Abstract
Amitraz has become one of the most extensively used chemical acaricide for control of cattle tick due to development of resistance against most of the organophosphates and synthetic pyrethroid acaricides. The resistance status of amitraz was evaluated against Rhipicephalus (Boophilus) microplus collected from Banaskantha district, Gujarat, India by adult immersion test (AIT). The different concentrations of amitraz utilized in the AIT were 125, 250, 500, 750 and 1,000 ppm. The adult female ticks showed an upward trend in the mortality percentage with increase in drug concentration. The regression graph of probit mortality of ticks plotted against log values of progressively increasing concentrations of amitraz was utilized for the determination of slope of mortality which was 1.868 ± 0.2068. The lethal concentration (LC95) was calculated as 3098.2 ppm and the RF was 24.78 which indicated level II resistance status. The dose response curves for egg masses, reproductive index and inhibition of oviposition of R. (B.) microplus were also validated and the slope was −0.5165 ± 0.08287, −0.1328 ± 0.04472 and 24.22 ± 8.160, respectively. The current study appears to be the pioneer report of amitraz resistance in R. (B.) microplus from India and the data generated could be of immense help to develop effective control strategies against ticks.
Keywords: Amitraz, Resistance, Rhipicephalus (Boophilus) microplus
Introduction
Ticks and tick borne diseases are a major constraint to livestock health in many parts of the world including India where Rhipicephalus (Boophilus) microplus is widely prevalent and most economically important tick infesting dairy animals (Ghosh et al. 2007). The losses caused by this tick to the cattle industry is a combination of both direct and indirect effects. Direct effects on production include skin damage from tick bites (Biswas 2003), blood loss, toxicity from the bites, reduced animal weight gain and milk production (Sajid et al. 2007), whereas the indirect effects are related to the transmission of tick borne diseases like babesiosis and anaplasmosis (Rodriguez-Vivas et al. 2004).
Acaricides have played a pivotal role in the control of R. (B.) microplus but its indiscriminate and incessant use with improper concentrations has probably contributed to the development of resistance to most of the acaricides in several countries (FAO 2004). There are several reports available on acaricidal resistance in R. (B.) microplus from various parts of the country (Chaudhary and Naithani 1964; Basu and Haldar 1997; Singh et al. 2010; 2012). Recently, large scale resistance to organophosphate (OP) compound diazinon (Kumar et al. 2011) and synthetic pyrethroids (SP) particularly cypermethrin and deltamethrin (Sharma et al. 2012) has been experimentally validated in Indian isolates of R. (B.) microplus. The development of resistance against OP and SP acaricides have increased the dependency of farmers on formamidine (amitraz) and hence increased its usage many fold in recent past. However, as reports of development of amitraz resistance in R. (B.) microplus are unavailable from this part of the world, the current study was undertaken to detect amitraz resistance in R. (B.) microplus.
Materials and methods
Study area
Live engorged adult female R. (B.) microplus ticks were collect from sheds of dairy animals comprising cross bred cattle as well as buffaloes from Banaskantha district, Gujarat, India in August, 2012. Besides, uniform tick infestation pattern, easy accessibility to these areas encouraged to select the regions. Also data related to frequency, type and mode of acaricide treatment adopted by the owners and their experiences about the commonly used acaricides efficacy was recorded. The ticks were collected in vials, closed with muslin cloth to allow air and moisture exchange, brought to the Entomology Laboratory, Department of Veterinary Parasitology, GADVASU, Ludhiana, cleaned, labelled and kept at 28 ± 1 °C and 85 ± 5 % relative humidity.
Acaricide
Technical grade amitraz 100 % pure (AccuStandard® Inc. U.S.A) was used to prepare the stock solution in methanol. For the experimental bioassay, different concentrations of the amitraz was prepared in distilled water from the stock solution and tested against the field isolates of R. (B.) microplus.
Adult immersion test (AIT)
AIT was conducted according to the method of Sharma et al. (2012) with minor modifications. Briefly, the pre weighed engorged females of R. (B.) microplus were immersed in different concentrations of amitraz (125, 250, 500, 750 and 1,000 ppm) for 2 min and then dried on filter paper before transferring into the petri dishes. After 24 h, ticks were transferred to the glass tubes covered with muslin cloth and were kept in desiccators kept in BOD incubator maintained at 28 ± 1 °C and 85 ± 5 % RH. The ticks which did not oviposit even after 14 days post treatment were considered as dead. The control group was treated in similar manner in distilled water. Each concentration was replicated twice and ten adults were used per replication and the following parameters were compared:
Mortality: recorded up to 14 days post treatment (dpt).
The egg masses laid by the live ticks.
Reproductive index (RI) = egg mass wt (EW)/engorged female wt.
Percentage inhibition of oviposition (IO%) = [(RI control − RI treated)/RI control × 100].
Dose response data were analyzed by probit method (Finney 1962) using Graph Pad Prism 4 software. The LC95 value of amitraz was determined by applying regression equation analysis to the probit transformed data of mortality.
Resistance diagnosis in field isolates
Resistance factors (RF) were worked out by the quotient between LC95 of field isolates and LC95 of susceptible isolate (half the recommended dose of amitraz as discriminating dose is twice the LC95). On the basis of RF, the resistance status of R. (B.) microplus was classified as susceptible (RF < 1.4), level I resistant (RF = 1.5 − 5.0), level II resistant (RF = 5.1 − 25.0), level III resistant (RF = 25.1 − 40) and level IV resistance (RF > 40.1) as per Sharma et al. (2012).
Results and discussion
Data on the effects of various concentration of amitraz on R. (B.) microplus is presented in Table 1. The mortality of ticks was found to increase with increasing concentrations of amitraz and maximum mortality of 80.0 % was recorded at 1,000 ppm. It was observed that exposure of ticks to the concentration at which amitraz is being widely used in field condition in India (250 ppm) could only achieve 30.0 % mortality and even much higher concentration of 1,000 ppm failed to produce 100 % mortality, thus indicating development of resistance against amitraz in these ticks.
Table 1.
Dose dependent response of amitraz against R. (B.) microplus collected from Gujarat
| Conc. (ppm) | No. of ticks | Weight (mg) (mean ± SE) | No. dead 14 dpt (mortality %) | EW (mg) | RI (egg mass/live tick wt) | IO% |
|---|---|---|---|---|---|---|
| 125 | 20 | 87.91 ± 4.33 | 4 (20) | 0.541 | 0.374 | 31.75 |
| 250 | 20 | 90.30 ± 2.93 | 6 (30) | 0.504 | 0.395 | 27.92 |
| 500 | 20 | 93.05 ± 4.07 | 10 (50) | 0.334 | 0.351 | 35.94 |
| 750 | 20 | 86.32 ± 4.02 | 14 (70) | 0.166 | 0.295 | 46.16 |
| 1000 | 20 | 89.70 ± 4.26 | 16 (80) | 0.099 | 0.259 | 52.73 |
| Control | 20 | 90.83 ± 3.77 | 0 | 0.996 | 0.548 | 0.0 |
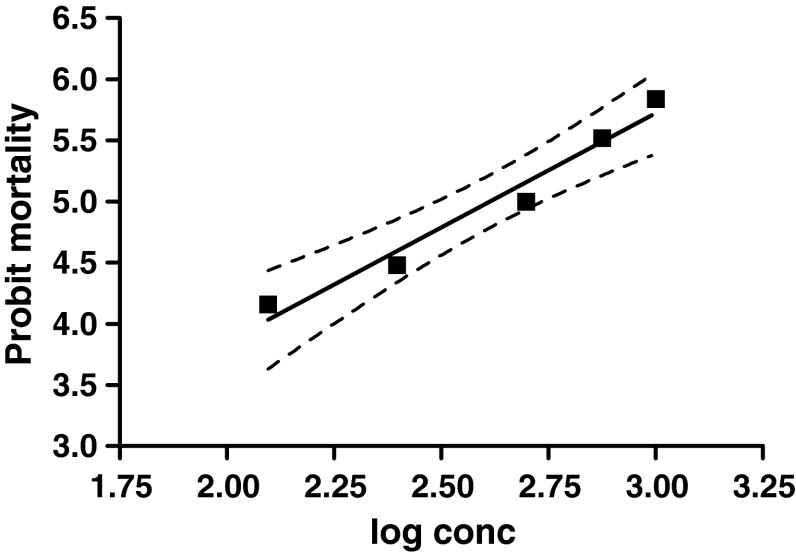
The regression graph of probit mortality of ticks plotted against log values of progressively increasing concentrations of amitraz is shown in Fig. 1. The dotted lines in the regression curve represented the 95 % confidence limits. The slope of mortality was 1.868 ± 0.2068 whereas, the value of goodness of fit (R2) was 0.9645. From the regression equation the LC95 value of amitraz was calculated as 3098.2 ppm and the RF was 24.78 which indicated level II resistance status.
Fig. 1.
Dose mortality curve of R. (B.) microplus against amitraz
The dose response curves for egg masses, RI and IO% of R. (B.) microplus were also validated by AIT. The egg mass of ticks treated with different concentrations of amitraz decreased with increasing concentrations of drug and the variation was statistically significant (p = 0.0083). The slope of egg mass was negative (−0.5165 ± 0.08287) because with the increasing concentrations of acaricide the ticks died. The RI of ticks treated with different concentrations of amitraz decreased with increasing concentrations of drug and the slope was negative (−0.1328 ± 0.04472). This indicated that the increase in concentration of the acaricide may have not caused 100 % mortality in ticks but the survived ticks showed decrease in their efficiency to convert the live weight into egg mass. Further, there was an increase in the IO% in ticks with increase in drug concentration and thus a positive slope (24.22 ± 8.160) was recorded.
In bioassays, technical grade amitraz was selected over commercial formulation as commercial products are prepared with many proprietary ingredients and it is difficult to assess the responses due to active ingredients (Shaw 1966). The stock solutions were prepared by dissolving in 100 % methanol and the working concentrations were prepared with distilled water. Use of organic solvents facilitates adsorption of compound over the surface area of target biological materials and also enhances penetration of active ingredients of the acaricide across the exoskeleton (Sharma et al. 2012).
Among the various acaricides used in India for the control of ticks in livestock, resistance has been reported against most of the acaricides in R. (B.) microplus (Chaudhary and Naithani 1964; Basu and Haldar 1997; Singh et al. 2010, 2012; Kumar et al. 2011; Sharma et al. 2012). Reports of resistance against amitraz are available against R. (B.) microplus ticks from various parts of world (Jonsson et al. 2000; Li et al. 2004; Veiga et al. 2012) but reports of amitraz resistance in R. (B.) microplus is currently not available from India. Further, resistance against amitraz has not been reported from Gujarat state, India probably because the use of amitraz for tick control started recently to control OP and SP resistant ticks (as stated by farmers). But now upon its indiscriminate and incessant use for past few years the problem of resistance is emerging in the ticks against amitraz and would soon be widespread if suitable measure are not taken.
The current study reports only the detection of resistance status of R. (B.) microplus against amitraz and the mechanism involved needs to be established. The mode of action of amitraz is believed to be interference with nervous system function of the targeted pest species by binding to the octopamine receptors (Evans and Gee 1980). Several types of octopamine receptors have been identified in insects (Blenau and Baumann 2001), and a putative octopamine-like, G-protein-coupled receptor has also been reported in R. (B.) microplus (Baxter and Barker 1999). However, reports from past suggest evidence regarding the involvement of metabolic detoxification mechanisms in amitraz resistance, mutation of the octopamine receptors was speculated to be the main mechanism of resistance to amitraz (Li et al. 2004).
The results revealed gradual development of resistance in R. (B.) microplus to amitraz. Based on the data obtained on the emerging problem of resistance, an alert on good practices aiming for tick control required to be recommended in order to monitor resistance. This will further prevent the development of resistance and at the same time will decrease environmental pollution, thus also causing reduction in the residual effect of acaricides in the animal products like milk and meat.
Acknowledgments
Authors are thankful to the Director of Research, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana for providing facilities to carry out the research work.
References
- Basu A, Haldar DP. A note on the effect of continuous use of Sevin 50 WP on some cattle ticks. J Vet Parasitol. 1997;11:183–184. [Google Scholar]
- Baxter GD, Barker SC. Isolation of a cDNA for an octopamine-like, G-protein coupled receptor from the cattle tick, Boophilus microplus. Insect Biochem Mol Biol. 1999;29:461–467. doi: 10.1016/S0965-1748(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Biswas S (2003) Role of veterinarians in the care and management during harvest of skin in livestock species. In: Proceedings of national seminar on leather industry in today’s perspective, Kolkata, pp 62–64
- Blenau W, Baumann A. Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch Insect Biochem Physiol. 2001;48:13–38. doi: 10.1002/arch.1055. [DOI] [PubMed] [Google Scholar]
- Chaudhary RP, Naithani RC. Resistance to BHC in the cattle tick Boophilus microplus in India. Bull Entamol Res. 1964;55:405–410. doi: 10.1017/S0007485300049555. [DOI] [Google Scholar]
- Evans PD, Gee JD. Action of formamidine pesticides on octopamine receptors. Nature. 1980;281:60–62. doi: 10.1038/287060a0. [DOI] [PubMed] [Google Scholar]
- FAO (2004) Resistance management and integrated parasite control in ruminants, Guidelines. Animal Production and Health Division, FAO, Rome, pp 25–77
- Finney DJ. Probit analysis—a statistical treatment of the response curve. Cambridge: Cambridge University Press; 1962. pp. 1–318. [Google Scholar]
- Ghosh S, Bansal GC, Gupta SC, Ray DD, Khan MQ, Irshad H, Shahiduzzaman M, Seitzer U, Ahmed JS. Status of tick distribution in Bangladesh, India and Pakistan. Parasitol Res. 2007;101:207–216. doi: 10.1007/s00436-007-0684-7. [DOI] [PubMed] [Google Scholar]
- Jonsson NN, Mayer DG, Green PE. Possible risk factors on Queensland farms for acaricide resistance in the cattle tick (Boophilus microplus) Vet Parasitol. 2000;88:79–92. doi: 10.1016/S0304-4017(99)00189-2. [DOI] [PubMed] [Google Scholar]
- Kumar S, Paul S, Sharma AK, Kumar R, Tewari SS, Chaudhuri P, Ray DD, Rawat AKS, Ghosh S. Diazinon resistant status in Rhipicephalus (Boophilus) microplus collected from different agroclimatic zones of India. Vet Parasitol. 2011;181:274–281. doi: 10.1016/j.vetpar.2011.04.030. [DOI] [PubMed] [Google Scholar]
- Li AY, Davey RB, Miller RJ, George JE. Detection and characterization of amitraz resistance in the southern cattle tick Boophilus microplus (Acari: Ixodidae) J Med Entomol. 2004;41:193–200. doi: 10.1603/0022-2585-41.2.193. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Vivas RI, Mata-Mendez Y, Perez-Gutierrez E, Wagner G. The effect of management factors on the seroprevalence of Anaplasma marginale in Bos indicus cattle in the Mexican tropics. Trop Anim Health Prod. 2004;36:135–143. doi: 10.1023/B:TROP.0000012105.19518.80. [DOI] [PubMed] [Google Scholar]
- Sajid MS, Iqbal Z, Khan MN, Muhammad G, Iqbal MU. Effect of Hyalomma ticks (Acari: Ixodidae) on milk production of dairy buffaloes (Bos Bubalus Bubalis) of Punjab (Pakistan) Ital J Anim Sci. 2007;6:939–941. [Google Scholar]
- Sharma AK, Kumar R, Kumar S, Nagar G, Singh NK, Rawat SS, Dhakad ML, Rawat AKS, Ray DD, Ghosh S. Deltamethrin and cypermethrin resistance status of Rhipicephalus (Boophilus) microplus collected from six agro-climatic regions of India. Vet Parasitol. 2012;188:337–345. doi: 10.1016/j.vetpar.2012.03.050. [DOI] [PubMed] [Google Scholar]
- Shaw RD. Culture of an organophosphorus resistant strain of Boophilus microplus (Canestrini) and assessment of its resistance spectrum. Bull Entomol Res. 1966;56:398–405. doi: 10.1017/S0007485300056480. [DOI] [PubMed] [Google Scholar]
- Singh NK, Jyoti, Haque M, Rath SS (2010) Studies on acaricide resistance in Rhipicephalus (Boophilus) microplus against synthetic pyrethroids by adult immersion test with a discriminating dose. J Vet Parasitol 24: 207–208
- Singh NK, Haque M, Jyoti, Rath SS. Deltamethrin resistance in Rhipicephalus microplus in Ludhiana. Indian Vet J. 2012;89:23–25. [Google Scholar]
- Veiga LPHN, de Souza AP, Bellato V, Sartor AA, Nunes AP, Cardoso HM. Resistance to cypermethrin and amitraz in Rhipicephalus (Boophilus) microplus on the Santa Catarina Plateau, Brazil. Rev Bras Parasitol Vet. 2012;21:133–136. doi: 10.1590/S1984-29612012000200011. [DOI] [PubMed] [Google Scholar]