Abstract
The prevalence of Theileria infection in tick vector Hyalomma anatolicum anatolicum collected from healthy animals of Ludhiana district, Punjab was recorded to assess the natural infection level of theilerial parasite in the field condition. A total of 60 semi-engorged H. a. anatolicum were collected from cattle and their salivary glands were dissected out. One half of the salivary gland was stained with methyl green pyronin (MGP) and the other half was utilized for DNA isolation for molecular detection of Theileria infection. A PCR and nested PCR assays were standardized for the detection of T. annulata infection in salivary gland of H. a. anatolicum. The prevalence of T. annulata infection was recorded as 8.3, 20.0 and 60.0 % by MGP staining, primary PCR and nested PCR, respectively. Further, the prevalence was higher in female ticks (8.8 %) than male ticks (6.6 %). The results demonstrated that both primary and nested PCR assays are a valuable technique for detection of T. annulata infection in vector tick under field conditions.
Keywords: Hyalomma anatolicum anatolicum, Methyl green pyronin (MGP) stain, Nested PCR, Theileria annulata
Introduction
Bovine tropical theileriosis is an economically important disease of cross-bred cattle in India transmitted by Hyalomma anatolicum anatolicum and causes heavy economic losses in terms of high morbidity, mortality and reduced production in recovered animals. In India around 10 million cattle are at risk for tropical theileriosis with an annual economic loss of US $800 million (Brown 1997). The detection of Theileria infection in the vector ticks is an important component in the study of epidemiology as it quantifies the flow of infection in nature. Detection of Theileria sporoblast in the salivary gland of H. a. anatolicum has been carried out by various staining techniques by several workers (Walker et al. 1979; Irvin et al. 1981; Buscher and Tangus 1986) for epidemiological studies. However, in India only limited work has been carried out in some parts of the country viz. Haryana (Sangwan et al. 1986), Tripura (Das and Sharma 1991), Uttar Pradesh (Srivastava and Khan 1997; Das and Ray 2003) and Punjab (Haque et al. 2010).
In comparison to staining techniques, polymerase chain reaction (PCR) assay is highly sensitive for detection of low level of infection because it is able to amplify even a minute amount of parasitic DNA. The sensitivity of the assay can be further enhanced by employing nested PCR assay on samples negative by primary PCR assay. PCR has been used for detection of Theileria infection in the vector ticks (D’Oliveira et al. 1995; Kirvar et al. 2000; Das and Ray 2003) but there is a paucity of information on molecular detection of disease from Punjab. The current study reports a nested PCR protocol employing primer sequences based on the major merozoite surface antigen gene sequence and its comparison over the methyl green pyronin (MGP) staining for detection of Theileria infection in the vector ticks of Ludhiana district, Punjab.
Materials and methods
Collection of ticks
Partially fed adult H. a. anatolicum ticks of both sexes were collected from healthy cattle from district Ludhiana, Punjab. The collection was done from January, 2012 to June, 2012. Those ticks were then brought to laboratory and transferred to desiccator in which 80–85 % relative humidity was maintained using 10 % KOH (Solomon 1951). The desiccator was kept in incubator at a constant temperature at 28 °C.
Dissection of ticks and removal of salivary gland
Ticks were embedded dorsal side up on low melting point paraffin in a petri dish and dissection of ticks was done under a dissecting microscope according to the method of Purnell and Joyner (1968). The salivary glands of dissected ticks were harvested and one half (left) was stained by MGP stain as per the method of Irvin et al. (1981) with slight modification for the detection of Theileria sporoblasts within the acini. The other half (right) of the salivary gland was transferred in 60 μl of lysis buffer in an eppendorf tube and stored at −20 °C until DNA extraction.
Staining of salivary glands
The salivary glands were fixed by dipping the slide in Cornoy’s fixative for 5 min. The slides were then rinsed in 70 % alcohol for 2 min followed by distilled water for 2 min and then air-dried. They were next immersed in MGP staining solution for 7 min and rinsed in distilled water and air-dried. Lastly when completely dry, the slides were dipped briefly in xylene before mounting in DPX.
Genomic DNA isolation
For conducting the PCR, whole genomic DNA was isolated from the right salivary gland using QIAamp® DNA mini kit (QIAGEN, GmbH, Germany) following the manufacturer’s recommendations with minor modifications. In the end DNA was extracted in 150 μl of elution buffer and stored at −20 °C for further use. Genomic DNA of T. annulata isolated from infected blood showing high parasitaemia in blood smear examination using standard protocols (Sambrook et al. 1989) and utilized as positive control. Genomic DNA was also isolated from the unfed larvae of H. a. anatolicum and used as negative control.
PCR protocol
The PCR (primary as well as nested) was optimized to identify the major merozoite surface antigen DNA of T. annulata from tick salivary gland as described by D’Oliveira et al. (1995). The sequences of oligonucleotide primers 721 bp employed in PCR assay were:
- Tann N516F:
5′ GTA ACC TTT AAA AAC GT 3′
- Tann N517R:
5′ GTT ACG AAC ATG GGT TT 3′
The sequences of oligonucleotide primers 572 bp employed in nested PCR assay are as follows:
- Ta14136 iF:
5′ AAG ACC CTT AAG GTC GGA GAC AAG A 3′
- Ta 294 iR:
5′ GTC GAC AAC TGG TTT GTA ATC 3′
Two rounds of PCR in a final volume of 25 μl were carried out in a PCR thermal cycler (Eppendorf, Germany). In the primary PCR assay, master solution consisted of 2.5 μl of 10× PCR buffer (MBI Fermentas), 0.5 μl of 10 mM dNTP mix (MBI Fermentas), 1.5 μl of 25 mM MgCI2 (MBI Fermentas), 1.0 U of Hotstart Taq DNA polymerase (recombinant) (MBI Fermentas), 1 μl each (20 pmol) of the primers and 5 μl of template DNA isolated from field samples. The volume was made up to 25 μl with nuclease free water. The cycling conditions were as: Initial denaturation at 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 1 min, annealing at 41 °C for 1 min and extension at 72 °C for 1 min and the final extension was performed at 72 °C for 10 min. In order to carry out the nested PCR, the master solution were the same as described above but instead of template DNA, 1 μl of the primary PCR product was used as template and amplified with 20 pmol, each of the primers. The cycling conditions were also same except an annealing temperature of 62 °C for 1 min.
The tick sample which were found negative both by primary and nested PCR were analyzed by PCR assay using the primer set encoding the mitochondrial 16S rDNA sequences of hard and soft ticks (Black and Piesman 1994) to establish that tick DNA was isolated but the infection was absent in those tick sample. The sequences of the primers are as under:
- Haa 16S.1
5′ CTG CTC AAT GAT TTT TTA AAT TGC TGT GG 3′
- 16S71
5′ CCG GTC TGA ACT CAG ATC AAG T 3′
The amplification was carried out in a final reaction volume of 25 μl, with a programme consisting of ten cycles of denaturation for 1 min at 92 °C, annealing for 1 min at 48 °C and extension for 1.5 min at 72 °C. This was followed by 32 cycles of denaturation for 1 min at 92 °C, annealing for 35 s at 54 °C and extension for 1.5 min at 72 °C and final extension for 7 min at 72 °C. The PCR products (primary, nested, 16S) were checked for amplification by electrophoresis on a 1.5 % agarose gel and visualized using gel documentation system (Syngene, U.K.). The results of PCR assay were compared with that of MGP stained salivary glands.
Results and discussion
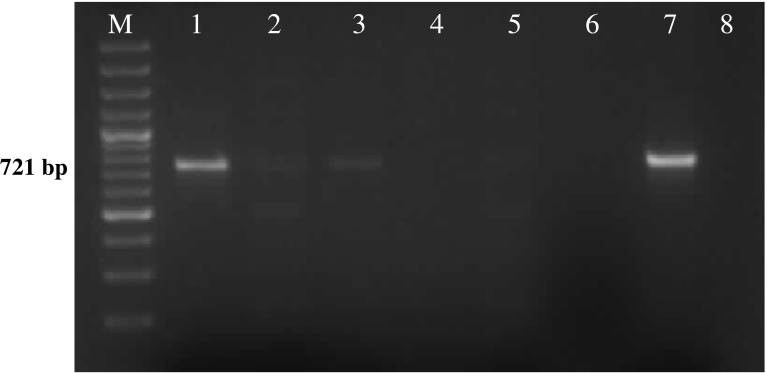
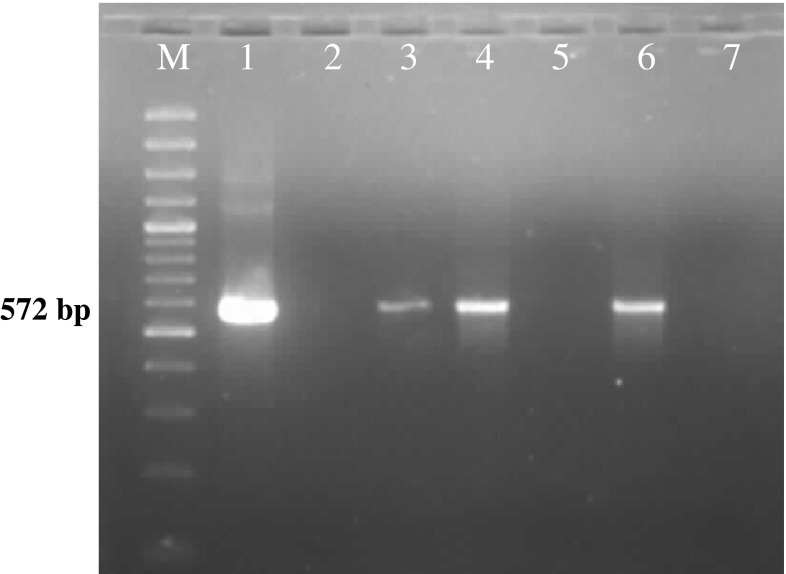
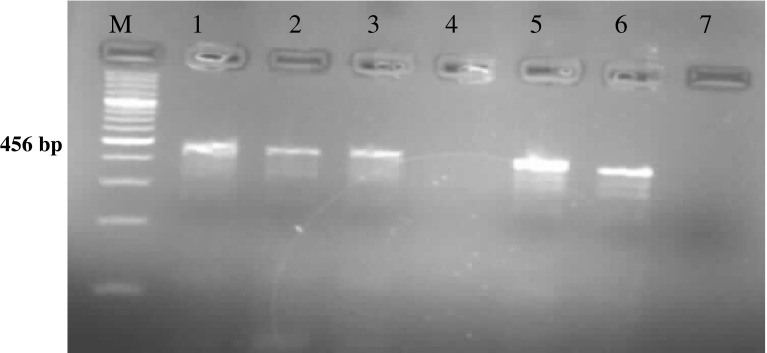
Among the total of 60 adult semiengorged H. a. anatolicum ticks examined, 8.3 % (5/60) were found positive for Theileria sporoblast by MGP staining. The infected acini of the salivary gland were hypertrophied and stained pinkish while round mass of Theileria sporoblast stained bluish green (Fig. 1) and uninfected salivary gland showed no sporoblastic mass. In order to assess the true status of T. annulata infection in these samples primary PCR followed by nested PCR assay was employed to detect any amplification in form of ethidium bromide-stained amplicons, after standardization of the assays. Of the total samples subjected to primary PCR, 20.0 % (12/60) were found to be positive for T. annulata infection as revealed by the amplification of 721 bp product (Fig. 2). PCR products obtained from primary PCR of T. annulata when employed as template in nested PCR produced the amplicons of desired size (572 bp) was recorded in 60.0 % (36/60) samples (Fig. 3). This validates that nested PCR, when coupled with primary PCR, resulted in increased sensitivity of the assay, as the products from the primary PCR (24 samples) that were not visualized in the ethidium bromide-stained agarose gel electrophoresis, when subjected to nested PCR, could be detected. Further, the genomic DNA isolated from H. a. anatolicum unfed larvae (used as negative control) did not showed the desired amplification in either of the PCR assay. The tick samples (24/60) which were found negative both by both primary and nested PCR were analyzed by 16S PCR assay and they produced the amplicons of desired size (456 bp) in all samples as shown in Fig. 4, thus establishing the fact that the DNA was isolated from the tick salivary gland but the infection was absent in these samples.
Fig. 1.
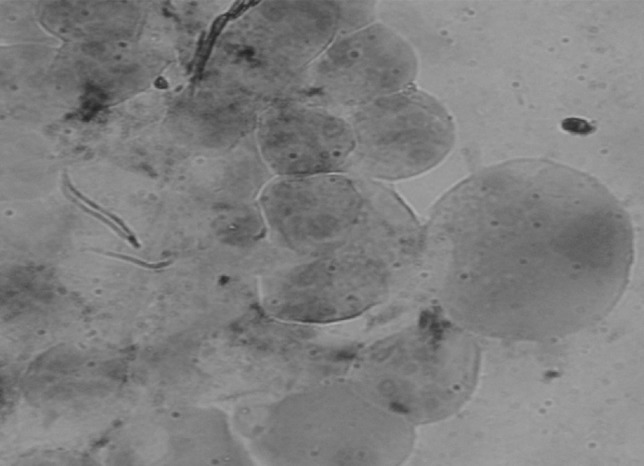
Methyl green pyronin (MGP) stained salivary gland showing Theileria sporoblast in Hyalomma anatolicum anatolicum. (Color figure online)
Fig. 2.
Amplification of 721 bp fragment of T. annulata. Lane M 100 bp DNA Ladderplus, lane 1 positive control, lane 8 negative control, lanes 2–7 field collected tick samples (salivary glands)
Fig. 3.
Amplification of 572 bp fragment of T. annulata by nested PCR. Lane M 100 bp DNA Ladderplus, lane 1 known positive nested product of T. annulata, lanes 2–6 field collected tick salivary glands, lane 7 negative control
Fig. 4.
Amplification of 456 bp fragment of H. a. anatolicum by 16S PCR. Lane M 100 bp DNA Ladderplus, lanes 1–6 field collected tick salivary glands, lane 7 negative control
Theileria annulata is transmitted trans-stadially mainly by nymphal and also adult stages of H. a. anatolicum. The detection of Theileria infection in tick in the field may prove to be crucial and rewarding approach to theileriosis epidemiology. Examination of tick salivary glands for Theileria infections presents a convenient method to determine the Theileria infection in the field condition and studies of Theileria in tick vector date back to 1906 when Koch described the developmental stages in ticks. The early methods for detection of Theileria parasite included embedding and cutting of serial sections of tick salivary glands followed by Giemsa staining of these sections (Cowdry and Ham 1930). Later MGP staining emerged as the most widely used staining method for detection of Theileria sporoblast in the salivary gland of H. a. anatolicum (Walker et al. 1979; Irvin et al. 1981; Buscher and Tangus 1986) for epidemiological studies. In Indian scenario similar studies has been carried out with prevalence rate of 32.8 % from Haryana (Sangwan et al. 1986), 43.38 % from Tripura (Das and Sharma 1991), 15.9–92.3 % from various villages of Uttar Pradesh (Srivastava and Khan 1997) and 11.65 % from Punjab (Haque et al. 2010).
Among a total of 15 adult male H. a. anatolicum ticks 1 (6.6 %) was found positive for Theileria sporoblast whereas, among 45 adult female ticks 4 (8.8 %) were found positive by MGP staining. The prevalence rate of Theileria infection was higher in female ticks than in males as has been earlier reported by several workers (Walker et al. 1983; Buscher and Tangus 1986; Sangwan et al. 1989; Haque et al. 2010). The female ticks play a more important role in the transmission of Theileria infection as they have more abundance of type III acini than male ticks and Theileria parasites were only detected in type III acini (Irvin et al. 1981). Hence the abundance of type III acini in female ticks favors the development of Theileria annulata thus causing an increased prevalence of infection in female ticks.
Staining techniques are used to detect theileriosis in vector ticks however, amplification of parasite DNA has been stated to be far more sensitive than the conventional methods. This is because sometimes the lower levels of infection cannot be detected by staining methods, but as the PCR assay has an extremely high sensitivity; it is able to amplify even a minute amount of parasitic DNA (Bose et al. 1995). Kirvar et al. (2000) detected up to 1 infected acinus/tick in resting and partially fed adult H. a. anatolicum ticks by PCR assay. Similarly from India, Das and Ray (2003) reported a higher rate (42.8 %) of T. annulata infection in H. a. anatolicum salivary gland by PCR in comparison to MGP staining (31.0 %).
The results obtained from the current study demonstrate that this PCR assays are much sensitive for the detection of T. annulata infection in H. a. anatolicum salivary gland and can be utilized in field condition for the study of the epidemiology of theileriosis.
Acknowledgments
Authors are thankful to The Dean, Postgraduate Studies, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana for providing facilities to carry out the research work.
Footnotes
This article is a part of M.V.Sc thesis of the first author submitted to GADVASU, Ludhiana, Punjab.
References
- Black WC, Piesman J. Phylogeny of hard and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci USA. 1994;91:10034–10038. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose R, Jorgensen WK, Delgliesh RJ, Friedhoff RK, De Vos AJ. Current state and future trends in diagnosis of Babesiosis. Vet Parasitol. 1995;57:61–74. doi: 10.1016/0304-4017(94)03111-9. [DOI] [PubMed] [Google Scholar]
- Brown CGD. Dynamics and impact of tick-borne diseases of cattle. Trop Anim Health Prod. 1997;29:1S–3S. doi: 10.1007/BF02632905. [DOI] [PubMed] [Google Scholar]
- Buscher G, Tangus J. Quantitative studies on Theileria in the salivary glands of Rhipicephalus appendiculatus adults: search for conditions for high infection. Int J Parasitol. 1986;16:121–129. doi: 10.1016/0020-7519(86)90097-4. [DOI] [PubMed] [Google Scholar]
- Cowdry EV, Ham AW. The life cycle of the parasite of East Coast Fever in ticks transmitting the disease. Science. 1930;72:461–462. doi: 10.1126/science.72.1870.461. [DOI] [PubMed] [Google Scholar]
- D’Oliveira C, vander Weide M, Habela MA, Jacquiet P, Jongejan F. Detection of Theileria annulata in blood samples of carrier cattle by PCR. J Clin Microbiol. 1995;33:2665–2669. doi: 10.1128/jcm.33.10.2665-2669.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G, Ray D. PCR-based detection of Theileria annulata in ticks collected from cattle of West Bengal India. J Vet Parasitol. 2003;17:11–14. [Google Scholar]
- Das SS, Sharma NN. Prevalence of Theileria infection in Hyalomma anatolicum anatolicum ticks in north districts of Tripura (India) J Vet Parasitol. 1991;5:25–27. doi: 10.1016/0304-4017(91)90093-b. [DOI] [PubMed] [Google Scholar]
- Haque M, Jyoti Singh NK, Rath SS. Prevalence of Theileria annulata infection in Hyalomma anatolicum anatolicum in Punjab state, India. J Parasit Dis. 2010;34:48–51. doi: 10.1007/s12639-010-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin AD, Boarer CDH, Dobbelaere DAE, Mohan SM, Marake R, Ocama JGR. Monitoring Theileria parva infection in adult Rhipicephalus appendiculatus ticks. Parasitology. 1981;82:137–147. doi: 10.1017/S0031182000041949. [DOI] [PubMed] [Google Scholar]
- Kirvar E, Ilhan T, Katzer F, Hooshmand-Rad P, Zweygarth E, Gerstenberg C, Phipps P, Brown CG. Detection of Theileria annulata in cattle and vector ticks by PCR using the Tams1 gene sequences. Parasitology. 2000;120:245–254. doi: 10.1017/S0031182099005466. [DOI] [PubMed] [Google Scholar]
- Purnell RE, Joyner LP. The development of Theileria parva in the salivary glands of the tick Rhipicephalus appendiculatus. Parasitology. 1968;58:725–732. doi: 10.1017/S0031182000029036. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Sangwan AK, Chhabra MB, Samantaray S. Theileria infectivity of Hyalomma ticks in Haryana, India. Trop Anim Health Prod. 1986;18:149–154. doi: 10.1007/BF02359525. [DOI] [PubMed] [Google Scholar]
- Sangwan AK, Chhabra MB, Samantaray S. Relative role of male and female Hyalomma anatolicum anatolicum ticks in Theileria transmission. Vet Parasitol. 1989;31:83–87. doi: 10.1016/0304-4017(89)90010-1. [DOI] [PubMed] [Google Scholar]
- Solomon ME. Control of humidity with potassium hydroxide, sulphuric acid or other solutions. Bull Entomol Res. 1951;42:543–554. doi: 10.1017/S0007485300028947. [DOI] [Google Scholar]
- Srivastava SC, Khan MH. Infection of Theileria annulata in Hyalomma a. anatolicum Koch. J Vet Parasitol. 1997;11:207–210. [Google Scholar]
- Walker AR, McKeller SB, Bell LJ, Brown CGD. Rapid quantitative assessment of Theileria infection in ticks. Trop Anim Health Prod. 1979;11:21–26. doi: 10.1007/BF02237760. [DOI] [PubMed] [Google Scholar]
- Walker AR, Latif AA, Morzaria SP, Jongejan F. Natural infection rates of Hyalomma anatolicum anatolicum with Theileria in Sudan. Res Vet Sci. 1983;35:87–90. [PubMed] [Google Scholar]