Abstract
Abstract
Two new compounds, maniesculentins A (1) and B (6), together with four known ones were isolated from the stems of Manihot esculenta Crantz. The structures of the new compounds were elucidated by extensive spectroscopic methods including NMR spectroscopy and mass spectrometry. The two new compounds (1, 6) were assayed for antibacterial activity against four tested bacteria lines.
Graphical Abstract
Electronic supplementary material
The online version of this article (doi:10.1007/s13659-015-0052-8) contains supplementary material, which is available to authorized users.
Keywords: Euphorbiaceae, Manihot esculenta, Chemical constituents, Diterpenoids
Introduction
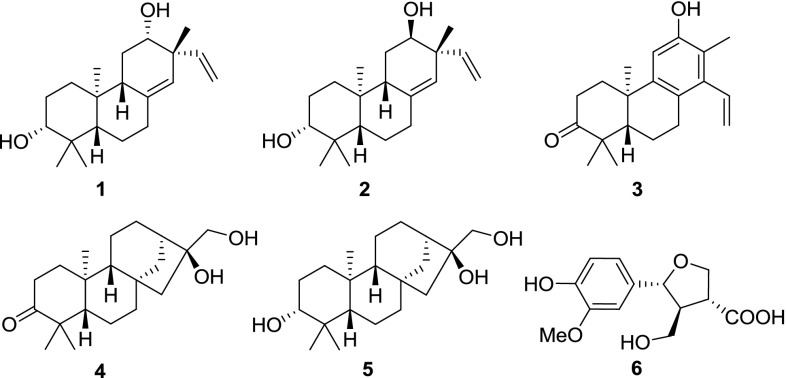
The Euphorbiaceae produce a diverse range of secondary metabolites. Recently, a series of secondary metabolites with fascinating structural features and significant biological activities from Euphorbiaceae family were reported. The main constitutes of Euphorbiaceae are diterpenoids with different skeletons such as jatrophanes, lathyranes, tiglianes, ingenanes, and myrsinanes with a wide array of pharmaceutical activities, such as antiproliferative, cytotoxic, antimicrobial and anti-inflammatory, anticancer and antioxidant activities [1–7]. Manihot (Euphorbiaceae), a shrub, has about 60 species and is widely cultivated in tropical regions. Two species were introduced in China, which are M. esculenta Crantz and M. glaziovii. M. esculenta is widely cultivated in Fujian, Guangdong, Guangxi, Guizhou, Hainan, Taiwan and Yunnan provences of China [8]. Previous study on M. esculenta roots reported various types of stress metabolites, which are predominantly steroids and diterpenoids produced in the damaged cassava root tissue by cutting and fungal-infection [9]. Recently investigations in China mainly focused on the roots distributed in Hainan [10]. However, the study on the plant distributed in Yunnan has not yet reported. Therefore, searching for novel structural and bioactive natural products from its stems led to the isolation of two new compounds 1 and 6 (Fig. 1), and four known diterpenoids, yucalexin P–21 (2) [10, 11], cleistanthene–type sonderianol (3) [12], calliterpenone (4) [13], ent-kauran-3α,16α, 17-triol (5) [14]. Herein, this paper describes the isolation, structure elucidation and antimicrobial activities of these compounds.
Fig. 1.
Chemical structures of compounds 1–6
Results and Discussion
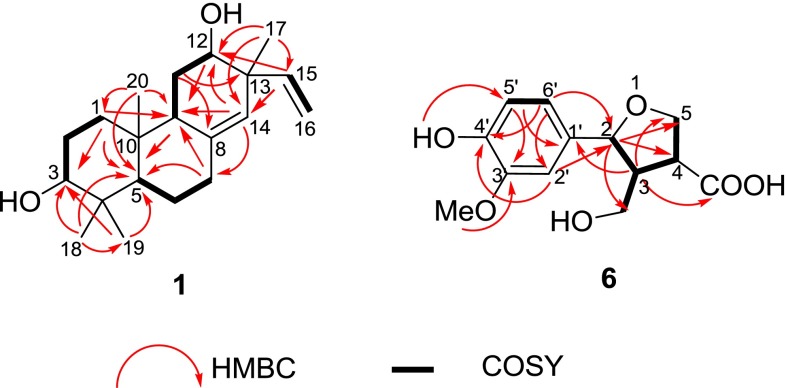
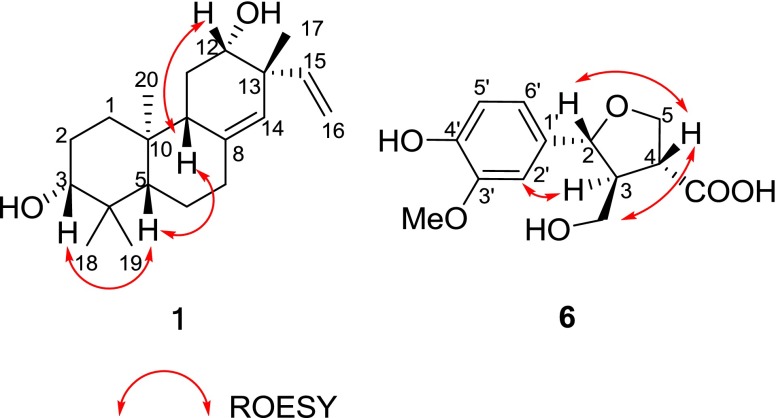
Maniesculentin A (1) was isolated as a white, amorphous powder. Its molecular formula C20H32O2 was determined by HREIMS ([M]+ m/z 304.2408, calcd. 304.2402), indicating five degrees of unsaturation. The IR absorption signals revealed the presence of hydroxyl (3430 cm−1) and olefinic (1634 cm−1) groups. The 1H NMR spectrum (Table 1) of 1 exhibited four angular methyl group (δ H 0.74, 0.82, 1.01 and 1.07), two oxygenated methines [δ H 3.26 (1H, dd, J = 11.4 and 4.1 Hz), 3.63 (1H, s); δ C 78.8, 72.7], one trisubstituted double bond [δ H (5.07, s); δ C 138.1, 125.7], and one terminal double bond [δ H 5.01(1H, d, J = 17.6 Hz), 5.00 (1H, d, J = 10.0 Hz), 5.74 (1H, dd, J = 17.6, 10.0 Hz); δ C 146.1, 114.2], which were supported by HSQC and HMBC experiments (Fig. 2). The above information suggested that compound 1 should be an ent-pimarane-type diterpenoid. Detailed analysis of NMR data indicated that 1 was an isomer of 2 [10, 11], as indicated by the significant variation of 13C NMR signals at δ C 25.9 for C-11, 43.7 for C-13 and 23.5 for Me-17 in 1 instead of δ C 36.8 for C-11, 47.5 for C-13 and 26.0 for Me-17 in 2, suggesting the β–orientation for 12-OH. In addition, the ROESY correlation (Fig. 3) of H-9/H-12 further indicated β–orientation for 12-OH. The above elucidation was further confirmed by 2D NMR (HSQC, 1H-1H COSY, and HMBC) (Fig. 2). Firstly, the 1H-1H COSY (Fig. 2) revealed the presence of partial structures of –CH2-CH2-CH (O)-, -CH-CH2-CH2- and –CH2-CH (O)-, as shown with bold lines in Fig. 2. Secondly, the HBMC correlations (Fig. 2) of both two methyl prontons H3-19 (δ H 0.82) and H3-18 (δ H 1.02) with C-3 (δ C)/C-5 (δ C 54.1)/C-4 (δ C 38.9); the other two methyl prontons H3-20 (δ H 0.74), and H3-17 (δ H 1.07) with C-1 (δ C 36.7)/C-5 (δ C 54.1)/C-9 (δ C 46.3)/C-10 (δ C 38.2), and C-12 (δ C 72.7)/C-13 (δ C 43.7)/C-14 (δ C 125.7), respectively; the H-7 (δ H 2.37 and 3.10) with C-5 (δ C 54.1)/C-10 (δ C 38.2)/C-14 (δ C 125.7); and the H-11 (δ H 1.65) with C-8 (δ C 138.1)/C-13 (δ C 43.7) constructed the typical fused A/B/C- ring system of the ent-pimarane-type diterpenoid core. The relative configuration of 3-OH was a–orientation determined by the ROESY correlation (Fig. 3) from H-3 to H-5 and the large coupling constant (dd, J = 11.4 and 4.1 Hz) of H-3 with H2-2 [15]. Thus, the structure of 1 was determined as 3a,12a-dihydroxy-pimara-8(14),15-dien.
Table 1.
NMR Data of 1 and 6 in CDCl3 (δ in ppm and J in Hz)
| No. | 1 | No. | 6 | ||
|---|---|---|---|---|---|
| δ H | δ C | δ H | δ C | ||
| 1 | 1.65 (m) | 36.7 | 2 | 4.62 (d, 7. 0) | 86.1 |
| 1.16 (td, 13.4, 3.6) | |||||
| 2 | 1.64 (m) | 27.4 | 3 | 3.13 (m) | 48.2 |
| 1.54 (m) | |||||
| 3 | 3.26 (dd, 11.4, 4.1) | 78.8 | 4 | 3.46 (td, 9.0, 3.9) | 46.0 |
| 4 | 38.9 | 5 | 4.37 (d, 9.0) | 69.8 | |
| 4.19 (dd, 9.0, 3.9) | |||||
| 5 | 1.09 (m) | 54.1 | 1′ | 130.5 | |
| 6 | 1.67 (m) | 22.2 | 2′ | 6.89 (br s) | 108.4 |
| 1.43 (dd, 13.0, 4.1) | |||||
| 7 | 2.37 (dd, 13.9, 5.0) | 35.4 | 3′ | 146.9 | |
| 2.10 (td, 13.9, 5.0) | |||||
| 8 | 138.1 | 4′ | 145.8 | ||
| 9 | 1.83 (t, 8.3) | 46.3 | 5′ | 6.91 (d, 8.1) | 114.4 |
| 10 | 38.2 | 6′ | 6.81 (dd, 8.1, 1.9) | 119.1 | |
| 11 | 1.68 (m) | 25.9 | CH2OH | 4.51 (dd, 9.8, 7.0) | 69.9 |
| 1.62 (m) | 4.33 (dd, 9.8, 1.9) | ||||
| 12 | 3.63 (s) | 72.7 | COOH | 178.6 | |
| 13 | 43.7 | OCH3 | 3.92 (s) | 55.9 | |
| 14 | 5.07 (s) | 125.7 | |||
| 15 | 5.74 (dd, 17.6, 10.0) | 146.1 | |||
| 16 | 5.01 (d, 17.6) | 114.2 | |||
| 5.00 (d, 10.0) | |||||
| 17 | 1.07 (s) | 23.5 | |||
| 18 | 1.02 (s) | 28.4 | |||
| 19 | 0.82 (s) | 15.6 | |||
| 20 | 0.74 (s) | 14.7 | |||
Fig. 2.
Key 1H-1H COSY and HMBC correlations of 1 and 6
Fig. 3.
Key ROESY correlations of 1 and 6
Maniesculentin B (6) was obtained as colorless oil. Its molecular formula was deduced to be C13H16O6 from the HREIMS at m/z 268.0943 (calcd. 268.0947), corresponding to six degrees of unsaturation. The IR spectrum showed absorption bands at 3442, 1764, 1631 and 1517 cm−1, indicating the presence of hydroxyl, carbonyl and aromatic groups, respectively. The 13C NMR and DEPT spectroscopic data (Table 1) exhibited 13 carbon signals, including one 1,3,4-trisubstituted aromatic ring (δ C 146.9, 145.8, 130.5, 19.1, 114.4, 108.4), one furan ring (δ C 86.1, 46.0, 48.2, 69.9), one hydroxymethyl (δ C 69.8), and one methoxy group (δ C 55.9). The 1H NMR spectrum also revealed characteristic signals of 1, 3, 4-trisubstituted aromatic ring [δ H 6.89 (br s, H-2), 6.91 (d, J = 8.1 Hz, H-5), 6.81 (br d, J = 8.1 Hz, H-6)]. The 1H NMR and 13C NMR spectra of 6 displayed similarity to the known lariciresinol, a furan methanol lignan isolated from Araucaria angustifolia [16]. The major difference is one carbonxyl group instead of 4′-hydroxy-3′-methoxyphenmethyl group at C-4 supported by the HMBC correlation (Fig. 2) from H-4 (δ H 3.46) to COOH (δ C 178.6). The hydroxymethy group was positioned at C-3 by means of the 1H–1H COSY correlation of H-3 (δ H 3.13) with the two protons of CH2OH (δ H 4.33 and 4.51), and the HMBC (Fig. 2) correlation from H-2 (δ H 4.62) to CH2OH (δ C 69.9). Addition, in the HMBC spectrum (Fig. 2), the correlations from OH (δ H 5.63) to C-5′ (δ C 114.4) and OMe (δ C 55.9) to C-3′ (δ C 146.9) indicated that OH and OMe were connected to C-4′ and C-3, respectively. On the basis of the above information, the planar structure of 6 was further confirmed in Fig. 1.
The ROESY correlation (Fig. 3) between H-2 and H-4 manifested that H-2 and H-4 were in the same side (assigned as β-orientation). The relative configuration of H-3 was a-orientation determined by coupling constants (J = 7.0 Hz), which was similar to the literature [17]. In addition, the ROESY correlations (Fig. 3) of CH2OH/H-4 and H-3/H-2′ further confirmed the relative configuration of H-3. Consequently, compound 6 was determined to be tetrahydro-2a-(4′-hydroxy-3′-methoxyphenyl)- 4a-carbonxyl-3b- hydroxymethyl furan (6), and named as maniesculentin B.
Maniesculentins A (1) and B (6) were assayed for antibacterial activicity against four bacteria lines (Staphylococcus aureus, Streptococcus, Escherichia.coli, Pseudomonas aeruginosa) by double–dilution [18, 19]. The results of antibacterial activity showed that the two new compounds were inactive against four tested bacteria lines.
Experimental Part
General Experimental Procedures
Optical rotations were detected with a JASCO P-1020 digital polarimeter. UV spectra were recorded on a Shimadzu UV–2401 PC spectrophotometer. IR spectra were scanned with Bruker Tensor–27 infrared spectrometer with a KBr disk. 1D and 2D NMR spectra were measured on Bruker AM–400, DRX–600 spectrometer using TMS as internal standard. MS and HREIMS spectra were carried out on Brucker HCT/E squire and Waters Autospec Premier P776 spectrum. HPLC analysis was performed on an Agilent 1100 liquid chromatograph equipped with a Waters X-Bridge C18 column (4.6 × 250 mm, 5 μm) with a flow rate of 3.0 mL/min, detected by a DAD detector. Column chromatography was carried out on silica gel (200–300 and 300–400 mesh; Qingdao Marine Chemical, Inc., Qingdao, P. R. China) and Sephadex LH–20 (40–70 μm, Amersham Pharmacia Biotech AB, Uppsala, Sweden). TLC spots were visualized under UV light and by dipping into 8 % H2SO4 in EtOH followed by heating.
Plant Material
The stems of M. esculenta Crantz were collected from Xishuangbanna, Yunnan Province, People’s Republic of China, in June 2012. The plant was authenticated by Mr. Yu Chen (Kunming Institute of Botany, Chinese Academy of Sciences). A voucher specimen (No. H201206162) was deposited at the Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences.
Extraction and Isolation
The air-dried and powdered stems of M. esculenta (13.0 kg) were extracted with 95 % aqueous EtOH (3 × 20 L) under reflux for three times (4, 3, and 3 h, respectively). The combined EtOH extracts were concentrated under vacuum to give a crude extract (750.0 g), which was suspended in water and then partitioned with EtOAc. The EtOAc extract (140.0 g) was subjected to a silica gel column, eluted with petroleum ether-ethyl acetate (from 9:1 to 5:5) and then eluted with chloroform–methanol (from 9:1 to 8:2) to yield seven fractions (A–J). Fr. C (30.0 g) was separated over an MCI–gel column (MeOH/H2O from 4:6 to 10:0) to obtain four fractions (Fr. C1–C4). Fr. C3 (800.0 mg) was chromatographed on Sephadex LH–20 (MeOH) to obtain Fr. C3A (400.0 mg), which was further purified by a silica gel column (petroleum ether–acetone, 9:1) to obtain Fr. C3A2 (200.0 mg).
Fr. C3A2 was further purified by Semi–preparative HPLC using a Waters X-bridge C18 (4.6 × 250 mm, 5 μm) column with 40 % MeOH/H2O to obtain compound 1 (10.0 mg) and 2 (16.0 mg). Fr.C2 (600.0 mg) was purified using Sephadex LH-20 (CHCl3–MeOH, 1:1) and then by the Waters X-bridge C18 (4.6 × 250 mm, 5 μm) column with 60 % MeOH/H2O to afford compounds 3 (8.0 mg), 4 (5.0 mg), 5 (20.0 mg). Fr.C1 (102.0 mg) was chromatographed on Sephadex LH–20 (MeOH) to obtain Fr. C1A (56.0 mg), and further purified by a silica gel column (CHCl3) to obtain compound 6 (9.0 mg).
Maniesculentins (1)
Amorphous powder. [a]23D = −51. 7 (c = 0.40, CHCl3), UV (MeOH) λ max (log ε) 204 (2.08); IR (KBr) ν max 3443, 1633, 1456, 1385, 1179, 1038, 596 cm−1; 1H NMR and 13C NMR data, see (Table 1); positive ESIMS m/z 305 [M + H]+; HREIMS m/z 304.2408 [M]+ (calcd for C13H16O6, 304.2402).
Maniesculentins B (6)
Colorless oil. [a]23D = + 14. 7 (c = 0.60, CHCl3), UV (CHCl3) λ max (log ε) 280 (2.39), 240 (2.33); IR (KBr) ν max 3442, 1764, 1631, 1517, 1384, 1277, 1038, 575 cm−1; 1H NMR and 13C NMR data, see (Table 1); negative ESIMS m/z 267 [M-H]−; HREIMS m/z 268.0943 [M]+ (calcd for C13H16O6, 268.0943).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported financially by National Natural Science Foundation of China (31170332), and the Candidates of the Young Academic and Technical Leaders of Yunnan Province (2010CI047). We thank Prof. Guo Ying Zuo′s group, Research Center of Natural Medicine, Clinical School of Kunming General Hospital of Chengdu Military Command, Kunming, China for bioassay.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Xiao-Jiang Hao, Email: haoxj@mail.kib.ac.cn.
Hong-Ping He, Email: hehongping@mail.kib.ac.cn, Email: hehongping@yahoo.com.
References
- 1.Gao S, Liu HY, Wang YH, He HP, Wang JS, Di YT, Li CS, Fang X, Hao XJ. Org. Lett. 2007;9:3453–3455. doi: 10.1021/ol701501p. [DOI] [PubMed] [Google Scholar]
- 2.Tang GH, Zhang Yu, Yuan CM, Li Y, Gu YC, Di YT, Wang YH, Zuo GY, Li SF, Li SL, He HP, Hao XJ. J. Nat. Prod. 2012;75:1962–1966. doi: 10.1021/np3006315. [DOI] [PubMed] [Google Scholar]
- 3.Li SF, Di YT, Li SL, Zhang Y, Yang FM, Sun QY, Simo JM, He HP, Hao XJ. J. Nat. Prod. 2011;74:464–469. doi: 10.1021/np1006444. [DOI] [PubMed] [Google Scholar]
- 4.Özbilgin S, Citoğlu GS. Turk J. Pharm. Sci. 2012;9:241–256. [Google Scholar]
- 5.Bittner M, Alarcon J, Aqueveque P, Becerra J, Hernandez V, Hoeneisen M, Silva YM. Bol. Soc. Chil. Quim. 2001;46:419–432. [Google Scholar]
- 6.Wang Q, Zhang DY, Wu XM. J. Pharm. Univ. 2009;40:474–480. [Google Scholar]
- 7.Liu JQ, Yang YF, Li XY, Liu EQ, Li ZR, Zhou L, Li Y, Qiu MH. Phytochemistry. 2013;96:265–272. doi: 10.1016/j.phytochem.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Editorial Committee Flora of China . Chinese Academy of Sciences. Beijing: Flora of China Science Press; 2008. pp. 275–276. [Google Scholar]
- 9.Sakai T, Nakagawa Y, Uritani I, Data ES. Agric. Biol. Chem. 1986;50:2905–2907. doi: 10.1271/bbb1961.50.2905. [DOI] [Google Scholar]
- 10.Li SS, Dai HF, Zhao YX, Zuo WJ, Li XN, Mei WL. J. Trop. Subtrop. Bot. 2012;20:197–200. [Google Scholar]
- 11.Tsutomu S, Yoshiko N. Phytochemistry. 1988;27:3769–3779. doi: 10.1016/0031-9422(88)83015-2. [DOI] [Google Scholar]
- 12.Grace MH, Jin YH, Wilson GR, Coates RM. Phytochemistry. 2006;67:1708–1715. doi: 10.1016/j.phytochem.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Liu G, Mvller R, Rvedi P. Helv. Chim. Acta. 2003;86:420–438. doi: 10.1002/hlca.200390043. [DOI] [Google Scholar]
- 14.Dou DQ, Tian F, Qiu YK, Xiang Z, Xu BX, Kang TG, Dong F. Nat. Prod. Res. 2010;24:40–47. doi: 10.1080/14786410802393621. [DOI] [PubMed] [Google Scholar]
- 15.Denton RW, Harding WW, Anderson CI, Jacobs H, McLean S, Reynolds WF. J. Nat. Prod. 2001;64:829–831. doi: 10.1021/np010098a. [DOI] [PubMed] [Google Scholar]
- 16.Fonseca SF, Cambello PI, Barata LES, Rubeda EA. Phytochemistry. 1978;17:499–502. doi: 10.1016/S0031-9422(00)89347-4. [DOI] [Google Scholar]
- 17.Junei K, Hiroyuki H, Katsura F, Toshihiro N. Chem. Pharm. Bull. 1991;39:2952–2955. doi: 10.1248/cpb.39.2952. [DOI] [Google Scholar]
- 18.Zuo GY, Meng FY, Hao XY, Zhang YL, Wang GC, Xu GL. J. Pharm. Pharm. Sci. 2008;11:90–94. doi: 10.18433/j3d30q. [DOI] [PubMed] [Google Scholar]
- 19.Tang GH, Zhang Y, Gu YC, Li SF, Di YT, Wang YH, Yang CX, Zuo GY, Li SL, He HP, Hao XJ. J. Nat. Prod. 2012;75:996–1000. doi: 10.1021/np3001128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.