Abstract
A study has been done to find out the prevalence of different kinds of parasites in Cirrhinus mrigala (Hamilton 1822) during 2010–2011. It has been found that the temperature variation affects some parasitic infestation over the fish species. It was found that ciliophoran and crustacean parasites are more prevalent from November to February whereas the myxozoan and monogenean parasites are more prevalent from January to April. Considering temperature variation throughout the year it has been inferred that most of the parasitic infections were found between November and April when the temperature range varies from 19 to 26 °C. So from the study it can be concluded that lower temperature elicits the parasitic growth in fishes while the higher temperature retards the growth.
Keywords: Prevalence, Parasites, Temperature variation
Introduction
Aquaculture is one of the most economically important applied strategies all over the world. Fishes are one of the most beneficial food and nutritional resources to human and other organism. But this fishes are facing various risks factors responsible for fish diseases due to infection of the microorganisms such as fungi, bacteria, virus and protozoa (Kabata 1985). Besides, helminth and crustacean parasites causes fatal diseases to the fishes (Smyth 1994). Amongst the protozoan parasites, Myxozoa and Ciliophora cause serious diseases in fishes (Lom 1960). The parasite community of fish shows considerable variation with the environmental conditions in which fish live (Hossain et al. 2008). During the study it has been found that the ciliates belonging to the genus Trichodina, Tripartiella, and Ichthyophthirius are more prevalent from November to February whereas, the myxozoan such as Myxobolus, Thelohanellus showed higher prevalence from January to April. Another myxozoan parasite, Henneguya sp. occurred in low concentration throughout the year. It has also been detected that helminth infections caused by Gyrodactylus and Dactylogyrus found all over the body surface and tail fin region. These parasites infect the fish and cause massive destruction to the skin and gills epithelium.
Materials and methods
The host fishes have been collected from the fish farms, lakes and ponds of West Bengal and brought alive to the laboratory. The fishes were cultured in the small cemented tanks along with some water plants for giving them natural nutritional support. During June 2010 to May 2011, more than 300 fishes were randomly collected for the study. The infected fishes were collected and examined in every month of the year. The smears of gills, body, and caudal fin were prepared on grease free clean slides with a drop of 0.5 % NaCl solution and air dried. The Indian ink method of Lom and Vavrá (1963) was followed to identify the myxozoan spore and for permanent preparation, the air dried smears were stained with Giemsa. The ciliophoran parasites were subjected to silver impregnation after the method of Klein (1958). The monogenean parasites were processed following the method of Yamaguti (1963).The crustacean parasites were stained with cotton blue stain for permanent preparation.
The months were divided into six groups namely, July–August, September–October, November–December, January–February, March–April, May–June. The prevalence was calculated as number of infested fish divided by number of observed fish multiplied by hundred. The three main water quality parameters like, water temperature; pH and dissolved oxygen were measured in each month. For the analysis of dissolved oxygen, water was collected from column region in DO bottles and fixed with MnSO4 and alkaline KI. Water temperature was measured by mercury thermometer and pH by Pen pH meter.
Result
The infected fishes were recognized by their irritating and sluggish movement. Heavily infected fishes showed reddish appearance and white spots appeared throughout their body surface including the gills.
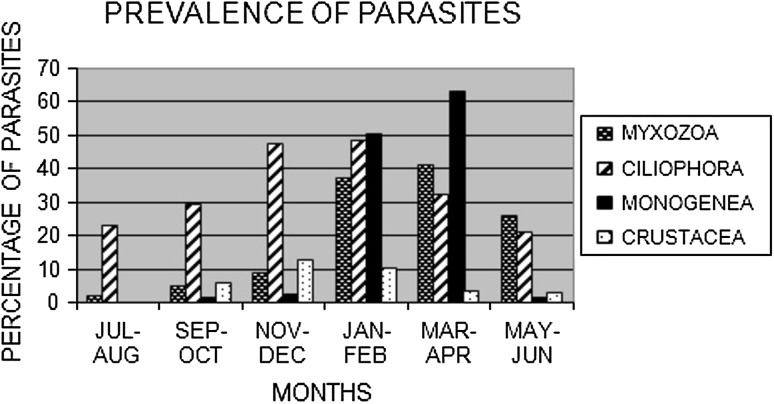
It was found that myxozoan and monogenean parasites were most prevalent during March and April whereas the ciliophorans were affecting the fishes throughout the year but mostly during the winter (November–February). Crustacean parasites were prevalent during November–December but their intensity was less.
Among the myxozoan parasite, Myxobolus infection were mostly found in May–June whereas, Thelohanellus was found in March and April but Henneguya was found to be very less throughout the year (Table 1). Among the ciliophorans, Tripertiella infection were most prevalent in November–December whereas Ichthyophthirius infection was prevalent in January–February. However, Trichodina was found to be less in number in fishes (Table 1). Between the two monogeneans, Dactylogyrus is more prevalent in infecting fishes than that of Gyrodactylus and found mainly from January to April (Table 2). It was reported that Dactylogyrus caused mass mortality in fishes (Subashinghe 1992; Shariff and Vijiarungam 1986). Both the crustaceans were found to be very less in number throughout the year but the prevalence of Ergasilus sp. was slightly higher than Argulus sp. (Table 1). The water temperature, pH and dissolved oxygen are the major contributory factors for transmission as they fluctuate more rapidly(Table 3). But ciliophoran except Ichthyophthirius multifiliis were encountered in sampled fish specimens more or less throughout the year and mainly found when the temperature dropped below 21 °C (Fig. 1). During the study the crustacean parasites like Ergasilus sp. and Argulus sp. have also been isolated. Its prevalence was found to be lower throughout the year. It was observed that the prevalence of these parasites was more in the winter season. This work corroborated with the work of Ahmed et al. (1991).
Table 1.
Prevalence of different parasites in Cirrhinus mrigala
| Months | Myxozoans | Prevalence (%) | Ciliophorans | Prevalence (%) | Monogeneans | Prevalence (%) | Crustacean | Prevalence (%) |
|---|---|---|---|---|---|---|---|---|
| July–August 2010 | Myxobolus sp. | 05 | Trichodina sp. | 10 | Dactylogyrus sp. Diesing, 1850 | 0 | Ergasilus sp. | 0 |
| Thelohanellus sp. | 0 | Tripertiella sp. | 58 | Gyrodactylus sp. | 0 | Argulus sp. | 0 | |
| Henneguya sp. | 0 | Ichthyophthirius multifilis. | 0 | |||||
| September–October 2010 | Myxobolus sp. | 12 | Trichodina sp. | 11 | Dactylogyrus sp. | 03 | Ergasilus sp. | 09 |
| Thelohanellus sp. | 02 | Tripertiella sp. | 62 | Gyrodactylus sp. | 0 | Argulus sp. | 02 | |
| Henneguya sp. | 0 | Ichthyophthirius multifilis. | 14 | |||||
| November–December 2010 | Myxobolus sp. | 20 | Trichodina sp. | 15 | Dactylogyrus sp. | 05 | Ergasilus sp. | 20 |
| Thelohanellus sp. | 05 | Tripertiella sp. | 82 | Gyrodactylus sp. | 0 | Argulus sp. | 5 | |
| Henneguya sp. | 0 | Ichthyophthirius multifilis. | 44 | |||||
| January–February 2011 | Myxobolus sp. | 66 | Trichodina sp. | 11 | Dactylogyrus sp. | 84 | Ergasilus sp. | 13 |
| Thelohanellus sp. | 45 | Tripertiella sp. | 55 | Gyrodactylus sp. | 17 | Argulus sp. | 07 | |
| Henneguya sp. | 0 | Ichthyophthirius multifilis. | 78 | |||||
| March–April 2011 | Myxobolus sp. | 62 | Trichodina sp. | 12 | Dactylogyrus sp. | 82 | Ergasilus sp. | 06 |
| Thelohanellus sp. | 56 | Tripertiella sp. | 66 | Gyrodactylus sp. | 44 | Argulus sp. | 0 | |
| Henneguya sp. | 05 | Ichthyophthirius multifilis. | 08 | |||||
| May–June 2011 | Myxobolus sp. | 68 | Trichodina sp. | 23 | Dactylogyrus sp. | 03 | Ergasilus sp. | 03 |
| Thelohanellus sp. | 07 | Tripertiella sp. | 40 | Gyrodactylus sp. | 0 | Argulus sp. | 02 | |
| Henneguya sp. | 02 | Ichthyophthirius multifilis. | 0 | |||||
| Average in total year | Myxozoans | Ciliophorans | Monogeneans | Crustacean |
Table 2.
Monthly prevalence (%) of ectoparasites of Cirrhinus mrigala
| Parasites | July–August 2010 | September–October 2010 | November–December 2010 | January–February 2011 | March–April 2011 | May–June 2011 |
|---|---|---|---|---|---|---|
| Myxozoans | 1.67 | 4.67 | 8.33 | 37 | 41 | 25.67 |
| Ciliophorans | 22.67 | 29 | 47 | 48 | 32 | 21 |
| Monogeneans | 0 | 1.5 | 2.5 | 50.5 | 63 | 1.5 |
| Crustaceans | 0 | 5.5 | 12.5 | 10 | 3 | 2.5 |
| Average of total parasitic prevalence | 6.09 | 10.17 | 17.58 | 36.38 | 34.75 | 12.67 |
Table 3.
Monthly fluctuations of water quality parameters in nursery pond
| Months | Water temp. (°C) | DO (ppm) | pH |
|---|---|---|---|
| July–August 2010 | 29 | 5.7 | 6.4 |
| September–October 2010 | 26 | 6.6 | 7.2 |
| November–December 2010 | 21 | 6.7 | 7.5 |
| January–February 2011 | 19 | 5.8 | 7.1 |
| March–April 2011 | 26 | 6.1 | 8.1 |
| May–June 2011 | 32 | 5.9 | 7.3 |
Fig. 1.
Graphical representation of prevalence of parasites throughout the year
Discussion
From the study it can be concluded that the myxozoan and monogenean parasites are found to be most frequent from January to April, when the temperature varies in between 19 and 26 °C (Fig. 1). But its number was reduced when the temperature increased. Thus it can be inferred that in high temperature the life cycle of these parasites is affected or mostly they remain in the dormant stage. So it can be inferred that the prevalence of the parasites are very much temperature dependent as most of the parasitic infection occurred during the winter, especially in January–February while minimum parasitic infection was recorded during rainy season (July–August). Hossain et al. (2008) also viewed that parasite community showed considerable variation with the environmental condition they live.
Acknowledgments
Authors (SM and SP) are thankful to the Department of Science and Technology, Govt. of India, New Delhi and University of Kalyani for financial support.
References
- Ahmed A, Ali SMK, Samad A. Probable cause of fish ulcer in Bangladesh. Nutri News. 1991;14(1):3. [Google Scholar]
- Hossain MD, Hossain MK, Rahaman MH, Akter A, Khanom DA. Prevalence of ectoparasites of carp fingerlings at Santaher, Bogra. Univ J Zool Rajshahi Univ. 2008;27:17–19. [Google Scholar]
- Kabata Z. Parasites and diseases of fish cultured in the tropics. London and Philadelphia: Taylor and Francis; 1985. [Google Scholar]
- Klein BM. The “dry” silver methods and its proper use. J Protozool. 1958;5:99–103. doi: 10.1111/j.1550-7408.1958.tb02535.x. [DOI] [Google Scholar]
- Lom J. Trichodina reticulata Hirschmann and Partsch 1955 from Crucian carp, and T. domergueif. latispina Dogel 1940 from Diaptomus. Acta Societatis Zologicke. 1960;3:246–257. [Google Scholar]
- Lom J, Vavrá J. Mucous envelope of spores of the subphylum Cnidospora (Deflein, 1901) Vist Esl Spol Zool. 1963;27:4–6. [Google Scholar]
- Shariff M, Vijiarungam A (1986) Occurrence of parasites at the fish breeding station in peninsular Malaysia and their control. In: Chan HH, Ang KJ, Law AT et al (ed) internatinal conference on development and management of tropical living aquatic resources, Universiti Pertanian Malaysia, 2–5 August 1983. pp 68–73
- Smyth JD. Introduction to animal parasitology. 3. Cambridge: Cambridge university press; 1994. [Google Scholar]
- Subashinghe RP. Hatchery disease of fresh water fishes in Srilanka. In: Sharif M, Subashinghe RP, Arthur JR, editors. Diseases in Asian aquaculture. 1. Philippines: Asian Fisheries Society; 1992. [Google Scholar]
- Yamaguti S. Systema Helminthum vol. Acanthocephala of vertebrates. New York: Interscience; 1963. [Google Scholar]