Abstract
Tropical bovine theileriosis, a tick borne disease, caused by, Theileria annulata with marked clinical signs of pyrexia (102–105 °F), enlargement of lymphnodes etc., causes heavy economic losses in terms of high mortality and morbidity rates. Diagnosis of theileriosis is mainly based on clinical symptoms and microscopic examination of stained blood smears and lymph node biopsy smears but limitations of these methods against Theileria sp. limits the specificity. Hence, to overcome the limitations, the present study reports the detection of T. annulata in blood samples of cattle by polymerase chain reaction. The study was conducted on 155 cattle having typical clinical symptoms and blood smear after staining with Giemsa stain was examined for the presence of T. annulata in RBC. The Primer sequences were used as per d’Oliveira et al. The assay employs primers specific for the gene encoding the 30-kDa major merozoite surface antigen of T. annulata and the amplification of 721 bp was done. Out of the total 155 animals, 34 were positive for T. annulata by blood smear method whereas 134 samples were positive by PCR. So diagnosis of blood samples by PCR is found to be the most sensitive and specific methodology as compared to cytological blood smear examination. The sensitivity was 23.88 % and specificity was 90.47 % of blood smear method considering PCR as gold standard and it was found that PCR is more sensitive than the conventional method of examination.
Keywords: Tropical bovine theileriosis, Polymerase chain reaction, Diagnosis, Cattle
Introduction
Livestock sector plays a critical role in India’s rural population by contributing 9 % to Gross Domestic Product and employing 8 % of the labour force. Cattle and buffalo are the preponderant and the most interactive species amongst the livestock, hence the concept of cross breeding was adopted in order to improve the genetic makeup of the low producing indigeneous cattle but it increases the susceptibility of vector borne haemoprotozoan diseases.
Bovine Tropical Theileriosis, a tick borne disease, is caused, Theileria annulata (Brown 1997; Preston 2001) shows the clinical signs of pyrexia (102–105 °F), enlargement of lymphnodes, depression, anorexia, anaemia, drop in milk production, nasal and ocular discharges and dyspnoea (Soulsby 1982)The disease causes heavy economic losses in terms of high mortality and morbidity rates (Brown 1990).In India economic loss due to ticks and tick borne diseases in animal has been estimated to the tune of USD 498.7 million per annum and BTT alone contributes USD 384.3 million per annum (Minjauw and Mc Leod 2003).
Diagnosis of Theileriosis is mainly based on clinical symptoms and microscopic examination of stained blood and lymph node biopsy smears. But smear method is often associated with technical problems, wrong diagnosing and low sensitivity in diagnosing the carrier cows (Shayan and Rahbari 2005). Serological tests like immunofluorescent antibody test has limitations of cross-reactivity with antibodies directed against other Theileria sp.
In order to overcome the limitations in routinely available diagnostic method, the present study was designed for the molecular polymerase chain reaction based diagnosis of Bovine Tropical Theileriosis and its comparison with traditional blood smear examination.
Materials and methods
The study was conducted on 155 cattle with the history of high temperature (102–106 °F), anorexia, depression, anaemia, loss of milk production, haemorrhagic conjunctiva. Blood smear were taken from the tip of the ear and stained with Giemsa stain. The blood samples were also collected for molecular identification of T.annulata. The genomic DNA from the blood samples were extracted out as per the protocol of Sambrook and Russell (2001). The assay employs primers specific for the gene encoding the 30-kDa major merozoite surface antigen of T. annulata (d’Oliveira et al. 1995).
Forward and reverse primer
| Sr. no. | Primer | Sequence |
|---|---|---|
| 1. Forward primer | N516 | 5″GTAACCTTTAAAAACGT3″ |
| 2. Reverse primer | N517 | 3″GTTACGAACATGGGTTT5″ |
The PCR reaction was set up into 25 μl volume containing 12.5 μl PCR Master Mix (0.05/μl Taq DNA polymerase in reaction buffer, 4 mM Mgcl2, 0.4 mM dATP, 0.4 mM dCTP, 0.4 mM dGTP, and 0.4 mM dTTP), 1.5 μl of each primer (Forward and Reverse), 2 μl of the DNA and total volume was made up to 25 μl using Nuclease free water. The reaction conditions was initial denaturation at 94 °C for 1 min. followed by 30 cycles each consisting of individual cycle consisting of 94 °C for 45 s, 55 °C for 1 min, 72 °C for 1 min. with final extension at 72 °C for 5 min. The PCR amplicons were analyzed by agar gel electrophoresis in 1.5 % agarose gel. The specificity and sensitivity of blood smear examination was evaluated by 2 × 2 contingency table (Thapliyal and Mishra 1996) by considering PCR as a gold standard.
Results and discussion
Out of the total 155 animals, 34 were found to be positive for T. annulata by blood smear method whereas 134 samples were positive by PCR. In the present study it was found that the samples that were negative by the blood smear examination had given specific band by PCR. The samples positive for blood smear were also positive by PCR test, while out of 134 samples, 100 samples positive by PCR were not detected by the microscopic examination.
The sensitivity was found to be 23.88 % and specificity was 90.47 % of blood smear method and it was found that PCR is more sensitive than the conventional method of examination (Table 1). The present study is in agreement with Rady et al. (2010) and Odongo et al. (2010). Altay et al. (2005), Durrani et al. (2008), Azizi et al. (2008), Bami et al. (2009) also reported that PCR was more sensitive than the traditional method of blood smear examination who also used the same primers for detection of Theileria organisms in blood.
Table 1.
2 × 2 Contingency table for blood smear method and PCR
| Blood smear | PCR | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 32a | 2b | 34 |
| Negative | 102c | 19d | 121 |
| Total | 134 | 21 | 155 |
aTrue positive
bFalse positive
cFalse negative
dTrue negative
Statistical comparision of PCR and blood smear method had shown significant difference between these methods at 1 % level (Table 2). Azizi et al. (2008) reported that sensitivity and accuracy of PCR method in diagnosing of T. annulata carriers was more than common method of smear examination. Sanchez et al. (1999) verified the utility of this technique in epidemiological studies and compared it with conventional diagnostic techniques and reported that PCR was more sensitive and specific test for detection of T. annulata infections.
Table 2.
Comparison between blood smear examination and PCR
| Parameters | Animals screened | Positive for theileriosis | Negative for theileriosis |
|---|---|---|---|
| Blood smear | 155 | 34 | 121 |
| PCR | 155 | 134 | 21 |
Variability of prevalence of theileriosis between the two methods was found to be significant (P < 0.05, 0.01), Chi Square value is 129.94
The primer specific for the gene i.e. N516 and N517 was used to detect T. annulata and the amplification of 721 bp (Fig 1) was detected in all 134 cases. So diagnosis of blood samples by PCR is found to be the most sensitive and specific methodology as compared to cytological blood smear examination. Thus PCR will be useful to detect subclinical infection of Theileriosis in animals. In the field conditions the cattle having subclinical infection of T.annulata are the major source of infection to the ticks (Fig. 2). The subclinical infection is not diagnosed on blood smear examination whereas PCR detects very minute infection of T. annulata also, as evident in the present study and can be used as an excellent tool for the diagnosis of T. annulata infections in carrier cattle (Fig. 3).
Fig. 1.

Photomicrograph of heavy infection of erythrocytes with T. annulata (1000×)
Fig. 2.
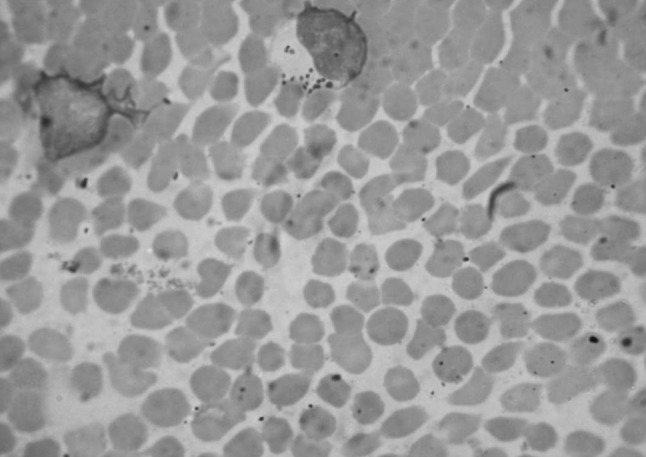
Photomicrograph of blood smear showing Koch’s blue body in lymphocytes (1000×). (Color figure online)
Fig. 3.
Agarose gel electrophoresis of amplified T. annulata DNA. Lane 1 100 bp DNA ladder Lane 2 Positive control. Lane 3 Negative control Lane 4–7 Positive samples. Lane 8 Negative sample
Acknowledgments
Authors acknowledge the HOD, Department of Veterinary Parasitology and Veterinary Pathology, Nagpur Veterinary College (NVC) for the materials and Associate Dean, for the facilities to carry out this study.
References
- Altay K, Nazir D, Patricia JH, Munir A. Detection of Theileria ovis in naturally infected sheep by nested PCR. Vet Parasitol. 2005;127:99–104. doi: 10.1016/j.vetpar.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Azizi H, Behroooz S, Amir FD, Fazlollah S, Camellia T. Detection of Theileria annulata by PCR and its comparision with smear method in native carrier cows. Biotechnology. 2008;7:1–4. doi: 10.3923/biotech.2008.1.9. [DOI] [Google Scholar]
- Bami MH, Khazraiinia P, Haddadzadeh HR, Kazemi B. Identification of Theileria species in sheep in the eastern half of Iran using nested PCR–RFLP and microscopic techniques. Iran J Vet Res. 2009;11(3):32. [Google Scholar]
- Brown GCD. Control of tropical theileriosis (Theileria annulata infection) of cattle. Parasitologia. 1990;32:23–31. [PubMed] [Google Scholar]
- Brown CGD. Dynamics and impact of tick borne diseases of cattle. Trop Anim Health Prod. 1997;29(Suppl 4):1–3. doi: 10.1007/BF02632905. [DOI] [PubMed] [Google Scholar]
- d’Oliveira C, Van-der Weide M, Habela A, Jacquiet P, Jongejan F. Detection of Theileria annulata in blood samples of carrier cattle by PCR. J Clin Microbiol. 1995;33(10):2665–2669. doi: 10.1128/jcm.33.10.2665-2669.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrani AZ, Ahmad M, Ashraf M, Khan MS, Khan JA, Kamal N, Mumtaz N. Prevalence of theileriosis in buffaloes and detection through blood smear examination and polymerase chain reaction test in district Lahore. J Anim Plant Sci. 2008;18:2–3. [Google Scholar]
- Minjauw B, Mc Leod A (2003) Tick-borne diseases and poverty: the impact of ticks and tick-borne diseases on the livelihood of small-scale and marginal livestock owners in India and eastern and southern Africa. Research report, DFID Animal Health Programme. 1–124
- Odongo OD, Jack DS, Henry KK. A nested PCR assay exhibits enhanced sensitivity for detection of Theileria parva infections in bovine blood samples from carrier animals. Parasitol Res. 2010;106:357–365. doi: 10.1007/s00436-009-1670-z. [DOI] [PubMed] [Google Scholar]
- Preston PM. The encyclopedia of arthropod transmitted infections. 1. Wallingford: CABI Publishing; 2001. pp. 487–504. [Google Scholar]
- Rady AA, Laila SA, Amr M, Amira AH. Using polymerase chain reaction (PCR) for diagnosis of bovine theileriosis in upper Egypt. IJAVMS. 2010;4(3):67–74. [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3. New York: Cold Spring Harbour Laboratory Press; 2001. [Google Scholar]
- Sanchez JM, Viseras J, Adroher FJ, Garcia FP. Nested polymerase chain reaction for detection of Theileria annulata and comparison with conventional diagnostic techniques: its use in epidemiology studies. Parasitol Res. 1999;85:243–245. doi: 10.1007/s004360050541. [DOI] [PubMed] [Google Scholar]
- Shayan P, Rahbari S. Simultaneous differentiation between Theileria sp. and Babesia sp. on stained blood smears using PCR. Parasitol Res. 2005;97:281–286. doi: 10.1007/s00436-005-1434-3. [DOI] [PubMed] [Google Scholar]
- Soulsby EJL. Helminths, artropods and protozoa of domesticated animals. 7. London: ELBS, Bailliere Tindall; 1982. [Google Scholar]
- Thapliyal DC, Mishra DS. Fundamentals of animal hygiene and epidemiology. 1. Charbagh: International Book Distributing. Co; 1996. p. 92. [Google Scholar]



