Abstract
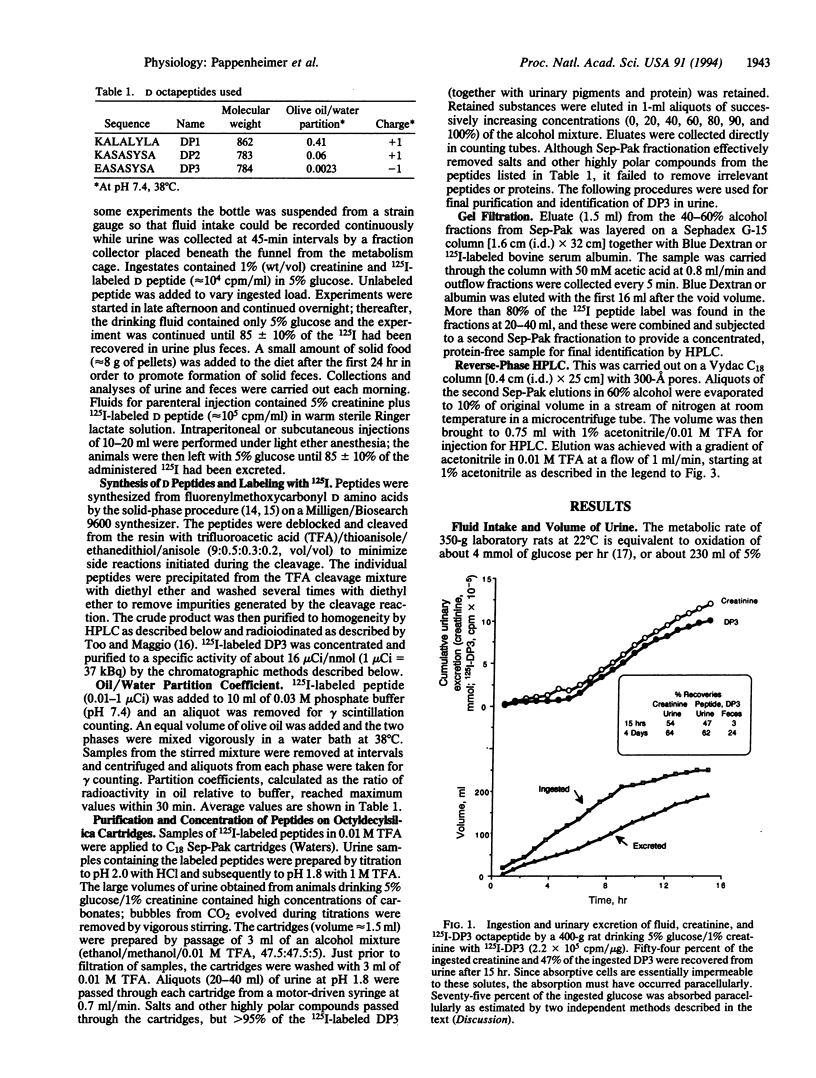
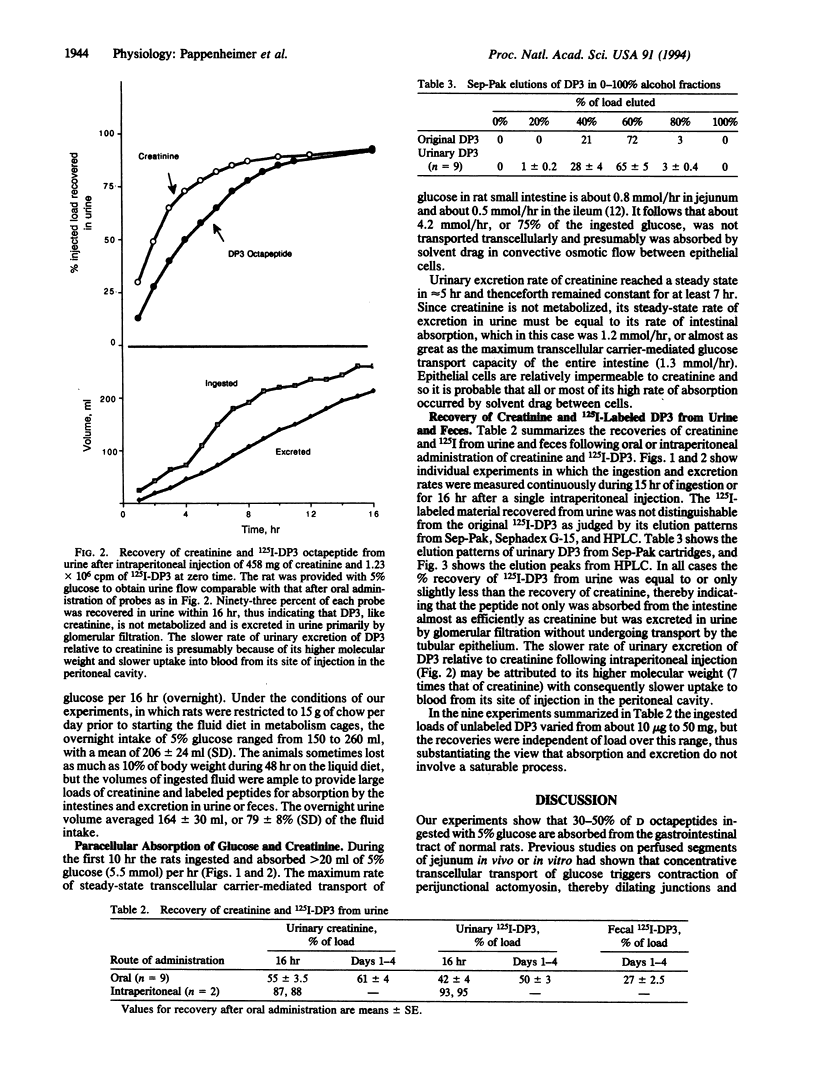
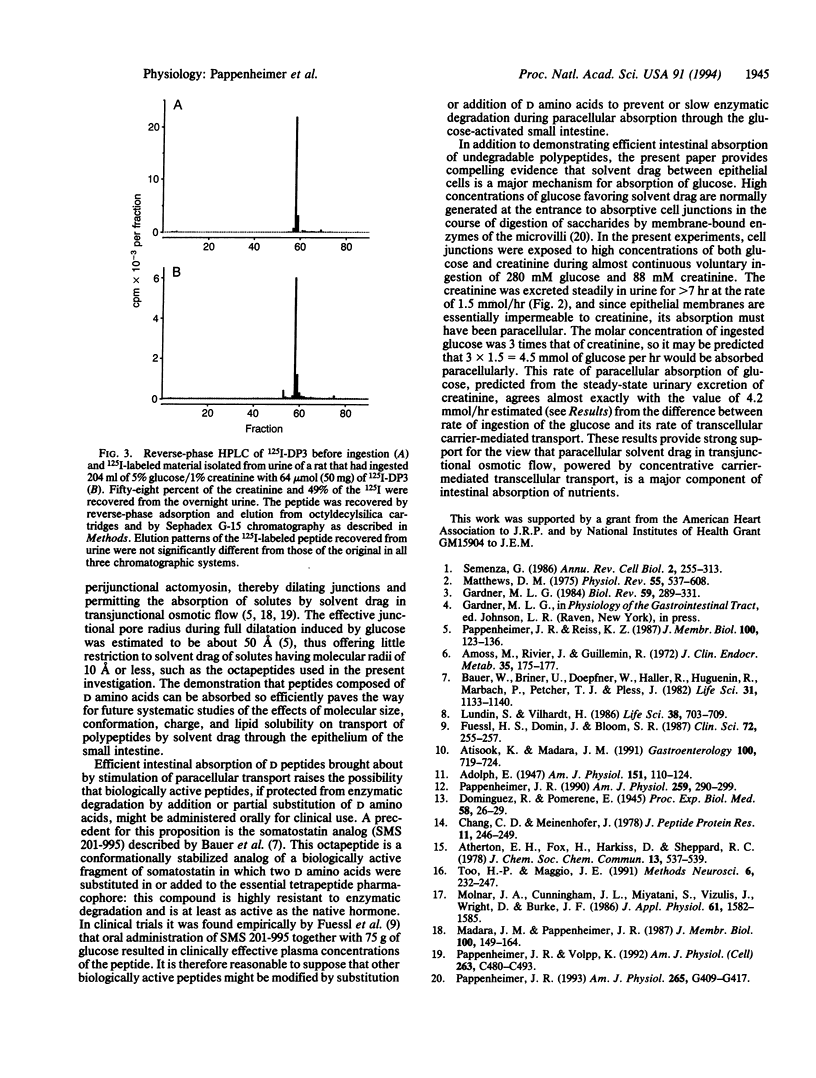
Octapeptides synthesized from D amino acids were absorbed from the intestine and excreted in urine of normal rats drinking 5% glucose/1% creatinine containing the 125I-labeled peptides at 0.1-25 mg/dl. The rats ingested fluid at the rate of about 20 ml/hr and produced urine at 15 ml/hr for several hours during the nocturnal feeding period. Sixty-one +/- 4% of the ingested creatinine and 50 +/- 3% of a lipid-insoluble D octapeptide (EASASYSA, 784 Da) were excreted intact in the urine. The steady-state molar rate of absorption-excretion of creatinine equaled or exceeded the maximum rate of carrier-mediated intestinal transport of glucose, suggesting that both the creatinine and the D octapeptide were transported paracellularly by solvent drag through absorptive cell junctions that were dilated by the glucose. More than 70% of the ingested glucose was also absorbed paracellularly. The results demonstrate that intact oligopeptides can be absorbed efficiently from the intestine when they are not hydrolyzed by membrane-bound peptidases of the brush border. The results also provide support for recent theories proposing that coupling of membrane digestion with paracellular solvent drag accounts for a major fraction of normal intestinal absorption of nutrients.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amoss M., Rivier J., Guillemin R. Release of gonadotropins by oral administration of synthetic LRF or a tripeptide fragment of LRF. J Clin Endocrinol Metab. 1972 Jul;35(1):175–177. doi: 10.1210/jcem-35-1-175. [DOI] [PubMed] [Google Scholar]
- Atisook K., Madara J. L. An oligopeptide permeates intestinal tight junctions at glucose-elicited dilatations. Implications for oligopeptide absorption. Gastroenterology. 1991 Mar;100(3):719–724. doi: 10.1016/0016-5085(91)80016-3. [DOI] [PubMed] [Google Scholar]
- Bauer W., Briner U., Doepfner W., Haller R., Huguenin R., Marbach P., Petcher T. J., Pless SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982 Sep 13;31(11):1133–1140. doi: 10.1016/0024-3205(82)90087-x. [DOI] [PubMed] [Google Scholar]
- Chang C. D., Meienhofer J. Solid-phase peptide synthesis using mild base cleavage of N alpha-fluorenylmethyloxycarbonylamino acids, exemplified by a synthesis of dihydrosomatostatin. Int J Pept Protein Res. 1978 Mar;11(3):246–249. doi: 10.1111/j.1399-3011.1978.tb02845.x. [DOI] [PubMed] [Google Scholar]
- Fuessl H. S., Domin J., Bloom S. R. Oral absorption of the somatostatin analogue SMS 201-995: theoretical and practical implications. Clin Sci (Lond) 1987 Feb;72(2):255–257. doi: 10.1042/cs0720255. [DOI] [PubMed] [Google Scholar]
- Gardner M. L. Intestinal assimilation of intact peptides and proteins from the diet--a neglected field? Biol Rev Camb Philos Soc. 1984 Aug;59(3):289–331. doi: 10.1111/j.1469-185x.1984.tb00708.x. [DOI] [PubMed] [Google Scholar]
- Lundin S., Vilhardt H. Absorption of intragastrically administered DDAVP in conscious dogs. Life Sci. 1986 Feb 24;38(8):703–709. doi: 10.1016/0024-3205(86)90584-9. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Pappenheimer J. R. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100(2):149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- Matthews D. M. Intestinal absorption of peptides. Physiol Rev. 1975 Oct;55(4):537–608. doi: 10.1152/physrev.1975.55.4.537. [DOI] [PubMed] [Google Scholar]
- Molnar J. A., Cunningham J. J., Miyatani S., Vizulis A., Wright J. D., Burke J. F. Closed-circuit metabolic system with multiple applications. J Appl Physiol (1985) 1986 Oct;61(4):1582–1585. doi: 10.1152/jappl.1986.61.4.1582. [DOI] [PubMed] [Google Scholar]
- Pappenheimer J. R. On the coupling of membrane digestion with intestinal absorption of sugars and amino acids. Am J Physiol. 1993 Sep;265(3 Pt 1):G409–G417. doi: 10.1152/ajpgi.1993.265.3.G409. [DOI] [PubMed] [Google Scholar]
- Pappenheimer J. R., Reiss K. Z. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 1987;100(2):123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- Pappenheimer J. R., Volpp K. Transmucosal impedance of small intestine: correlation with transport of sugars and amino acids. Am J Physiol. 1992 Aug;263(2 Pt 1):C480–C493. doi: 10.1152/ajpcell.1992.263.2.C480. [DOI] [PubMed] [Google Scholar]
- Semenza G. Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli. Annu Rev Cell Biol. 1986;2:255–313. doi: 10.1146/annurev.cb.02.110186.001351. [DOI] [PubMed] [Google Scholar]


