoG (D’Ambrosio et al., 2009), our surgical procedures incorporate routine precautions to minimize thermal and mechanical damage. During drilling, the skull and drill bit are cooled with room-temperature sterile saline to minimize frictional heating, and particular care is taken to avoid deforming or tearing the dura. In addition, the depth of anchoring screws and screw electrodes is carefully controlled to avoid brain compression. During the exothermic phase of the curing process, the acrylic headset is cooled with compressed air to prevent overheating of the brain.
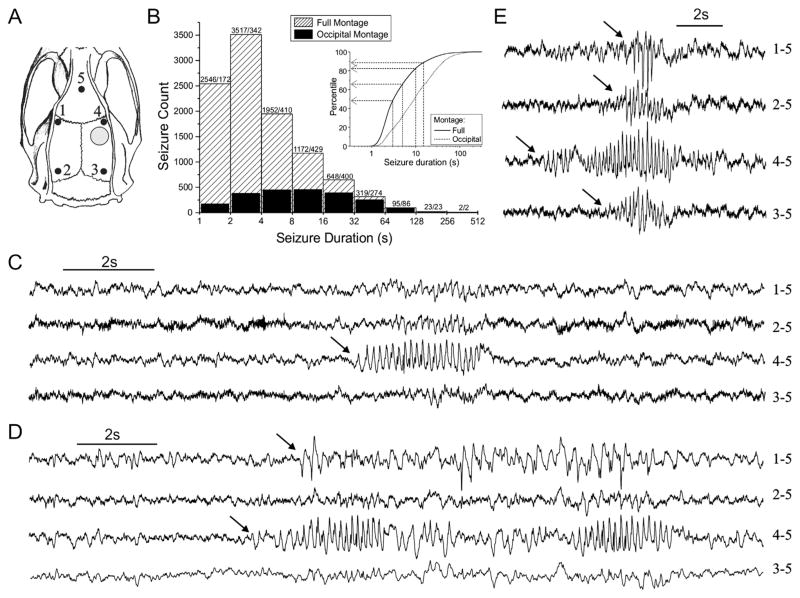
Fig. 1.
Distribution of seizure durations as observed by continuous ECoG with the full or occipital montages. A) Spontaneous seizures were observed with a week of continuous epidural ECoG recording 1 month post-injury either by the full montage (electrodes 1–5), or by the occipital montage (electrodes 2, 3, and 5). Black dots indicate the location of the numbered epidural electrodes in the rat skull. Electrode 5 is the reference in both montages. Gray circle indicates the rostral parasagittal site of FPI. B) Histogram shows counts of seizures in logarithmically scaled (i.e. unequal width) bins. Numbers above the columns indicate count for full and occipital montages, respectively. The occipital montage fails to detect a large number of shorter focal seizures that are detected by the full montage. Empirical cumulative frequency distributions are shown in the inset. Arrows indicate the proportion of seizures that are excluded under the indicated duration-based seizure definitions. C–E) Focal seizures observed 1 month postinjury include both perilesional cortical seizures (C) and spreading seizures (D and E). Note that the focal perilesional type (C) and the focal seizures spreading frontally (D) can be detected only with the full montage, while the seizures spreading to the occipital cortex (E) are ultimately detected also by the occipital montage.
Video/ECoG monitoring and seizure identification
The ECoG was acquired continuously for 1 week with the full 5-electrode montage, in seven sequential one-day epochs from the 22nd to the 28th day after rpFPI. Animals were tethered to the amplifier headstage. Brain electrical activity was amplified (×5000) and filtered (0.3 Hz high-pass, 300 Hz low-pass) using a Neurodata 12 or a M15 amplifier (Grass Instruments, Quincy, MA), acquired at 600 Hz per channel on computers equipped with SciWorks 4.1 or Experimenter V3 software (Datawave Technologies Inc., Longmont, CO), and DT3010 acquisition boards (DataTranslation Inc., Marlboro, MA). Videos were recorded in VHS using digital cameras connected to VCRs. Each camera monitored a maximum of two cages (1 animal per cage).
Data analysis was conducted blind to subject and day postinjury. ECoG was visualized in Matlab (MathWorks Inc., Natick, MA) and manually scrolled offline. This approach is laborious and requires expert raters, but it permits reliable analysis of all seizure activity generated by the epileptic focus and it is the approach used to evaluate human ECoG data at the UW Regional Epilepsy Center. Seizures were characterized by 1) focal trains of ~150 ms long spikes lasting at least 1 s that are clearly distinct from baseline, 2) a sudden increase in spectral power in the theta band over the baseline (Butler et al., 2013; D’Ambrosio et al., 2004; Ikeda et al., 2009), and 3) simultaneous stereotyped ictal behavioral changes according to a behavioral scale previously described (D’Ambrosio et al., 2009) and according to the clinical practice of seeking evidence of abnormal neuronal activity paired to behavioral signs (D’Ambrosio et al., 2009; D’Ambrosio and Miller, 2010; Fisher et al., 2005). Trains of spikes shorter than 1 s were considered likely interictal (D’Ambrosio et al., 2009), and were excluded from study. Identified seizures ranged from 1 s to over 5 min (Table 1). Events occurring within 5 s of each other were defined as a single seizure. In accord with previous work (D’Ambrosio et al., 2005; Pearce et al., 2014; Willoughby and Mackenzie, 1992), age-dependent idiopathic seizures, were not seen in this study because that seizure type typically appears after 5–6 months of age in this rat strain and gender. The following data were extracted for each seizure: 1) onset time, 2) duration, and 3) ECoG channel(s) at which the event was detected.
Table 1.
Effect of recording montage and seizure definition on experimental epilepsy outcome measures.
| Duration-based seizure
definition |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Full montage |
Occipital montage |
||||||||||
| ≤1 s | >3 s | >5 s | >10 s | >15 s | >1 s | >3 s | >5 s | >10 s | >15 s | ||
| Rats | |||||||||||
| Mean frequency (seizures/h) | 3.7 | 1.9 | 1.3 | 0.6 | 0.4 | 0.8 | 0.7 | 0.6 | 0.4 | 0.3 | |
| Range (seizures/h) | Low | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| High | 17.4 | 10.9 | 7.6 | 4.4 | 2.8 | 4.3 | 3.9 | 3.4 | 2.5 | 1.9 | |
| Time seizing (s/h) | 28.1 | 24.5 | 22.1 | 17.7 | 15.0 | 14.9 | 14.6 | 14.1 | 12.9 | 11.7 | |
| Range (s/h) | Low | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| High | 180.3 | 167.3 | 154.8 | 132.1 | 111.9 | 103.4 | 103.0 | 100.9 | 94.4 | 86.7 | |
| Incidence | 94% | 94% | 88% | 88% | 88% | 88% | 82% | 76% | 71% | 71% | |
| Epileptic seizures | |||||||||||
| Seizure count | 10,274 | 5296 | 3517 | 1783 | 1148 | 2138 | 1765 | 1488 | 1069 | 815 | |
| Mean duration (s) | 7.6 | 12.9 | 17.5 | 27.7 | 36.2 | 19.3 | 22.9 | 26.4 | 33.9 | 40.7 | |
| Duration range (s) | Low | 1.0 | 3.0 | 5.0 | 10.0 | 15.0 | 1.1 | 3.0 | 5.0 | 10.0 | 15.0 |
| High | 318.3 | 318.3 | 318.3 | 318.3 | 318.3 | 318.3 | 318.3 | 318.3 | 318.3 | 318.3 | |
Summary statistics are shown for the 17 rats used and the 10,274 seizures observed. Time seizing, and seizure frequency, duration and incidence are computed on the basis of the indicated subsets of the data obtained from week-long continuous video-ECoG recordings from rpFPI rats.
Power analyses
The ECoG data acquired from week-long continuous recordings of 17 rats were used in Monte-Carlo simulations to conduct bootstrapped non-parametric statistical power analyses. These simulations were similar in principle to those we reported previously (Eastman et al., 2010, 2011), but were designed to assess the effects of different experimental variables (Table 2), such as seizure definitions, recording montages, and duration of recordings on the statistical power to detect the treatment effects. The seizure activity of each rat was represented as a list of all the seizure onset times observed during the week of continuous monitoring. These lists were aggregated in a database, which was indexed by subject, that contained the onset times of all seizures longer than 1 s that were detected using the full 5-electrode recording montage. This database was filtered to produce subset databases representing the seizures that would have been detected using the occipital montage and/or other seizure definitions based on duration. Thus, a database filtered to remove seizures that were shorter than 5 s and/or were not detected by at least one of electrodes 2 and 3 (Fig. 1A) represented the seizures that would have been detected in these animals using the occipital montage and defining seizures to last at least 5 s. A total of 10 databases were used to examine 2 recording montages and 5 duration based seizure definitions. Recordings of different lengths were simulated by changing the length of the interval in which onsets were counted, and measurement-to-measurement variation was modeled by choosing intervals of the specified length at random, with replacement, within the week-long records. The frequency of seizure assigned to each subject in a simulated experiment was determined by counting seizure onsets within a specified time interval and dividing by the length of the interval. Thus, seizure frequencies were simulated based on the actual temporal patterns of seizure onsets recorded from rpFPI rats. Ten thousand experiments were simulated for each set of variables investigated, and the statistical power to detect a specified treatment effect was determined as the fraction of experiments in which significant differences in seizure frequency between the control and treated groups were detected using the statistical test appropriate for the different types of experiment: seizure control and seizure prevention.
Table 2.
Variables investigated by bootstrap power analyses.
|
Seizure-control experiments (Fig. 7) are conducted to determine whether a treatment has an AS effect, i.e. suppresses seizures once the epileptic focus has developed. Such experiments are best conducted with a repeated measures design (Eastman et al., 2010), in which each subject serves as its own control, to minimize the impact of subject-to-subject variability. Rats are recorded at baseline and epileptic rats (defined as rats exhibiting seizures during the baseline observation period) are administered treatment. They are recorded again after a specified period on treatment, and seizure frequencies at baseline and “on-treatment” are compared to assess the effect of treatment. For each simulated seizure-control experiment a group of subjects was chosen randomly, with replacement, from a database. Baseline seizure frequencies for each subject were determined as described above, and any subjects without seizures at baseline were counted and replaced with another random selection from the database. A specified treatment effect was simulated by tagging each seizure within the selected time window with a uniform random real number (0–100) and eliminating seizures tagged with random numbers less than the target percent drug effect. Treated seizure frequencies were determined based on a count of seizure onsets remaining within the observation interval. We have previously shown that seizure frequency has a log-normal distribution in rpFPI rats 1 month after injury, and that the seizure frequency data acquired in seizure control experiments are best analyzed after logarithmic transformation (Log10), using a paired t-test (Eastman et al., 2010). Thus, that strategy was adopted for the simulated seizure control experiment, and results are reported in terms of the effective group size, that is the number of animals that must actually be injured and recorded (at least once), required to provide 80% power to detect the indicated effects.
In seizure prevention experiments (Fig. 6), seizure frequencies are compared in independent groups of untreated and treated subjects to assess whether a treatment administered after an epileptogenic insult prevents or diminishes the later development of CSRSs. For each simulated seizure prevention experiment, independent groups of control and treatment subjects were chosen randomly, with replacement, from a database. Control seizure frequencies were determined as described above for baseline seizure frequency in seizure-control experiments, except that control subjects without seizures were not replaced. A statistical comparison of control and treatment groups was performed after each simulated experiment. Reductions in seizure frequency were assessed using a one-tailed Mann–Whitney U test (α = 0.05) with tie correction (Siegel, 1956). All power analyses were performed using the Statistics101 resampling statistics program (http://www.statistics101.net/). Other statistical procedures, outside the power analyses, were conducted using SPSS v17 (IBM inc., NY).
Results
Data for power analyses were acquired from seventeen rats that were each recorded continuously for 1 week 1 month after rpFPI. Seizures were assessed using either the full montage, with one rostral electrode that monitors the vicinity of the perilesional epileptic focus, or the occipital montage (Fig. 1A), and data were analyzed using several common duration-based seizure definitions. The full montage detected 10,274 seizures ranging from 1 s to over 5 min in duration. The occipital montage detected just 2138 seizures, ranging from 1.1 s to over 5 min in duration (Table 1), which consisted primarily of spreading seizures that had propagated to electrodes 2–3 from the perilesional electrode (Fig. 1E), while it missed all the focal perilesional seizures (Fig. 1C) and the focal seizures spreading contralaterally but not caudally (Fig. 1D). Although the ranges of seizure durations detected by the full and the occipital montages were nearly identical, the latter can be seen to systematically exclude seizures in inverse proportion to their duration (Fig. 1B). Therefore, the mean duration of seizures detected by the occipital electrodes (19.3 s; range: 1.1 s–318.3 s) was longer than that of those detected by the full montage (7.6 s; range: 1.0 s–318.3 s). Arbitrarily defining seizures as events lasting longer than 3–15 s resulted in greater exclusion of seizures with the full montage. The exclusion of seizures, either by definition or by reliance on the occipital electrodes alone, caused the apparent incidence of epilepsy to vary from 94% when all seizures detected by the full montage were considered to 71% when seizures longer than 15 s were assessed with the occipital montage, and the mean frequency of seizures to vary from 3.7 seizures/h to 0.3 seizures/h, respectively (Table 1).
Because the perilesional neocortical epileptic focus generates seizures of a wide range of durations, we assessed the relationship between long seizures and the short seizures that are systematically excluded by suboptimal ECoG montage or arbitrary duration-based definitions.
To this end, we examined the frequency of short and long seizures in the 17 week-long recordings, and found them to be highly significantly correlated regardless of the definition of long and short. For example, the correlation was highly significant when short or long seizures were defined either as ≤ 5 s and > 5 s (r = 0.94, p < 10−5; Pearson) or, more restrictively, as ≤3 s and >10 s (r = 0.88, p < 10−5; Pearson). The correlation is evident also when examining the frequency of short and long seizures in the 7 non-overlapping 24 h epochs in each of 17 rats (119 epochs total; Fig. 2). Regardless of the definition, long seizures were not observed in the absence of short seizures in any 24 h observation period.
Fig. 2.
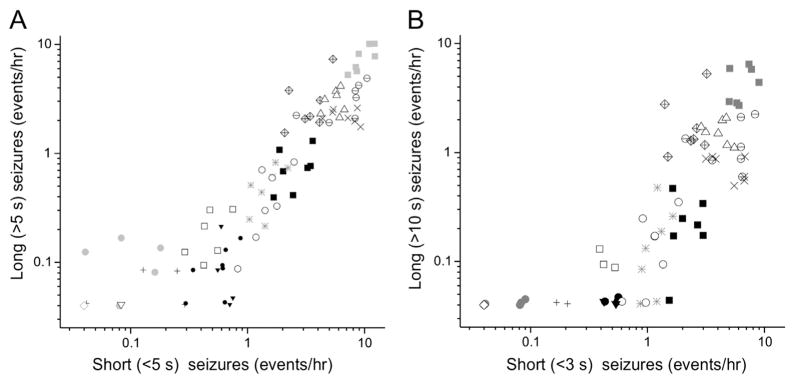
The frequencies of short and long seizures are highly correlated. The frequencies of long seizures are plotted as a function of the frequencies of short seizures, as observed with the full montage in 7 consecutive non-overlapping 24 h recordings from 16 epileptic rats. Individual rats are indicated by different plotting symbols (up to 7 points per rat). A) Short and long seizures were defined as lasting up to 5 s and longer than 5 s, respectively. The correlation between their frequencies (r = 0.94, n = 17; p < 10−5; Pearson). B) Short and long seizures were defined as lasting up to 3 s and longer than 10 s, respectively. The correlation between their frequencies remains highly significant (r = 0.88, p < 10−5; Pearson).
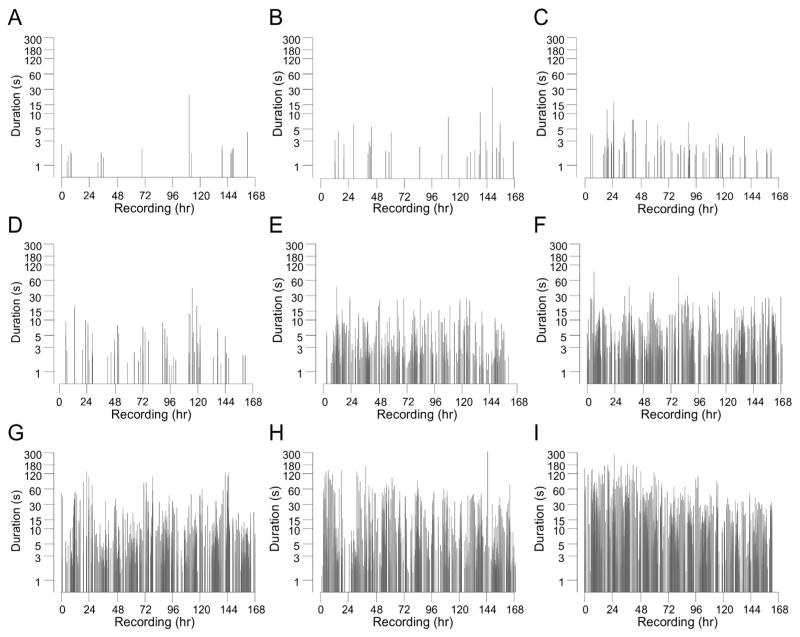
A representative sample of the temporal patterns of ictal activity observed in rpFPI animals during continuous ECoG recordings with the full montage is shown in Fig. 3. Records from animals with lower seizure frequency (Figs. 3A–D) display some long (12–40 h) interseizure intervals, but seizure onsets in Figs. 3B–C appear to be approximately randomly dispersed. In contrast, circadian patterns of seizure clustering are most evident in Figs. 3A and D. While some prolonged (several hours) seizure-free intervals are evident even in rats exhibiting seizure frequencies of 3–17 seizures/h (Figs. 3E–I), these display no striking temporal patterns. The seizure frequencies determined in individual rats monitored with the full montage ranged from 1 seizure/week (0.006/h) to 17.4 seizures/h when averaged over the week of recording, while frequencies determined using the occipital montage were lower (Fig. 4).
Fig. 3.
Rat-to-rat and temporal variability of ictal activity a month after rpFPI. Duration of seizures, as observed by the full montage, plotted by their chronology during 1 week of continuous recording for 9 representative rats with seizure frequencies ranging from 0.1 seizures/h (A) to 17.4 seizures/h (I). The effect of duration-based seizure definitions on the apparent frequency and temporal pattern of ictal activity can be visualized by focusing on the region above any selected criterion duration on the y-axis. Note the circadian clustering in A, D.
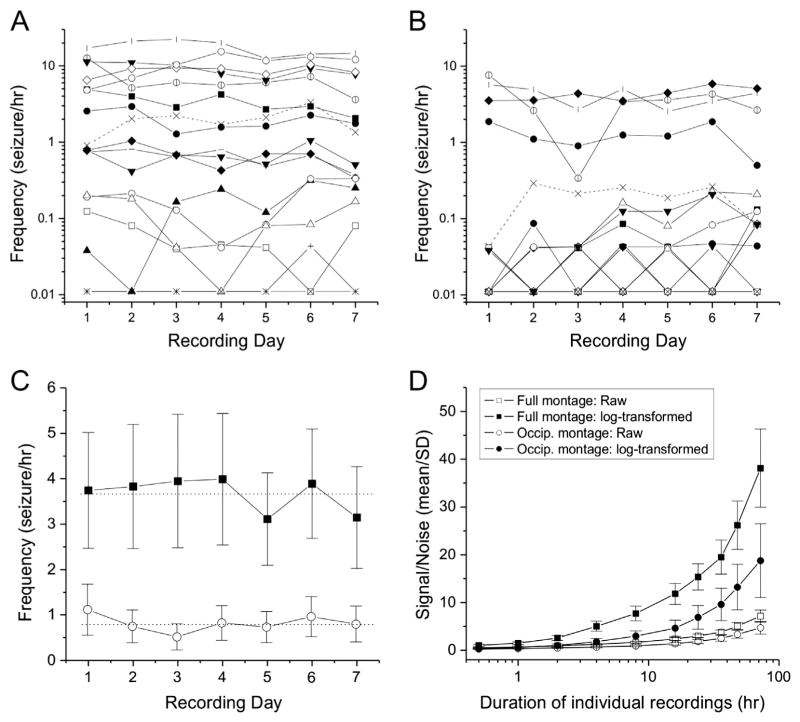
Fig. 4.
Seizure frequency variation in rpFPI rats monitored with the full or occipital ECoG montages. A and B) Day-to day variation in the frequency of seizures detected by the full montage (A) or the occipital montage (B) in individual rats. Data points represent seizure frequencies of 17 rats determined from 7 consecutive non-overlapping 24 h recording epochs extracted from 1 week of continuous recording. Plotting symbols identify the same rats in both panels. Note that the intra-subject day-to-day variation in seizure frequency is negligible compared to the variability among rats. C) Seizure frequencies determined daily with the full (filled squares) or occipital (hollow circles) ECoG recording montages. Note that the deviation of the daily means from the weekly mean (dotted lines) is small compared to the inter-subject variation as indicated by the standard error bars. Plot shows means ± SEM. D) Monte Carlo simulations of the effect of recording duration on the signal/noise ratio of 104 repeated seizure frequency determinations. For each rat and each duration of recording, the signal/noise ratio is computed as mean seizure frequency over standard deviation for seizures detected with the full montage, with and without Log10 transformation. Use of the Log10 transformation increase the signal/noise ratio, as expected for log normal distributions, at all durations of recordings tested. Plot shows means ± SEM of the signal/noise ratios in rats with at least one seizure in the week-long recording.
Consistent with previous reports (Curia et al., 2011; Eastman et al., 2010, 2011), seizure frequencies varied greatly among the 16 rats in which seizures were detected, but each rat’s seizure frequency remained relatively stable from day to day regardless of montage. Thus, when seizure frequencies were determined on the basis of 24 h recordings, the daily mean frequency of the full cohort of 17 rats fluctuated modestly about the overall weekly mean, with absolute deviations from the weekly mean no greater than 15% or 37% for seizures detected with the full or occipital montages, respectively (Fig. 4C).
Statistical power analyses
The experimental data were used to conduct bootstrapped non-parametric power analyses to determine how best to detect rpFPI-induced PTE treatment-induced effects. We specifically determined the duration of continuous ECoG monitoring required for reliable detection of treatment effects, and assessed the impact of different ECoG montages and seizure definitions on the power to detect treatment effects (Table 2). We first examined the probability of detecting epilepsy (observing at least one seizure) using the full montage (Fig. 5A) or just its two occipital electrodes (Fig. 5B). This was higher using the full montage, rather than just the occipital electrodes, regardless of seizure definition, and it increased with the inclusiveness of the seizure definition, regardless of montage. When seizures were detected only by the occipital electrodes that do not sample the perilesional neocortex (Fig. 5B), increasing the duration of recording resulted in steady improvement in the probability of detecting epilepsy regardless of how seizures were defined. The detection of epilepsy with the full montage depended more weakly on the duration of recording, especially under the most inclusive seizure definition (>1 s). Indeed, when all seizures detectable (>1 s) with the full montage were considered, increasing the duration of monitoring beyond 24 h resulted in only marginal increases in the probability of detecting epilepsy.
Fig. 5.
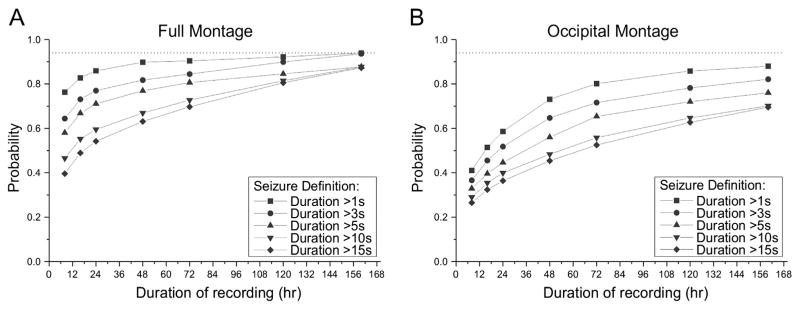
Effect of recording montage, seizure definition and duration of monitoring on the probability of detecting epilepsy. The probability of detecting at least one seizure during a recording of specified duration was determined in Monte-Carlo simulations using different seizure definitions based on duration. Each plotted point is based on 104 simulated experiments. A) Probability of detecting epilepsy using the full montage. Epilepsy is equally well detected using all but the most exclusive seizure definition (>15 s), when animals are recorded continuously for a week (160 h). Note that recording beyond 24 h provides only a marginal increase in the probability of detecting epilepsy using the most inclusive seizure definition (>1 s), while the advantage of prolonged recordings increases with the magnitude of the duration criterion for seizure identification. B) Probability of detecting epilepsy using the occipital montage. The probability of detecting epilepsy increases with recording duration for each seizure definition, and varies with seizure definition even with a full week of recording. In all cases, epilepsy is better detected with the full montage. Dotted lines indicate the probability of detecting epileptic seizures with the full montage, all seizures considered, and with the full week of recording. Note that suboptimal montages and seizure definitions introduce large errors in this estimate.
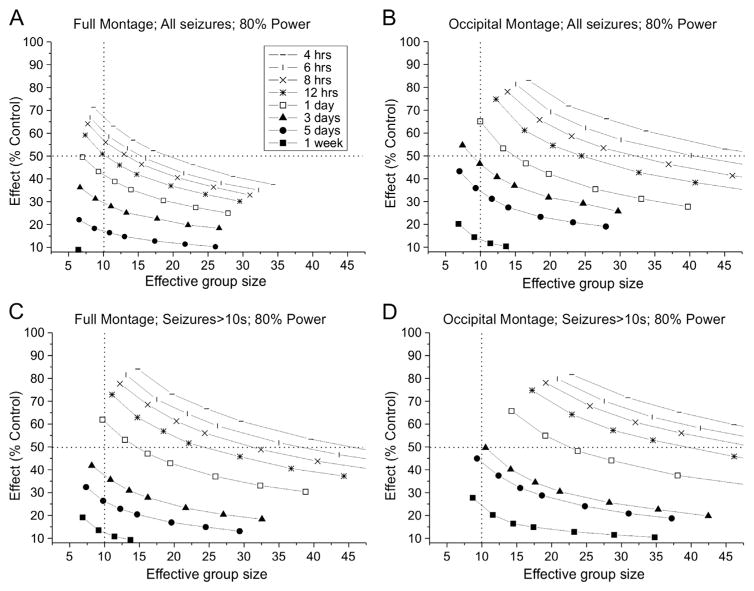
We next examined the effect of montage design, recording duration and seizure definition on statistical power to detect a treatment-induced prevention of seizures, in an independent groups comparison (Fig. 6). Seizure detection using the occipital montage was highly sensitive to both group size and duration of monitoring (Fig. 6B). Taking all detectable seizures into account, large group sizes of 20 or more animals per group were required to attain 80% power to detect 90% prevention, and this could be achieved only with recordings lasting 5 days or more per week. In contrast, the statistical power to detect a 90% prevention of seizures using the full montage was sensitive to group size, but not to duration of recording, and attained an acceptable level of 80% with groups of just 16 animals and recording for just 16 h a week (Fig. 6A). Interestingly, recordings ranging in duration from 16 h to 7 days provided identical power to detect the treatment effect, and recordings as short as 8 h provided only slightly less power. Monte Carlo simulations of experiments in which groups of 24 animals are monitored with the full montage revealed an interactive effect of seizure definition and duration of recordings on the power to detect a 90% prevention of seizures: prolonged recording improved statistical power when seizures are defined as lasting longer than 10 s, but not when seizures are defined more inclusively (Fig. 6C). When all seizures are counted, prolonged recording with the occipital montage provides statistical power approaching which can be obtained with the full montage, but the full montage achieves that power with much shorter recordings. We also examined the impact of using different duration-based definitions, rather than all clinical seizures (>1 s), on the power to detect prevention of seizures (Fig. 6D). Including all seizures allowed experiments with just 16 animals per group and 1 day of recording per week to reach 80% power to detect an effect. However, this performance degraded rapidly with progressively less inclusive seizure definitions and 30 animals per group are required to achieve 80% power when seizures are defined as lasting >10 s.
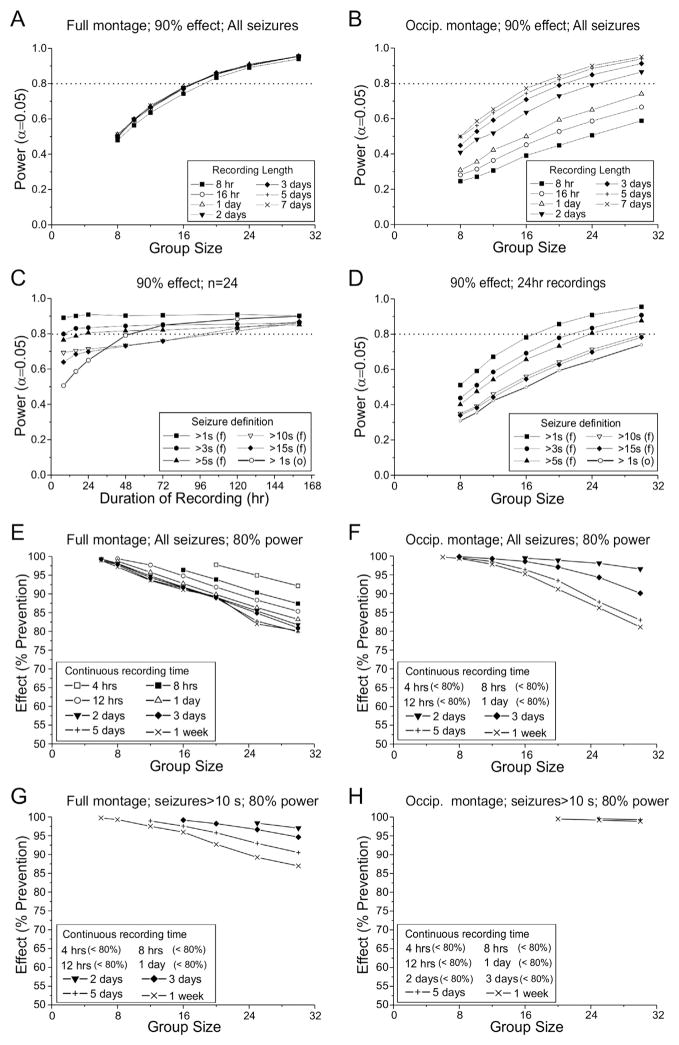
Fig. 6.
Effect of group size, recording length, recording montage and seizure definition on statistical power to detect treatment-induced prevention of seizures in independent-group comparisons. Each data point is based on 104 simulated experiments. A) Effect of group size on the power to detect a 90% prevention of seizures with 8 h to 7 days of continuous recording using the full montage and considering all seizures. Statistical power is much more sensitive to group size than duration of recording, and increasing the recording duration beyond 16 h results in no gain in statistical power. B) Effect of group size on the power to detect a 90% prevention of seizures with 8 h to 7 days of continuous recording using the occipital montage and considering all seizures. Statistical power increases with both group size and recording duration. Note that the performance of the occipital montage approaches that obtained with 16 h of recording with the full montage only when animals are recorded continuously for the entire week. C) Effect of recording time and seizure definition on power to detect a 90% decrease in seizure frequency using groups of 24 subjects each using the full (f) or occipital (o) montages. With the full montage, recording longer than 24 h results in substantial gains in statistical power only for seizures longer than 15 s. D) Effect of group size and seizure definition on the power to detect a 90% prevention of seizures in animals recorded for 24 h using the full (f) or occipital (o) montages. Increasing the duration-based seizure identification criterion decreases statistical power to detect a treatment effect. E–H) Group sizes required to detect indicated prevention of seizures with specified durations of continuous recording with either full or occipital montage. The plots illustrate the trade-off between group size and the magnitude of effect that can be detected when all seizures (E and, F) or seizures longer than 10 s (G and H) are monitored using the full (E and G) or occipital (F and, H) montage. Note that treatment effects are most sensitively detected when all seizures are monitored with the full montage (E), and that monitoring seizures longer than 10 s with the occipital montage (H) precludes reliable detection of any treatment effect with practical experiments.
When monitoring all seizures with the full montage (Fig. 6E), sizeable prevention of seizures (>80%) can be reliably detected (80% power) comparing groups of 16–30 animals continuously recorded for 24 h or more; and there is little advantage to monitoring longer than 48 h. However, when monitoring either all seizures with the occipital montage (Fig. 6F) or seizures longer than 10 s with the full montage (Fig. 6G), reliable detection of comparable seizure prevention requires continuous monitoring of experimental groups of 30 animals each for 7 days, which would be too costly to be practical. When seizures longer than 10 s are monitored with the occipital montage (Fig. 6H), week-long continuous recordings from groups of 30 animals each are insufficient to provide adequate power to detect prevention of seizures of less than 99%.
Finally, we examined the effect of montage, recording duration and seizure definition on statistical power to detect a treatment-induced seizure control in repeated measure experiments (Fig. 7). In these experiments, seizure frequencies before and during treatment are compared in the same group of epileptic rats, such that each animal serves as its own control. Since animals that do not exhibit seizures during baseline cannot be used in this kind of experiment, power analyses are displayed in terms of the numbers of animals that must be prepared and recorded to provide 80% power to detect decreases in seizure frequency. In all cases, the number of rats required to detect a specified effect is progressively diminished by increases in the duration of recording, and even very small (<10%) treatment-induced reductions in seizure frequency could be detected reliably (80% power) using week-long recordings of fewer than 8 rats when all detectable seizures (>1 s) were monitored with the full montage (Fig. 7A). However, larger, more clinically interesting effects can be reliably detected using far shorter recordings. When all detectable seizures (>1 s) are monitored, 24-hour recordings from a group of 7 animals provides 80% power to detect a clinically significant 50% decrease in seizure frequency. This performance is substantially better than that obtained using a suboptimal montage and/or seizure definition. When all detectable (>1 s) seizures are monitored with the occipital montage (Fig. 7B), the same 80% power to detect a 50% decrease in seizure frequency requires 24 h recordings from a group of 14 rats. Conversely, imposing a 10 s duration criterion for seizure identification increases the number of rats required to 24 (Fig. 7D).
Fig. 7.
Effect of recording montage, seizure definition and duration of recording on the number of subjects required to detect anti-seizure effects in repeated-measures experiments. Plotted lines depict the number of animals required to detect the indicated decrease in seizure frequency with 80% power (α = 0.05). Each plotted symbol corresponds to 104 simulated trials. The effective group size includes subjects that must be excluded from the study because no seizures were detected prior to treatment with the specified recording time. The symbols plotted on each line show the number of animals required to constitute groups of 6, 8, 10 12, 16, 20 and 24 animals with seizures during baseline recording. A) All seizures longer than 1 s detected with the full montage. With week-long continuous recordings, even trivial (<10%) decreases in seizure frequency can be detected using groups as small as 6 animals. One to three days of continuous recording are adequate to detect clinically interesting decreases (>50%) with small groups of animals. Preparation of just 7 rats is required to provide 80% power to detect such decreases with 1 day long recordings. B) All seizures longer than 1 s detected by an occipital montage. Performance is degraded when only spreading seizures are detected. Preparation of 8–16 rats is required for 80% power to detect a 50% reduction in seizure frequency in rats recorded continuously for 1–3 days. C) All seizures longer than 10 s detected with the full montage. Although performance is degraded compared to (A) when seizures are defined as events longer than 10 s, clinically interesting decreases in seizure frequency can still be detected in groups smaller than 15 animals recorded for 1–3 days. Preparation of 11–16 rats is required for 80% power to detect a 50% reduction in seizure frequency in rats recorded continuous for 1–3 days. D) Seizures longer than 10 s detected with the occipital montage. Preparation of 12–25 rats is required for 80% power to detect a 50% reduction in seizure frequency in rats recorded continuously for 1–3 days.
Discussion
We obtained continuous week-long 5-electrode epidural ECoG recordings 1 month after rpFPI, and used a Monte-Carlo-based non-parametric bootstrap strategy to test the impact of ECoG montage design, duration-based seizure definitions, group size and duration of recordings on the assessment of PTE, and on the statistical power to detect AS and AEG/DM effects of investigational treatments. Bootstrap methods are well-suited to conduct power analyses (Efron and Tibshirani, 1998) and, in contrast to parametric power analyses, they permit comparison of the performance of different statistics, regardless of their underlying distributions. The principal findings are that: 1) knowing the location of the acquired epileptic focus and closely monitoring its activity with a customized ECoG montage provides a better evaluation of the PTE syndrome and increases the power to detect the effects of AS and AEG/DM treatments, 2) arbitrary duration-based seizure definitions reduce the apparent incidence and frequency of CSRSs and diminish the power to detect treatment effects, 3) in seizure-prevention experiments, costly and laborious long-term continuous recording is neither required nor advantageous because recording longer than 24 h has no impact on the statistical power to detect treatment effects when all clinical seizures are monitored with a montage that samples the region of the epileptic focus, and 4) in seizure-control experiments, statistical power to detect AS effects increased with the duration of recordings but clinically interesting (>50% seizure reduction) effects can be detected in small groups of animals recorded for just 24 h before and during treatment using an optimized montage.
Posttraumatic epilepsy 1 month after rostral parasagittal FPI
Our previous work has shown that, while rpFPI is progressive in seizure frequency and duration, behavioral correlates, and underlying pathology, the frequency of focal neocortical seizures originating from the perilesional epileptic focus appears relatively stable for a period of several weeks around 1 month post-injury (Curia et al., 2011; D’Ambrosio et al., 2005, 2013). This is, thus, a convenient time for the assessment of investigational treatments, and we have successfully used periodic recordings to evaluate both AS and AEG/DM effects on frontal neocortical PTE at 1 month postinjury (D’Ambrosio et al., 2013; Eastman et al., 2010, 2011). In the present study, continuous ECoG recordings conducted 1 month postinjury detected numerous CSRSs with focal perilesional onset that did (Figs. 1D and E) or did not (Fig. 1C) appear to spread to other cortical areas. We have previously termed these seizure types grade 2 and grade 1, respectively, and shown that both originate in the frontal perilesional neocortex, as demonstrated in rpFPI animals by paired epidural and depth-electrode recordings (D’Ambrosio et al., 2005), by perilesional grid recordings D’Ambrosio et al., 2009), and by focal cooling of the perilesional neocortex D’Ambrosio et al., 2013), as well as by the induction of similar focal seizures in naive animals by infusion of autologous blood serum in the frontal neocortex (T.H. Stewart et al., 2010). Both seizure types have electrical and behavioral correlates that appear remarkably similar to human frontal neocortical focal seizures originating in homologous cortical areas (Bagla and Skidmore, 2011; Bancaud and Talairach, 1992; Butler et al., 2013; Williamson et al., 1985; Williamson and Spencer, 1986). Neocortical epilepsy is a very common form of human PTE (Diaz-Arrastia et al., 2009; Gupta et al., 2014). In particular, the initial visible onset is characterized by a spindle-like high voltage activity with a dominant power in the theta band (D’Ambrosio et al., 2004), similar to that reported for human focal seizures originating from the homologous frontal neocortex (Butler et al., 2013; Ikeda et al., 2009).
While our previous work based on periodic ECoG recordings (8–72 h per week) estimated the incidence of CSRSs in the 85–95% range 1 month post-injury (Curia et al., 2011; D’Ambrosio et al., 2005, 2009; Eastman et al., 2010, 2011), the present week of continuous recording using the full montage estimates it at 94%. Also consistent with previous reports, the frequency of seizures varied greatly among subjects, ranging from 0 to 17 seizures/h (Table 1). This intersubject variation was much greater than the within-subject day-to-day variation (Figs. 4A and B). We previously inferred some modest clustering of CSRSs in epileptic rpFPI rats (Eastman et al., 2010), and this was confirmed by the temporal structure of seizure onsets (Fig. 3). Circadian clustering was evident in some cases (e.g. Figs. 3A and D), but the overall temporal pattern of seizure onset appeared random.
These studies also found seizure frequency to be log normally distributed and that logarithmic transformation of seizure frequency data potently increased the power to detect AS effect (Eastman et al., 2010). We now extend these findings, and show that the log normal distribution is evident also when seizures are monitored with week-long continuous recordings (Fig. 8), and that the logarithmic transformation improves the seizure frequency signal/noise ratio with any duration of periodic-to-continuous recordings (Fig. 4D). Thus, we used logarithmic transformation to evaluate the impact of ECoG montage, seizure definitions, and duration of recordings on the power to detect AS and AEG/DM effects of treatments.
Fig. 8.
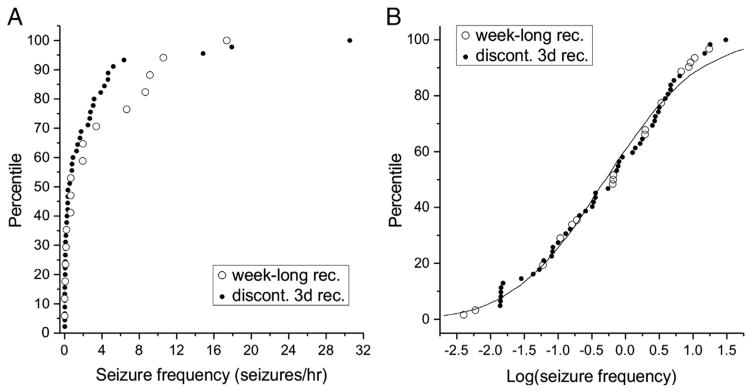
Log-normal distribution of seizure frequencies 1 month after FPI. A) A cumulative frequency histogram comparing the seizure frequencies of 45 rats each recorded 72 h/week (filled symbols; Eastman et al., 2010), to those of the 17 rats recorded continuously for a week in the present work (open symbols). The distributions of seizure frequencies in the two studies do not differ significantly (p > 0.4; two sample Kolmogorov–Smirnov test). B) After logarithmic transformation, the cumulative frequency histogram of the combined data from A has a sigmoid appearance that is consistent with a log-normal distribution with mean = −0.275 and S.D. = 1.09 (continuous line).
Optimized ECoG montage and realistic seizure definition maximize detection of epilepsy and treatment effects
The reliable development of an epileptic focus in the frontal perilesional neocortex is an advantageous feature of the rpFPI model, and likely of other models in which epilepsy arises after a discrete brain insult. The predictable focus permits the design of a montage for very sensitive measurements of its ictal activity (full montage; Fig. 1A) — a strategy that is not available when the location of the focus is not known. The use of such a montage would detect numerous focal perilesional seizures that would be missed by montages that are not designed to directly sample the epileptic focus. Indeed, the occipital montage missed all of the numerous focal perilesional seizures (Fig. 1C), as well as a smaller number of seizures that spread contralaterally but not caudally (Fig. 1D). This was reproduced in our study by counting only seizures that were detected by the two occipital electrodes in our standard montage (Fig. 1A). These were only 22% of the total (Table 1). While the two recording montages detected seizures spanning a similar range of durations, a large proportion of the shorter seizures, which are particularly prominent in the early months after injury (Curia et al., 2011; D’Ambrosio et al., 2005), was not detected by the occipital montage (Fig. 1B). Thus, the apparent incidence, frequency and time spent seizing observed with the occipital montage were all significantly lowered, and the mean seizure durations increased, in respect to those observed with the full montage (Table 1). Also, imposing arbitrary duration-based criteria for seizure identification had the predictable effect of dramatically reducing the seizure incidence and frequency, and increasing their mean duration, whether they were detected by the occipital or the full montage (Table 1). Thus, depending exclusively upon the ECoG recording montage and seizure definition, the same set of recordings supports starkly different characterizations of the same FPI-PTE syndrome with different implications for the utility of FPI-PTE for therapy development (Figs. 6 and 7). These observations also highlight both the critical importance of providing detailed and comprehensive accounts of recording protocols and seizure identification criteria when reporting spontaneous seizure data to permit comparison of results across laboratories.
We conducted a series of statistical power analyses to assess the value of the perilesional electrode and duration-based seizure definitions on the power to detect treatment effects. When seizures are monitored with an ECoG montage that includes an electrode placed near the focus at which they originate, the statistical power to detect treatment effect is maximized for both seizure prevention (Fig. 6) and seizure control (Fig. 7). Conversely, using recording montages that do not closely monitor the epileptic focus (Fig. 1) or by use of arbitrary duration-based seizure definitions, diminishes statistical power to detect treatment effects.
Why use a clinical definition of epileptic seizure in preclinical studies?
Strict adherence to ILAE definition of epileptic seizures (Fisher et al., 2005), which imposes no restriction on the duration of seizures permits shorter preclinical studies using fewer subjects. We have previously shown that both short and long seizures are clinical, i.e. are associated with simultaneous behavioral changes (D’Ambrosio et al., 2009), and satisfy the clinical criteria for diagnosis of epileptic seizures used to evaluate patients (Fisher et al., 2005; D’Ambrosio and Miller, 2010). Short and long seizures also respond similarly to treatment. Seizures of all durations were prevented by mild focal cooling (D’Ambrosio et al., 2013) and not by carisbamate (Eastman et al., 2011), and were poorly responsive to subchronic treatment with carbamazepine, valproate (Eastman et al., 2010) and carisbamate (Eastman et al., 2011). The short and long seizures in the rpFPI model are also clearly related. The week-long analysis of ictal patterns vs. seizure duration clearly shows that long seizures are not observed in the absence of shorter ones (Fig. 3). Using two different definitions of short (<5 s or <10 s) and long (>5 s or >10 s), we found that the correlation between the frequencies of short and long seizures (Fig. 2), and the relationship between the incidences of short and long seizures, are both highly significant. Thus, counting all clinical seizures in the rpFPI-PTE model can be recommended on several grounds: 1) they are a consistent feature of rpFPI-PTE, particularly in the early weeks after injury, 2) using them allows one to shorten experimental studies, 2) their frequency and incidence are closely related to the frequency and incidence of longer seizures, 3) they respond to treatment similar to longer seizures, and 4) they increase statistical power to detect changes in the epileptic syndrome and effects of investigational treatments with much briefer periods of ECoG monitoring.
Continuous recording is not needed for therapy development
The probability of detecting rpFPI-induced PTE critically depended on ECoG montage, seizure definition and duration of recording, but monitoring all clinical seizures with the full montage allowed close approximation of seizure incidence as measured by the full week of recording in just 24 h (Fig. 5). In contrast, using the occipital montage required the full week of recording to achieve comparable estimates. Regardless of montage, performance is degraded with longer duration-based seizure definitions. Duration of recording being equal, the worst performance was obtained using the occipital montage to monitor seizure longer than 15 s (Fig. 5). Our power analyses also show that the detection of AEG/DM effects on rpFPI-PTE does not require, or even benefit from, continuous recording longer than 24 h when seizures are optimally monitored (Figs. 6A and C). This is consistent with the observation that day-to-day variation in seizure frequency is negligible compared to inter-subject variability (Fig. 4), and accounts for the reproducibility of seizure frequency and incidence in studies using varying durations of ECoG monitoring (D’Ambrosio et al., 2005, 2009, 2013; Eastman et al., 2010, 2011). Thus, rpFPI-PTE appears to differ from CSRS models based on systemic administration of kainate or pilocarpine, which have been reported to require long-term continuous monitoring due to a high incidence of extreme clustering of CSRSs (Bajorat et al., 2011; Goffin et al., 2007; Williams et al., 2009). Our data specifically show that AEG/DM treatments of rpFPI-PTE are optimally assessed by monitoring all seizures detectable with an ECoG montage including an epidural electrode that samples the perilesional neocortex (Fig. 6). While monitoring all seizures (>1 s) that spread to the occipital electrodes approaches the sensitivity of the full montage when large groups are monitored continuously for a week, this entails collection and analysis of 6720 h (2 groups × 20 rats × 1 week) of ECoG to detect an 90% decrease in seizure frequency, compared to the 960 h (2 groups × 20 rats × 24 h) required with the full montage (Figs. 6E and F). Equivalent sensitivity cannot be obtained in practical experiments when seizures longer than 10 s are monitored with the occipital montage (Fig. 6H). These data also suggest that efforts to further optimize therapy testing should focus on measures that might decrease inter-subject variability.
Our power analyses also show that short recordings are adequate to detect AS effects. Seizure control is assessed in repeated measures experiments in which each subject serves as its own control. We have previously shown that this approach is best suited to limit the impact of the high intersubject variation in seizure frequency after rpFPI (Eastman et al., 2010). Measurement-to-measurement variability assumes a more important role in determining the statistical power to detect AS effects than in AEG/DM studies. Prolonged continuous recording consistently and progressively increases the sensitivity of AS effect detection such that even clinically inconsequential decreases in seizure frequency can be reliably detected using manageable numbers of animals monitored continuously for a week (Fig. 7). However, detection of clinically interesting AS effects does not require long-term continuous recording. Most clinical trials identify as “responders” those patients who experience at least a 50% reduction in seizure frequency during the investigational treatment. Our power analyses show that seven rats must be prepared to produce a group of six animals that provides 80% power to detect a 50% decrease in seizure frequency when all seizures (>1 s) detectable with a montage that samples the perilesional epileptic focus are monitored for 24 h before and during treatment (Fig. 7A). This performance is greatly degraded when seizures are recorded using a montage that does not sample the region of the epileptic focus or if duration-based criteria are used for seizure identification (Figs. 7B – D). It should be noted that when the objective is to determine whether a treatment diminishes seizure frequency in a group of rats, it does not really matter that an occasional rat presents with some interseizure intervals longer than 24 h (Fig. 3A). The impact of rats like these is accurately accounted for by the Monte Carlo approach to computing the non-parametric power analyses.
Implications for rigorous preclinical epilepsy therapy development
Rigorous preclinical testing of investigational treatments requires 1) models relevant to the conditions to treat, and 2) the knowledge of the power available to detect treatment effects. Only when these conditions are met, the decision to exclude drugs from further study can be properly made.
Etiologically realistic acquired epilepsy models are based on experimental brain insults that closely mimic insults that are epileptogenic in man and, thus, likely capture the full range of mechanisms that operate in the corresponding patient populations. These models have the potential to revolutionize epilepsy therapy development and greatly enhance bench to bedside translation because they may incorporate distinct mechanisms peculiar to the modeled etiology and display correspondingly distinct pharmacological profiles. There are already data that suggest that rpFPI-induced epileptic seizures differ mechanistically from both evoked seizure models and SE-induced CSRSs. Carisbamate, an investigational ASD that was withdrawn from the regulatory process for epilepsy treatment due to weak efficacy against non-convulsive focal seizures in pharmacoresistant patients (Bialer et al., 2010), was effective in controlling motor seizures in a wide range of evoked seizure models and in a SE-based epilepsy model (Bialer et al., 2010; Grabenstatter and Dudek, 2008), but had no detectable effect on non-convulsive focal CSRSs in our rpFPI-PTE model (Eastman et al., 2011). Similarly, carbamazepine controlled motor seizures in the chronic phase after kainate-induced SE (Grabenstatter et al., 2007), but had no detectable effect on rpFPI-PTE (Eastman et al., 2010). Importantly, most etiologically realistic models prominently feature non-convulsive focal seizures (Buckingham et al., 2011; Dubé et al., 2010; Kelly et al., 2001; Rakhade et al., 2011; K.A. Stewart et al., 2010), which are difficult to treat in humans and predominate in the pharmacoresistant patients participating in add-on clinical trials (Juul-Jensen, 1986; Mattson et al., 1996; Semah et al., 1998). Video/ECoG monitoring permits accurate quantitation of the entire spectrum of epileptic seizures including the non-convulsive seizures that have been largely overlooked in most preclinical studies.
Our data clearly show that etiologically realistic acquired epilepsy models can be adapted for practical use in ECoG-based drug discovery. We have identified FPI parameters that modulate the progression after injury and result in an incidence and frequency of seizures and speed of epileptogenesis comparable or superior to that reported in SE-based models (Curia et al., 2011, 2015). The use of the clinical definition of epileptic seizure (Fisher et al., 2005; D’Ambrosio and Miller, 2010) permits the inclusion of numerous short clinical seizures, a common feature of the early months postinjury, thus permitting studies to be conducted with fewer animals and earlier in the progression of the syndrome. In this optimized rpFPI model, the screening of AS treatments can be conducted particularly efficiently with just two 24-hour long recordings (one before and one during treatment) and a single group of just 7 rats, which provide 80% power to detect a 50% decrease in seizure frequency (Fig. 7A). Because epileptogenesis is rapid after optimized rpFPI, a laboratory that produces animals continuously can complete an ECoG-based evaluation of a AS drug within a few days, depending on the desired duration of treatment. Larger groups are required to evaluate AEG/DM effects in independent groups of treated and untreated rats injured by optimized rpFPI, but just 16 animals per group provide 80% power to detect a treatment-induced 90% reduction in seizure frequency 1 month post-injury (Fig. 6A). Because seizure frequency can be assessed in a single 24 h recording, about 1 month is required for testing a single compound.
Similar power analyses should be conducted for all other etiologically realistic acquired epilepsy models employed in therapy development. Although the insight that the power to detect treatment effects is diminished by systematic exclusion of arbitrarily defined classes of seizures is quite general, the power analyses we conducted can be confidently applied only to the rpFPI model that generated the seizure data that were bootstrapped in the Monte-Carlo simulations. They may also apply to other variants of FPI resulting in similar seizure durations and frequencies (Goodrich et al., 2013), or to other models, like the recently described perinatal hypoxia model that also generates a similar distribution of seizure frequency and durations (Rakhade et al., 2011), but it remains to be determined that the temporal pattern of seizure onsets is comparable to that of rpFPI (Fig. 3). The findings we present will most likely not apply to models like SE-based motor seizures, with significant seizure clustering. These would require dedicated power analyses.
Acknowledgments
National Institutes of Health (NS076570) and Citizens United for Research in Epilepsy (Prevention of Acquired Epilepsies Award) provided funding to R.D.
References
- Bagla R, Skidmore CT. Frontal lobe seizures. Neurologist. 2011;17 (3):125–135. doi: 10.1097/NRL.0b013e31821733db. [DOI] [PubMed] [Google Scholar]
- Bajorat R, Wilde M, Sellmann T, Kirschstein T, Köhling R. Seizure frequency in pilocarpine-treated rats is independent of circadian rhythm. Epilepsia. 2011;52:e118–e122. doi: 10.1111/j.1528-1167.2011.03200.x. http://dx.doi.org/10.1111/j.1528-1167.2011.03200.x. [DOI] [PubMed] [Google Scholar]
- Bancaud J, Talairach J. Clinical semiology of frontal lobe seizures. In: Chauvel P, Delgado-Escueta AV, Halgren E, Bancaud J, editors. Advances in Neurology. Vol. 57. Raven Press; New York: 1992. pp. 3–58. [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILATX) Epilepsy Res. 2010;92 (2–3):89–124. doi: 10.1016/j.eplepsyres.2010.09.001. http://dx.doi.org/10.1016/j.eplepsyres.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T, Sontheimer H. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17 (10):1269–1274. doi: 10.1038/nm.2453. http://dx.doi.org/10.1038/nm.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Ichise M, Teich AF, Gerard E, Osborne J, French J, Devinsky O, Kuzniecky R, Gilliam F, Pervez F, Provenzano F, Goldsmith S, Vallabhajosula S, Stern E, Silbersweig D. Imaging inflammation in a patient with epilepsy due to focal cortical dysplasia. J Neuroimaging. 2013;23 (1):129–131. doi: 10.1111/j.1552-6569.2010.00572.x. http://dx.doi.org/10.1111/j.1552-6569.2010.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Levitt M, Fender JS, Miller JW, Ojemann J, D’Ambrosio R. Impact of injury location and severity on posttraumatic epilepsy in the rat: role of frontal neocortex. Cereb Cortex. 2011;21:1574–1592. doi: 10.1093/cercor/bhq218. http://dx.doi.org/10.1093/cercor/bhq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Eastman CL, Miller JW, D’Ambrosio R. Modeling posttraumatic epilepsy for therapy development. In: Grant J, Laskowitz D, editors. Frontiers in Neurosciences: Translational Research in Traumatic Brain Injury. CRC Press; Boca Raton, FL: 2015. (in press) [Google Scholar]
- D’Ambrosio R, Fairbanks JP, Fender JS, Doyle D, Born DE, Miller JW. Posttraumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. http://dx.doi.org/10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Fender JS, Fairbanks JP, Simon E, Born DE, Doyle D, Miller JW. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;128:174–188. doi: 10.1093/brain/awh337. http://dx.doi.org/10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Miller JW. What is an epileptic seizure? Unifying definitions in clinical practice and animal research to develop novel treatments. Epilepsy Curr. 2010;10:61–66. doi: 10.1111/j.1535-7511.2010.01358.x. http://dx.doi.org/10.1111/j.1535-7511.2010.01358.x (Comment p. 90) [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Hakimian S, Stewart T, Verley DR, Fender JS, Eastman CL, Sheerin AH, Gupta P, Diaz-Arrastia R, Ojemann J, Miller JW. Functional definition of seizure provides new insight into post-traumatic epileptogenesis. Brain. 2009;132:2805–2821. doi: 10.1093/brain/awp217. http://dx.doi.org/10.1093/brain/awp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Eastman CL, Darvas F, Fender JS, Verley DR, Farin FM, Wilkerson HW, Temkin NR, Miller JW, Ojemann J, Rothman SM, Smyth MD. Mild passive focal cooling prevents epileptic seizures after head injury in rats. Ann Neurol. 2013;73 (2):199–209. doi: 10.1002/ana.23764. http://dx.doi.org/10.1002/ana.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Arrastia R, Agostini MA, Madden CJ, Van Ness PC. Post-traumatic epilepsy: the endophenotypes of a human model of epileptogenesis. Epilepsia. 2009;50 (S2):14–20. doi: 10.1111/j.1528-1167.2008.02006.x. http://dx.doi.org/10.1111/j.1528-1167.2008.02006.x. [DOI] [PubMed] [Google Scholar]
- Dubé CM, Ravizza T, Hamamura M, Zha Q, Keebaugh A, Fok K, Andres AL, Nalcioglu O, Obenaus A, Vezzani A, Baram TZ. Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci. 2010;30 (22):7484–7494. doi: 10.1523/JNEUROSCI.0551-10.2010. http://dx.doi.org/10.1523/JNEUROSCI.0551-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CL, Verley DR, Fender JS, Temkin NR, D’Ambrosio R. ECoG studies of valproate, carbamazepine and halothane in frontal-lobe epilepsy induced by head injury in the rat. Exp Neurol. 2010;224:369–388. doi: 10.1016/j.expneurol.2010.04.013. http://dx.doi.org/10.1016/j.expneurol.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CL, Verley DR, Fender JS, Stewart TH, Nov E, Curia G, D’Ambrosio R. Antiepileptic and antiepileptogenic performance of carisbamate after head injury in the rat: blind and randomized studies. J Pharmacol Exp Ther. 2011;336:779–790. doi: 10.1124/jpet.110.175133. http://dx.doi.org/10.1124/jpet.110.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Chapman & Hall/CRC; New York: 1998. p. 437. [Google Scholar]
- Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J., Jr Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46 (4):470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- Gilliam F, Carter J, Vahle V. Tolerability of antiseizure medications: implications for health outcomes. Neurology. 2004;63 (10 Suppl 4):S9–S12. doi: 10.1212/wnl.63.10_suppl_4.s9. [DOI] [PubMed] [Google Scholar]
- Goodrich GS, Kabakov AY, Hameed MQ, Dhamne SC, Rosenberg PA, Rotenberg A. Ceftriaxone Treatment after Traumatic Brain Injury Restores Expression of the Glutamate Transporter, GLT-1, Reduces Regional Gliosis, and Reduces Post-Traumatic Seizures in the Rat. J Neurotrauma. 2013;30:1434–1441. doi: 10.1089/neu.2012.2712. http://dx.doi.org/10.1089/neu.2012.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin K, Nissinen J, Van Laere K, Pitkänen A. Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Exp Neurol. 2007;205:501–505. doi: 10.1016/j.expneurol.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Grabenstatter HL, Dudek FE. A new potential AED, carisbamate, substantially reduces spontaneous motor seizures in rats with kainate-induced epilepsy. Epilepsia. 2008;49 (10):1787–1794. doi: 10.1111/j.1528-1167.2008.01657.x. http://dx.doi.org/10.1111/j.1528-1167.2008.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenstatter HL, Clark S, Dudek FE. Anticonvulsant effects of carbamazepine on spontaneous seizures in rats with kainate-induced epilepsy: comparison of intraperitoneal injections with drug-in-food protocols. Epilepsia. 2007;48 (12):2287–2295. doi: 10.1111/j.1528-1167.2007.01263.x. (40) [DOI] [PubMed] [Google Scholar]
- Gupta P, Ding K, Sayed N, Agostini M, Van Ness P, Madden C, Mickey B, D’Ambrosio R, Diaz-Arrastia R. Subtypes of post-traumatic epilepsy: clinical, electrophysiologic, and imaging features. J Neurotrauma. 2014 doi: 10.1089/neu.2013.3221. http://dx.doi.org/10.1089/neu.2013.3221. [DOI] [PMC free article] [PubMed]
- Ikeda A, Hirasawa K, Kinoshita M, Hitomi T, Matsumoto R, Mitsueda T, Taki JY, Inouch M, Mikuni N, Hori T, Fukuyama H, Hashimoto N, Shibasaki H, Takahashi R. Negative motor seizure arising from the negative motor area: is it ictal apraxia? Epilepsia. 2009;50 (9):2072–2084. doi: 10.1111/j.1528-1167.2009.02097.x. http://dx.doi.org/10.1111/j.1528-1167.2009.02097.x. [DOI] [PubMed] [Google Scholar]
- Juul-Jensen P. Epidemiology of intractable epilepsy. In: Schmidt D, Morselli P, editors. Intractable Epilepsy. Raven Press; New York: 1986. pp. 5–11. [Google Scholar]
- Kelly KM, Kharlamov A, Hentosz TM, Kharlamova EA, Williamson JM, Bertram EH, III, Kapur J, Armstrong DM. Photothrombotic brain infarction results in seizure activity in aging Fischer 344 and Sprague Dawley rats. Epilepsy Res. 2001;47 (3):189–203. doi: 10.1016/s0920-1211(01)00294-7. [DOI] [PubMed] [Google Scholar]
- Löscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia. 2011;52 (4):657–678. doi: 10.1111/j.1528-1167.2011.03024.x. http://dx.doi.org/10.1111/j.1528-1167.2011.03024.x. [DOI] [PubMed] [Google Scholar]
- Löscher W, Schmidt D. Seizing the moment for the future: the U.S. Anticonvulsant Screening Project. Epilepsia. 2012;53 (10):1841–1842. doi: 10.1111/j.1528-1167.2012.03647.x. http://dx.doi.org/10.1111/j.1528-1167.2012.03647.x. [DOI] [PubMed] [Google Scholar]
- Löscher W, Klitgaard H, Twyman RE, Schmidt D. New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov. 2013;12 (10):757–776. doi: 10.1038/nrd4126. http://dx.doi.org/10.1038/nrd4126. [DOI] [PubMed] [Google Scholar]
- Mattson RH, Cramer JA, Collins JF. Prognosis for total control of complex partial and secondarily generalized tonic clonic seizures. Department of Veterans Affairs Epilepsy Cooperative Studies No 118 and No 264 Group. Neurology. 1996;47 (1):68–76. doi: 10.1212/wnl.47.1.68. [DOI] [PubMed] [Google Scholar]
- Pearce PS, Friedman D, Lafrancois JJ, Iyengar SS, Fenton AA, Maclusky NJ, Scharfman HE. Spike-wave discharges in adult Sprague–Dawley rats and their implications for animal models of temporal lobe epilepsy. Epilepsy Behav. 2014;32:121–131. doi: 10.1016/j.yebeh.2014.01.004. http://dx.doi.org/10.1016/j.yebeh.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhade S, Klein P, Huynh T, Hilario-Gomez C, Kosaras B, Rotenberg A, Jensen F. Development of later life spontaneous seizures in a rodent model of hypoxia induced neonatal seizures. Epilepsia. 2011;52 (4):753–765. doi: 10.1111/j.1528-1167.2011.02992.x. http://dx.doi.org/10.1111/j.1528-1167.2011.02992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51 (5):1256–1262. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- Siegel S. Nonparametric Statistics for the Behavioral Sciences. McGraw-Hill; New York: 1956. [Google Scholar]
- Smith M, Wilcox KS, White HS. Discovery of antiepileptic drugs. Neurotherapeutics. 2007;4 (1):12–17. doi: 10.1016/j.nurt.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart KA, Wilcox KS, Fujinami RS, White HS. Development of postinfection epilepsy after Theiler’s virus infection of C57BL/6 mice. J Neuropathol Exp Neurol. 2010a;69:1210–1219. doi: 10.1097/NEN.0b013e3181ffc420. http://dx.doi.org/10.1097/NEN.0b013e3181ffc420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart TH, Eastman CL, Groblewski PA, Fender JS, Verley DR, Cook DG, D’Ambrosio R. Chronic dysfunction of astrocytic inwardly rectifying K+ channels specific to the neocortical epileptic focus after fluid percussion injury in the rat. J Neurophysiol. 2010b;104 (6):3345–3360. doi: 10.1152/jn.00398.2010. http://dx.doi.org/10.1152/jn.00398.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50 (Suppl 2):10–13. doi: 10.1111/j.1528-1167.2008.02005.x. http://dx.doi.org/10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- White HS. Chemoconvulsants. In: Peterson SL, Albertson TE, editors. Neuropharmacology Methods in Epilepsy Research. CRC Press; Boca Raton, FL: 1998. pp. 27–40. [Google Scholar]
- Williams PA, Dudek FE. A chronic histopathological and electrophysiological analysis of a rodent hypoxic-ischemic brain injury model and its use as a model of epilepsy. Neuroscience. 2007;149:943–961. doi: 10.1016/j.neuroscience.2007.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. http://dx.doi.org/10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson PD, Spencer SS. Clinical and EEG features of complex partial seizures of extratemporal origin. Epilepsia. 1986;27 (Suppl 2):S46–S63. doi: 10.1111/j.1528-1157.1986.tb05740.x. [DOI] [PubMed] [Google Scholar]
- Williamson PD, Spencer DD, Spencer SS, Novelly RA, Mattson RH. Complex partial seizures of frontal lobe origin. Ann Neurol. 1985;18 (4):497–504. doi: 10.1002/ana.410180413. [DOI] [PubMed] [Google Scholar]
- Willoughby JO, Mackenzie L. Nonconvulsive electrocorticographic paroxysms (absence epilepsy) in rat strains. Lab Anim Sci. 1992;42 (6):551–554. [PubMed] [Google Scholar]