Abstract
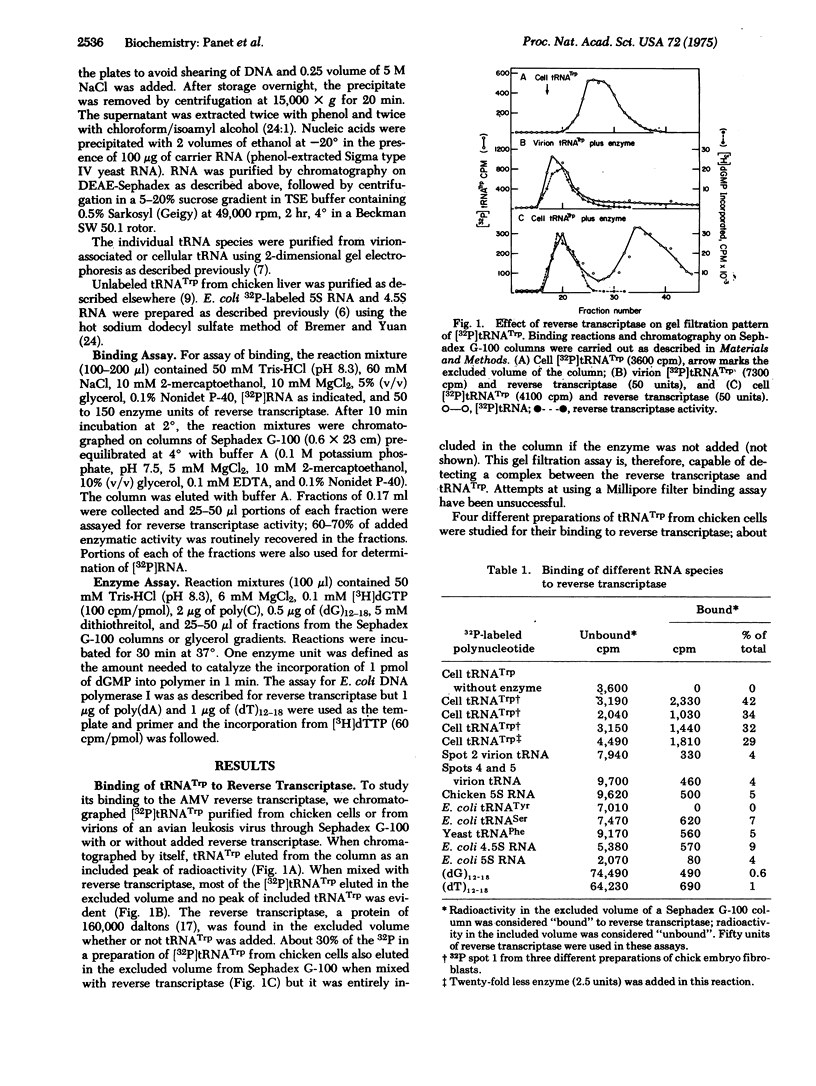
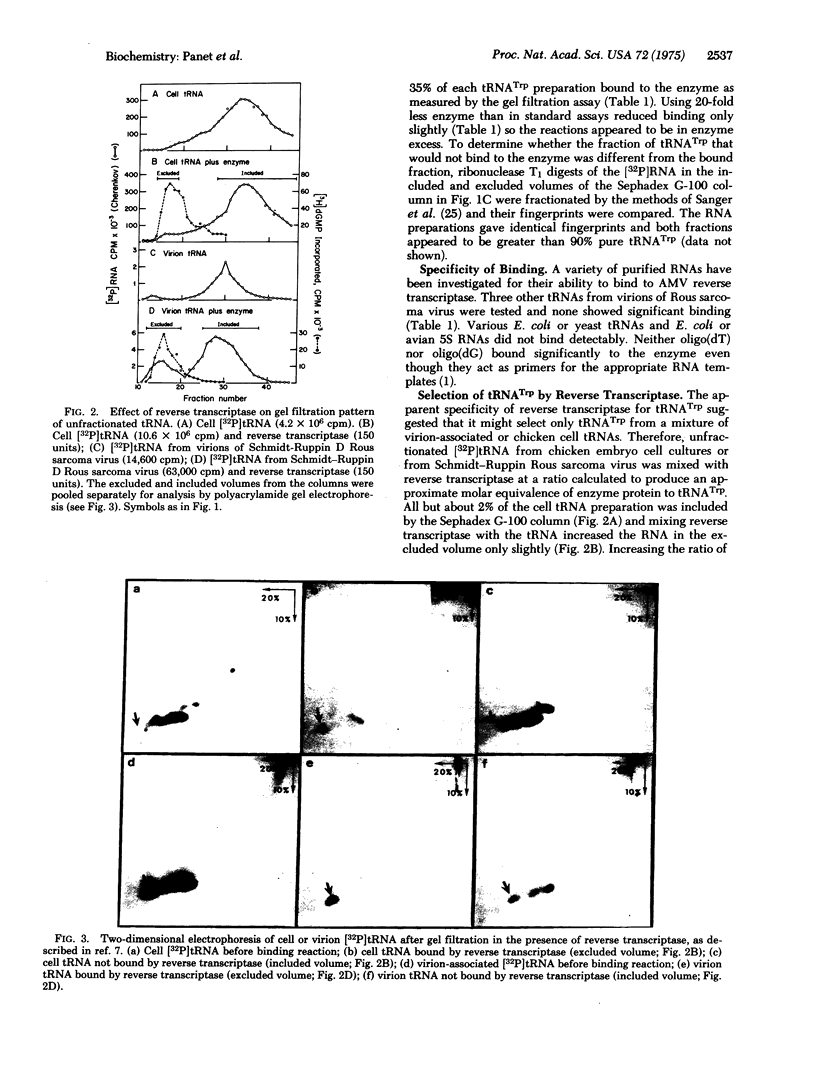
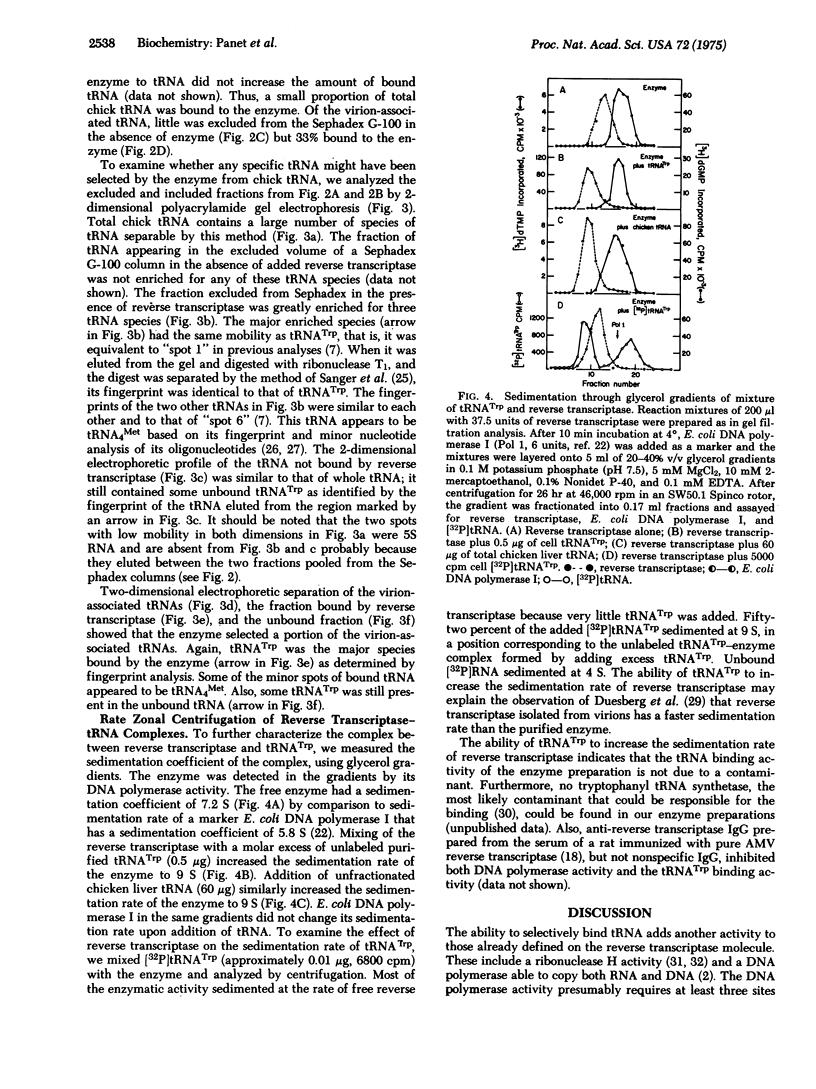
The ability of tryptophan tRNA (tRNATrp) to initiate reverse transcription of the 70S RNA of avian RNA tumor viruses suggested that the reverse transcriptase (RNA-dependent DNA polymerase; deoxynucleosidetriphosphate: DNA deoxynucleotidyltransferase; EC 2.7.7.7) might have a specific binding site for the tRNA. A complex of tRNATrp and the avian myeloblastosis virus reverse transcriptase has been demonstrated using chromatography on Sephadex G-100 columns. Of all the chicken tRNAs, only tRNATrp and a tRNA4Met bind to the enzyme with high enough affinity to be selected from a mixture of the chicken cell tRNAs. The ability of tRNATrp to change the sedimentation rate of the enzyme indicates that tRNATrp is not binding to a contaminant in the enzyme preparation. Treatment of the enzyme with monospecific antibody to reverse transcriptase prevented binding of tRNA as well as inhibited the DNA polymerase activity of the enzyme. The ability of reverse transcriptase to utilize tRNATrp aa a primer for DNA synthesis, therefore, appears to involve a highly specific site on the enzyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Smoler D. F. Association of an endoribonuclease with the avian myeloblastosis virus deoxyribonucleic acid polymerase. J Biol Chem. 1972 Nov 25;247(22):7282–7287. [PubMed] [Google Scholar]
- Baltimore D., Smoler D. Primer requirement and template specificity of the DNA polymerase of RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1507–1511. doi: 10.1073/pnas.68.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H., Yuan D. Chain growth rate of messenger RNA in Escherichia coli infected with bacteriophage T4. J Mol Biol. 1968 Jun 28;34(3):527–540. doi: 10.1016/0022-2836(68)90178-2. [DOI] [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Sawyer R. C., Taylor J. M., Faras A. J., Levinson W. E., Goodman H. M., Bishop J. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. I. Identification of a specific 4S RNA which serves as primer. J Virol. 1974 May;13(5):1126–1133. doi: 10.1128/jvi.13.5.1126-1133.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P., Helm K. V., Canaani E. Properties of a soluble DNA polymerase isolated from Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):747–751. doi: 10.1073/pnas.68.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Association of 4S ribonucleic acid with oncornavirus ribonucleic acids. J Virol. 1971 Aug;8(2):254–256. doi: 10.1128/jvi.8.2.254-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Transfer ribonucleic acid synthetase activity associated with avian myeloblastosis virus. J Virol. 1972 Feb;9(2):231–233. doi: 10.1128/jvi.9.2.231-233.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Dahlberg J. E., Sawyer R. C., Harada F., Taylor J. M., Levinson W. E., Bishop J. M., Goodman H. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. II. Structure of a 4S RNA primer. J Virol. 1974 May;13(5):1134–1142. doi: 10.1128/jvi.13.5.1134-1142.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Dibble N. A. RNA-directed DNA synthesis by the DNA polymerase of Rous sarcoma virus: structural and functional identification of 4S primer RNA in uninfected cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):859–863. doi: 10.1073/pnas.72.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. I. Characterization by polyacrylamide gel electrophoresis and fingerprint analysis. J Biol Chem. 1973 Jul 25;248(14):5024–5032. [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- Kornberg A. Active center of DNA polymerase. Science. 1969 Mar 28;163(3874):1410–1418. doi: 10.1126/science.163.3874.1410. [DOI] [PubMed] [Google Scholar]
- Lagerkvist U., Rymo L., Waldenström J. Structure and function of transfer ribonucleic acid. II. Enzyme-substrate complexes with valyl ribonucleic acid synthetase from yeast. J Biol Chem. 1966 Nov 25;241(22):5391–5400. [PubMed] [Google Scholar]
- Mölling K., Bolognesi D. P., Bauer H., Büsen W., Plassmann H. W., Hausen P. Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids. Nat New Biol. 1971 Dec 22;234(51):240–243. doi: 10.1038/newbio234240a0. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Weinstein I. B. Fractionation of rat liver transfer ribonucleic acid. Isolation of tyrosine, valine, serine, and phenylalanine transfer ribonucleic acids and their coding properties. Biochemistry. 1969 Mar;8(3):832–842. doi: 10.1021/bi00831a011. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Watson K. F., Yaniv A., Spiegelman S. Serological analysis of the deoxyribonucleic acid polymerase of avian oncornaviruses. II. Comparison of avian deoxyribonucleic acid polymerases. J Virol. 1972 Nov;10(5):959–964. doi: 10.1128/jvi.10.5.959-964.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Verma I. M., Baltimore D. Role of the subunits of the avian RNA tumor virus reverse transcriptase. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):919–923. doi: 10.1101/sqb.1974.039.01.107. [DOI] [PubMed] [Google Scholar]
- Piper P. W., Clark B. F. The nucleotide sequences of cytoplasmic methionine and valine tRNAs from mouse myeloma cells. FEBS Lett. 1974 Oct 1;47(1):56–59. doi: 10.1016/0014-5793(74)80425-4. [DOI] [PubMed] [Google Scholar]
- Piper P. W. The nucleotide sequence of a methionine tRNA which functions in protein elongation in mouse myeloma cells. Eur J Biochem. 1975 Feb 3;51(1):283–293. doi: 10.1111/j.1432-1033.1975.tb03928.x. [DOI] [PubMed] [Google Scholar]
- Rogg H., Wehrli W., Staehelin M. Isolation of mammalian transfer RNA. Biochim Biophys Acta. 1969 Nov 19;195(1):13–15. doi: 10.1016/0005-2787(69)90597-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Sawyer R. C., Dahlberg J. E. Small RNAs of Rous sarcoma virus: characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J Virol. 1973 Dec;12(6):1226–1237. doi: 10.1128/jvi.12.6.1226-1237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Harada F., Dahlberg J. E. Virion-associated RNA primer for Rous sarcoma virus DNA synthesis: isolation from uninfected cells. J Virol. 1974 Jun;13(6):1302–1311. doi: 10.1128/jvi.13.6.1302-1311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Bromfeld E., Manly K. F., Baltimore D. Covalently linked RNA-DNA molecule as initial product of RNA tumour virus DNA polymerase. Nat New Biol. 1971 Sep 29;233(39):131–134. doi: 10.1038/newbio233131a0. [DOI] [PubMed] [Google Scholar]
- Watson K. E., Nowinski R. C., Yaniv A., Spiegelman S. Serological analysis of the deoxyribonucleic acid polymerase of avian oncornaviruses. I. Preparation and characterization of monospecific antiserum with purified deoxyribonucleic acid polymerase. J Virol. 1972 Nov;10(5):951–958. doi: 10.1128/jvi.10.5.951-958.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein I. B., Ochoa M., Jr, Friedman S. M. Fidelity in the translation of messenger ribonucleic acids in mammalian subcellular systems. Biochemistry. 1966 Oct;5(10):3332–3339. doi: 10.1021/bi00874a036. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Flügel R. M., Larson J. E., Schendel P. F., Sweet R. W. Comparison of some reactions catalyzed by deoxyribonucleic acid polymerase from avian myeloblastosis virus, Escherichia coli, and Micrococcus luteus. Biochemistry. 1972 Feb 15;11(4):621–629. doi: 10.1021/bi00754a025. [DOI] [PubMed] [Google Scholar]
- Yarus M., Berg P. Recognition of tRNA by aminoacyl tRNA synthetases. J Mol Biol. 1967 Sep 28;28(3):479–490. doi: 10.1016/s0022-2836(67)80098-6. [DOI] [PubMed] [Google Scholar]



