Abstract
Objective:
Obesity and insulin resistance (IR) predispose to type 2 diabetes mellitus. Yet only half of obese adolescents have IR and far fewer progress to type 2 diabetes mellitus. We hypothesized that amino acid and fatty acid metabolites may serve as biomarkers or determinants of IR in obese teens.
Research Design and Methods:
Fasting blood samples were analyzed by tandem mass spectrometry in 82 obese adolescents. A principal components analysis and multiple linear regression models were used to correlate metabolic components with surrogate measures of IR: homeostasis model assessment index of insulin resistance (HOMA-IR), adiponectin, and triglyceride (TG) to high-density lipoprotein (HDL) ratio.
Results:
Branched-chain amino acid (BCAA) levels and products of BCAA catabolism were higher (P < .01) in males than females with comparable body mass index (BMI) z-score. In multivariate analyses, HOMA-IR in males correlated positively with BMI z-score and a metabolic signature containing BCAA, uric acid, and long-chain acylcarnitines and negatively with byproducts of complete fatty acid oxidation (R2 = 0.659, P < .0001). In contrast, only BMI z-score correlated with HOMA-IR in females. Adiponectin correlated inversely with BCAA and uric acid (R2 = 0.268, P = .0212) in males but not females. TG to HDL ratio correlated with BMI z-score and the BCAA signature in females but not males.
Conclusions:
BCAA levels and byproducts of BCAA catabolism are higher in obese teenage boys than girls of comparable BMI z-score. A metabolic signature comprising BCAA and uric acid correlates positively with HOMA-IR in males and TG to HDL ratio in females and inversely with adiponectin in males but not females. Likewise, byproducts of fatty acid oxidation associate inversely with HOMA-IR in males but not females. Our findings underscore the roles of sex differences in metabolic function and outcomes in pediatric obesity.
Obesity and insulin resistance are the major determinants of risk for type 2 diabetes mellitus (T2D) in children as well as adults. Yet only one-half of obese adolescents are insulin resistant (1, 2), and a relatively small proportion of these progress to T2D (3). To identify those at highest risk of glucose intolerance, it is essential to characterize metabolic markers that predict the development of insulin resistance (4).
We hypothesized that baseline levels of amino acids and amino acid and fatty acid metabolites may serve as biomarkers or determinants of insulin resistance (IR) in obese teens. To test that hypothesis, we used metabolomic profiling, principal components analysis, and multiple linear regression models to assess the correlations between amino acids and amino acid and fatty acid metabolites and surrogate markers of IR including homeostasis model assessment index of insulin resistance (HOMA-IR), adiponectin, and the triglyceride (TG) to high-density lipoprotein (HDL) ratio. Leptin was included as a surrogate measure of white adipose tissue mass. Unexpectedly, our findings revealed striking differences in metabolic biomarkers among boys and girls, indicating a critical role for sex differences in the pathogenesis of pediatric insulin resistance.
Research Design and Methods
Subjects
Subjects were identified prior to enrollment in Duke's multicomponent childhood obesity treatment program, a lifestyle modification program for obese children and adolescents. Inclusion criteria included new patient to the multicomponent childhood obesity treatment program; age 12 to 18 (inclusive) years; overweight or obese [body mass index (BMI) ≥ 85th percentile for sex and age as assessed using Centers for Disease Control and Prevention standard growth curves]; and ability of the subject and one parent/guardian to speak and read English well enough to complete questionnaires and intake forms. Subjects were excluded if they had diabetes mellitus or had taken weight-reducing agents, systemic corticosteroids, atypical antipsychotics, oral contraceptives, or medroxyprogesterone within the past 6 months. Subjects were removed if they did not provide fasting blood samples within 2 weeks of the first visit. Informed consent for participation was obtained from at least one parent/legal guardian for all children younger than 18 years and from the single 18-year-old patient at the time of entry to the study. The protocol was approved by Duke's Institutional Review Board.
Ninety subjects were enrolled; eight subjects were withdrawn because they did not supply a fasting blood sample. Thus, the study cohort consisted of 82 overweight and obese adolescents (41 males, 41 females).
Blood samples
Baseline blood samples were obtained after an 8- to 12-hour overnight fast. Plasma was stored at −80°C until analyzed.
Anthropometric measurements
Body weight and height were measured by standard methods. Blood pressure was measured twice; the average blood pressure was used for statistical analyses. Age, gender, and height-specific normal values for children are available at http://www.nhlbi.nih.gov/guidelines/hypertension/child_tbl.htm. BMI, BMI percentiles, and BMI z-scores were calculated using an age- and sex-specific pediatric z-score calculator (http://stokes.chop.edu/web/zscore). Body fat percentage (BF%) was estimated by electrical impedance using a Tanita BC-418 segmental body composition analyzer. A physical examination was performed by a pediatrician or a nurse practitioner. Clinic providers received training in pubertal staging.
Hormone analysis
Hormones were measured on a Meso Scale Discovery SI-2400 electrochemiluminescent imager (Meso Scale Discovery). Assay kits, also from Meso Scale Discovery, included a duplex for insulin (assay range 7.5–50 000 pg/mL) and leptin (43–100 000 pg/mL) and a single-plex for total adiponectin (5–106 ng/mL). Precision on duplicate measurements was less than 10% coefficient of variation. HOMA-IR was calculated as (fasting insulin in milliinternational units per liter × fasting glucose in milligrams per deciliter)/405 (5).
Conventional metabolite analysis
Measurements of conventional metabolites were performed using a Beckman-Coulter clinical analyzer. Plasma glucose, total cholesterol, HDL, low-density lipoprotein, TGs, and high-sensitivity C-reactive protein (CRP) were measured using reagents from Beckman. Total nonesterified fatty acids, total ketones, and 3-hydroxyburytate were measured with reagents from Wako. Precision was less than 5% coefficient of variation.
Plasma acylcarnitines and amino acids
Acylcarnitines (0.01–40 μmol/L, <15%) and amino acids (5–1000 μmol/L, <15%) were analyzed by tandem mass spectrometry using a Waters TQD instrument as described previously (6–10).
Statistical analyses
In this unbiased analysis, sample size was calculated to detect correlations of 0.4 or greater between hormones/metabolites and outcomes of interest (HOMA-IR, adiponectin, TG to HDL ratio). In linear regression models using five explanatory variables, a sample size of 76 provided a correlation of 0.4 with power of 0.8 and P < .05; thus, our sample size (n = 82) provides adequate statistical power. Unpaired t tests were used to compare anthropometric values and metabolites among females and males at baseline. We calculated Pearson correlation coefficients to analyze bivariate relationships between surrogate measures of IR, leptin, and blood metabolites. HOMA-IR, adiponectin, TG to HDL ratio, and leptin were natural log transformed to approximate normality. Associations between the ratio of C2 to (C3 + C5) and surrogate measures of IR were also investigated. In comparison tests and bivariate correlation analysis, we used a conservative P < .01 and R2 ≥ 0.1 for assigning statistical significance.
Principal components analysis
Principal components analysis (PCA) was used to reduce the large number of correlated metabolites into clusters of fewer components that were not correlated with each other (11). These factors were used as explanatory variables in multiple linear regression models of IR.
All the metabolites were assessed for normality. Variables that were not normally distributed were transformed using a ln(metabolite + 1) function. Kaiser-Guttman criteria were applied to select the principal components. Components with eigenvalues greater than 1.00 were retained for further analysis. Seven acylcarnitines (C6 %84.15, C5-OH/C3-DC %21.95, C7-DC %92.68, C12-OH/C10-DC %20.73, C18-OH/C16-DC %29.27 C10:2%9.76 C16-OH/C14-DC %6.10) were removed from the analysis because more than 5% of subjects had levels below the lower limits of quantification. Seventeen principal components were retained; these explained 80% of the variance of all metabolites used for the analysis. Associations between the metabolites and components were established using varimax-rotated factor loadings. Metabolites with an absolute value of factor loading of 0.4 or greater were considered to constitute that component.
For modeling HOMA-IR, adiponectin, leptin, and the TG to HDL ratio, the components retained in the PCA were used in multiple linear regression models as explanatory variables. All models were adjusted for age, sex, and BMI z-score. Statistically significant factors were determined using a stepwise linear regression analysis. In a secondary analysis, we determined whether the effects of these factors on dependent variables varied according to sex; to that end, we added sex interaction effects to models selected in the primary analyses. If any of the interaction effects was found to be statistically significant, we estimated sex-stratified regression models.
Tanner stage information was available for 53 subjects. Models were reestimated for the smaller sample by controlling for Tanner stage, sex, age, and BMI z-score.
For regression models, P < .05 was considered statistically significant; analysis was performed using SAS version 9.3 (SAS Institute Inc.).
Results
Baseline anthropometric and metabolic characteristics
Eighty-two subjects were studied; 50% were female, 53% African-American, 21% Caucasian, and 10% Hispanic. Fifty-nine had a BMI at the 99th percentile or greater, 19 were at the 95–99th percentile, and four were at the 92nd to the 94th percentile. At baseline, males and females were comparable in age, weight, BMI, BMI percentile, BMI z-scores, systolic and diastolic blood pressures, and birth weight, but females had higher body fat percent.
Baseline anthropometric characteristics and metabolites stratified by sex are presented in Table 1 and Supplemental Tables 1 and 2. Females had higher leptin levels but lower uric acid, glutamate/glutamine, valine, leucine/isoleucine, and combined branched-chain amino acid (BCAA) levels than males. Plasma C3 acyl (propionyl) carnitine and C5 (isovaleryl) carnitine, products of BCAA catabolism, were also lower in females than males. The variability in the ranges of the anthropometric characteristics and metabolites was similar in both sexes. Complete plasma amino acid and acylcarnitine profiles for males and females are reported in Supplemental Tables 1 and 2.
Table 1.
Comparisons of Anthropometric Values and Metabolites Across Sex
| Male Mean (SE) (n = 41) | Female Mean (SE) (n = 41) | P Value | |
|---|---|---|---|
| Anthropometric values | |||
| Age, y | 13.76 (0.26) | 14.0 (0.22) | .4752 |
| Weight, kg | 100.61 (4.12) | 94.19 (2.86) | .2043 |
| BMI | 34.82 (1.00) | 35.34 (0.94) | .7080 |
| BMI, % | 98.37 (0.26) | 98.27 (0.19) | .7624 |
| BMI z-score | 2.37 (0.06) | 2.24 (0.05) | .1040 |
| BF% | 39.11 (1.17, n = 39) | 44.03 (1.12, n = 37) | .0033 |
| Systolic blood pressure, mm Hg | 121.50 (2.00, n = 31) | 117.10 (1.94, n = 32) | .1189 |
| Diastolic blood pressure, mm Hg | 64.56 (1.46, n = 31) | 63.67 (1.47, n = 32) | .6674 |
| Birth weight, kg | 3.30 (0.13, n = 36) | 3.22 (0.10, n = 38) | .5960 |
| Metabolites | |||
| Adiponectin, ng/mL | 13915.54 (809.31) | 14896.76 (823.19) | .3979 |
| Leptin, pg/mL | 42346.02 (4654.15) | 63102.59 (4231.03) | .0014 |
| Insulin, pg/mL | 730.71 (82.45) | 657.10 (46.55) | .4392 |
| Glucose, mg/dL | 93.59 (1.09) | 90.27 (0.98) | .0262 |
| HOMA-IR | 4.08 (0.48) | 3.56 (0.27) | .3449 |
| Cholesterol, mg/dL | 162.68 (5.63) | 154.66 (4.67) | .2762 |
| LDL, mg/dL | 102.47 (4.76) | 92.25 (4.27) | .1139 |
| HDL, mg/dL | 44.48 (1.61) | 47.33 (1.49) | .1982 |
| TGs, mg/dL | 88.56 (8.65) | 80.85 (7.14) | .4940 |
| Uric acid, mg/dL | 6.39 (0.19) | 5.41 (0.16) | .0002 |
| NEFA, mmol/L | 0.47 (0.03) | 0.48 (0.03) | .7346 |
| CRP, mg/L | 3.44 (0.56) | 4.75 (0.81) | .1896 |
| β-Hydroxybutyrate, μmol/L | 75.77 (17.66) | 56.67 (9.11) | .3394 |
| Glutamate/glutamine, μM | 88.34 (3.08) | 75.80 (2.31) | .0016 |
| Valine, μM | 289.48 (6.83) | 259.00 (6.27) | .0011 |
| Leucine/isoleucine, μM | 202.78 (4.02) | 176.70 (3.47) | <.0001 |
| BCAA, μM | 492.26 (10.09) | 435.69 (9.26) | <.0001 |
| C2 acylcarnitine, μM | 6.64 (0.45) | 5.93 (0.33) | .2031 |
| C3 acylcarnitine, μM | 0.41 (0.02) | 0.31 (0.02) | .0001 |
| C5 acylcarnitine, μM | 0.14 (0.01) | 0.11 (0.01) | .0084 |
| C2 to (C3 + C5) ratio | 12.97 (1.04) | 15.35 (1.01) | .1034 |
| TG to HDL ratio | 2.18 (0.24) | 1.87 (0.22) | .3547 |
Abbreviations: LDL, low-density lipoprotein; NEFA, nonesterified fatty acid. Statistically significant differences are highlighted in bold.
Bivariate associations between baseline metabolites and HOMA-IR, adiponectin, leptin, and TG to HDL ratio
In bivariate analyses (Table 2), HOMA-IR in females correlated positively with TGs and negatively with HDL. In contrast, HOMA-IR in males correlated positively with BMI z-score and TGs and negatively with HDL and adiponectin. Adiponectin in females did not correlate with any of the variables. In contrast, adiponectin in males correlated positively with HDL and negatively with insulin and uric acid. In females, the TG to HDL ratio correlated positively with BMI z-score, insulin, uric acid, HOMA-IR, and BCAA. In males, TG to HDL ratio correlated positively with insulin, uric acid, and HOMA-IR and negatively with adiponectin (Table 2).
Table 2.
Correlates of HOMA-IR, Adiponectin, TG to HDL Ratio, and Leptin
| Correlate | Male (R2) | Female (R2) |
|---|---|---|
| Correlates of HOMA-IR | ||
| BMI z-score | 0.31 | 0.14 |
| BF% | 0.15 | 0.02 |
| HDL (−) | 0.25 | 0.19 |
| TGs | 0.23 | 0.19 |
| Adiponectin (−) | 0.28 | 0.06 |
| Uric acid | 0.03 | 0.14 |
| BCAA | 0.03 | 0.07 |
| C2 to (C3 + C5) ratio (−) | 0.35 | 0.02 |
| Correlates of adiponectin | ||
| BMI z-score | 0.07 | 0.02 |
| BF% | 0.02 | 0.02 |
| HDL | 0.31 | 0.05 |
| TGs (−) | 0.14 | 0.06 |
| Insulin (−) | 0.24 | 0.10 |
| Uric acid (−) | 0.25 | 0.08 |
| BCAA (−) | 0.12 | <0.01 |
| C2 to (C3 + C5) ratio | 0.06 | 0.01 |
| Correlates of TG to HDL ratio | ||
| BMI z-score | 0.13 | 0.16 |
| BF% | 0.13 | 0.06 |
| Insulin | 0.28 | 0.34 |
| Uric acid | 0.17 | 0.33 |
| BCAA | 0.01 | 0.30 |
| C2 to (C3 + C5) ratio | 0.06 | <0.01 |
| HOMA-IR | 0.32 | 0.23 |
| Adiponectin (−) | 0.27 | 0.02 |
| Correlates of leptin | ||
| BMI z-score | 0.32 | 0.42 |
| BF% | 0.36 | 0.35 |
| CRP | 0.08 | 0.24 |
Statistically significant correlations are highlighted in bold.
The level of C2 acylcarnitine (an end product of complete fatty acid oxidation) divided by the sum of C3 + C5 acylcarnitines (byproducts of BCAA and methionine catabolism) correlated inversely with HOMA-IR (R2 = 0.15, P = .0003) in the cohort as a whole. In sex-stratified models, HOMA-IR correlated negatively with the ratio C2 to (C3 + C5) in males (R2 = 0.35 P < .0001) but not in females (Table 2). Leptin in females correlated positively with BMI z-score, BF%, and CRP; leptin in males also correlated with BMI z-score and BF% but not with CRP. Leptin did not correlate with HOMA-IR or adiponectin in either males or females.
Multivariate analysis: PCA
A PCA was used to consolidate the metabolites into 17 components. The constituents for each component are shown in Table 3. To control for age, sex, and BMI z-score and the effects of other factors, we performed multivariate linear regression analyses. We used HOMA-IR, adiponectin, and the TG to HDL ratio as dependent variables to identify metabolic markers of insulin resistance and insulin sensitivity. We also performed the same analyses using leptin as a dependent variable.
Table 3.
Factors Obtained From PCA
| Factor | Metabolites Within Factor | Description | Eigenvalue | Proportion Variance | Cumulative Variance |
|---|---|---|---|---|---|
| 1 | C2 βOH-butyrate KET C14:1 C16:1 C4-OH C18:1 C16:2 C14:2 NEFA C14 C12 C12:1 C16 C6-DC/C8-OH C10-OH/C8-DC C18:1OH/C16:1-DC C10 C18:2 C16:1-OH/C14:1-DC | FAO byproducts | 13.79 | 0.23 | 0.23 |
| 2 | LEU/ILE GLX valine C3 URIC C5 | BCAA-related and uric acid | 5.64 | 0.09 | 0.32 |
| 3 | C8 C10 C10:1 C10-OH/C8-DC | Medium-chain acylcarnitines | 3.96 | 0.07 | 0.39 |
| 4 | C10:3 C8:1 C12:1 | Medium-chain acylcarnitines | 3.14 | 0.05 | 0.44 |
| 5 | C20:4 C18 C14:1-OH C16 C14 | Long-chain acylcarnitines | 2.67 | 0.04 | 0.49 |
| 6 | C8:1-DC C8:1-OH/C6:1-DC C14:1-OH | Medium-chain acylcarnitines | 2.41 | 0.04 | 0.53 |
| 7 | ARG HIS ORN CIT | Urea cycle amino acids and HIS | 2.15 | 0.04 | 0.56 |
| 8 | C5-DC C4-DC/Ci4-DC | Short-chain acylcarnitines | 1.88 | 0.03 | 0.59 |
| 9 | SER GLY ASX C4-Ci4 (−) | Glucogenic amino acids | 1.82 | 0.03 | 0.62 |
| 10 | TYR CRP PHE MET | Large neutral amino acids and CRP | 1.66 | 0.03 | 0.65 |
| 11 | PRO ALA | Glucogenic amino acids | 1.57 | 0.03 | 0.68 |
| 12 | C5:1 ASX C18:1-DC (−) C20-OH/C18-DC (−) | C5:1, ASX, and long-chain dicarboxyl acylcarnitines | 1.47 | 0.02 | 0.70 |
| 13 | GLU C18:2-OH | Miscellaneous | 1.31 | 0.02 | 0.72 |
| 14 | C22 URIC (−) | Miscellaneous | 1.24 | 0.02 | 0.75 |
| 15 | C16:1-OH/C14:1-DC C18:1-DC LACT (−) | Long-chain acylcarnitines and LACT | 1.18 | 0.02 | 0.76 |
| 16 | C20 C18:1-OH/C16:1-DC | Long-chain acylcarnitines | 1.10 | 0.02 | 0.78 |
| 17 | C14-OH/C12-DC | Miscellaneous | 1.03 | 0.02 | 0.80 |
Abbreviations: NEFA, nonesterified fatty acid; ARG, arginine; HIS, histidine; ORN, ornithine; CIT, citrulline; SER, serine; GLY, glycine; ASX, aspartate/asparagine; TYR, tyrosine; CRP, C-reactive protein; PHE, phenylalanine; MET, methionine; HIS, histidine; LACT, lactate; KET, Ketones; LEU/ILE, Leucine/Isoleucine; GLX, Glutamate/Glutamine; URIC, Uric Acid; PRO, Proline; ALA, Alanine. Negative sign (−) indicates negative correlation with the associated factor.
Homeostasis model assessment index of insulin resistance
Independent of age, sex, and BMI z-score, three components were positively associated with HOMA-IR in the cohort as a whole (Table 4): factor 2 (BCAA related and uric acid), factor 11 (glucogenic amino acids), and factor 12 (C5:1, aspartate/asparagine, and long chain dicarboxyl acylcarnitines). Conversely, factor 1 (fatty acid oxidation byproducts) and factor 14 (long chain acylcarnitines and uric acid) correlated negatively with HOMA-IR. Together these factors explained 55% of the variance in HOMA-IR (n = 82, R2 = 0.549, P < .0001). In this multivariate model, HOMA-IR levels were significantly higher in females than males. This relationship was not apparent in the comparisons presented in Table 1 before the adjustment for age, BMI z-score, and factors 1, 2, 11, 12, and 14.
Table 4.
Summary of Baseline Factors Associated With HOMA-IR, Adiponectin, TG to HDL Ratio, and Leptin (Sex, Age, and BMI Z-Score Adjusted)
| Full Sample | Parameter Estimate | t Value | P Value |
|---|---|---|---|
| HOMA-IR (n = 82, R2 = 0.549, P < .0001) | |||
| Female | 0.283 | 2.60 | .0112 |
| BMI z-score | 0.625 | 4.57 | <.0001 |
| Factor 1: FAO byproducts | −0.156 | −3.39 | .0011 |
| Factor 2: BCAA-related and uric acid | 0.184 | 3.30 | .0015 |
| Factor 11: glucogenic amino acids | 0.134 | 2.95 | .0043 |
| Factor 12: C5:1, ASX, and long-chain dicarboxyl acylcarnitines | 0.177 | 3.84 | .0003 |
| Factor 14: miscellaneous | −0.151 | −3.17 | .0023 |
| Adiponectin (n = 82, R2 = 0.171, P = .0124) | |||
| Age | −0.064 | −2.58 | .0119 |
| Factor 2: BCAA-related and uric acid | −0.116 | −2.58 | .0117 |
| Factor 4: medium-chain acylcarnitines | −0.080 | −2.09 | .0403 |
| TG to HDL ratio (n = 82, R2 = 0.607, P < .0001) | |||
| BMI z-score | 0.411 | 2.79 | .0067 |
| Factor 2: BCAA-related and uric acid | 0.246 | 4.09 | .0001 |
| Factor 9: glucogenic amino acids (SER GLY ASX C4-Ci4) | −0.150 | −3.05 | .0032 |
| Factor 11: glucogenic amino acids (PRO ALA) | 0.203 | 4.15 | <.0001 |
| Factor 12: C5:1, ASX, and long-chain dicarboxyl acylcarnitines | 0.294 | 5.93 | <.0001 |
| Factor 14: miscellaneous | −0.146 | −2.85 | .0057 |
| Leptin (n = 82, R2 = 0.537, P < .0001) | |||
| Female | 0.707 | 6.29 | <.0001 |
| BMI z-score | 1.125 | 7.53 | <.0001 |
| Factor 15: long-chain acylcarnitines and LACT | −0.210 | −3.70 | .0004 |
Abbreviations: ASX, aspartate/asparagine; LACT, lactate. Negative sign (−) indicates negative correlation with the associated factor. Sex, age, and BMI z-score are included in to all models; however, only statistically significant variables are reported for the full sample.
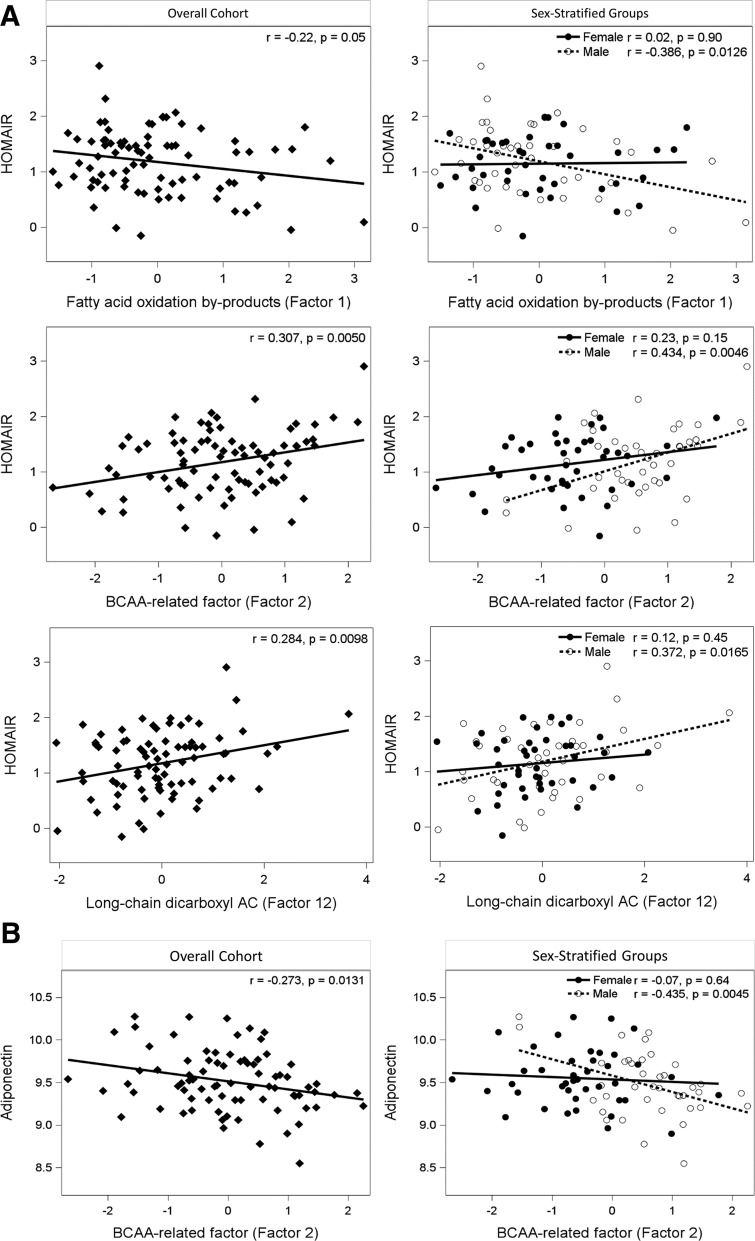
Secondary analyses showed significant sex interaction effects; we therefore analyzed sex-stratified regression models. These models revealed that HOMA-IR correlated positively with BMI z-score (P = .0167) and negatively with factor 14 (P = .0195) in females; in contrast, HOMA-IR in males correlated positively with factor 2 (BCAA related factor, P = .0119), factor 12 (P = .0007), and BMI z-score (P = .0023) and negatively with factor 1 (fatty acid oxidation byproducts, P = .0158) (Table 5). There was a positive linear relationship between the BCAA-related factor (factor 2), factor 12, and HOMA-IR and a negative association between the byproducts of fatty acid oxidation (FAO) and HOMA-IR (Figure 1A) in males but not females.
Table 5.
Summary of Relationships Between HOMA-IR, Adiponectin, TG to HDL Ratio, Leptin, and Metabolite Components in Females and Males (Age and BMI Z-Score Adjusted)
| Variables | Parameter Estimate | t Value | P Value |
|---|---|---|---|
| Male | |||
| HOMA-IR (n = 41, R2 = 0.659, P < .0001) | |||
| BMI z-score | 0.641 | 3.30 | .0023 |
| Factor 1: FAO byproducts | −0.166 | −2.55 | .0158 |
| Factor 2: BCAA-related and uric acid | 0.244 | 2.66 | .0119 |
| Factor 12: C5:1, ASX, and long-chain dicarboxyl acylcarnitines | 0.222 | 3.75 | .0007 |
| Adiponectin (n = 41, R2 = 0.268, P = .0212) | |||
| Factor 2: BCAA-related and uric acid | −0.189 | −2.81 | .0080 |
| TG to HDL ratio (n = 41, R2 = 0.601, P < .0001) | |||
| Factor 9: glucogenic amino acids (SER GLY ASX C4-Ci4) | −0.179 | −2.31 | .0274 |
| Factor 11: glucogenic amino acids (PRO ALA) | 0.246 | 2.84 | .0077 |
| Factor 12: C5:1, ASX and long-chain dicarboxyl acylcarnitines | 0.279 | 4.05 | .0003 |
| Factor 14: miscellaneous | −0.162 | −2.21 | .0342 |
| Leptin (n = 41, R2 = 0.505, P < .0001) | |||
| Age | −0.150 | −2.48 | .0177 |
| BMI z-score | 1.499 | 5.83 | <.0001 |
| Factor 15: long-chain acylcarnitines and LACT | −0.232 | −2.29 | .0028 |
| Female | |||
| HOMA-IR (n = 41, R2 = 0.441, P = .0046) | |||
| BMI z-score | 0.556 | 2.52 | .0167 |
| Factor 14: miscellaneous | −0.239 | −2.46 | .0195 |
| Adiponectin (n = 41) | |||
| No factor is statistically significant | |||
| TG to HDL ratio (n = 41, R2 = 0.662, P < .0001) | |||
| BMI z-score | 0.628 | 2.99 | .0052 |
| Factor 2: BCAA-related and uric acid | 0.282 | 3.64 | .0009 |
| Factor 11: Glucogenic amino acids (PRO ALA) | 0.155 | 2.37 | .0239 |
| Factor 12: C5:1, ASX, and long-chain dicarboxyl acylcarnitines | 0.326 | 3.83 | .0006 |
| Leptin (n = 41, R2 = 0.624, P < .0001) | |||
| Age | 0.095 | 2.86 | .0069 |
| BMI z-score | 1.075 | 7.41 | <.0001 |
| Factor 15: long-chain acylcarnitines and LACT | −0.124 | −2.75 | .0092 |
Abbreviations: ASX, aspartate/asparagine; LACT, lactate.
Figure 1.
A, Correlations between HOMA-IR and FAO byproducts (factor 1), BCAA-related factor (factor 2), and long-chain dicarboxyl acylcarnitine (factor 12). B, Correlation between adiponectin and BCAA-related factor (factor 2). Correlations are shown for the overall cohort (left side) and sex-stratified groups (right side). HOMA-IR values and adiponectin are natural log transformed.
Adiponectin
Independent of age, sex, and BMI z-score, two components were negatively associated with adiponectin (n = 82, R2 = 0.171, P = .0124) in the cohort as a whole (Table 4): factor 2 (BCAA related and uric acid), and factor 4 (medium chain acylcarnitines). In sex-stratified models, only factor 2 (P = .0080) remained statistically significant in males (Table 5, Figure 1B).
TG to HDL ratio
Independent of age, sex, and BMI z-score (Table 4), the TG to HDL ratio correlated positively with factor 2 (BCAA related and uric acid), factor 11, and factor 12 and negatively with factors 9 and 14. Factors 11 and 12 correlated positively with the TG to HDL ratio in sex-stratified models (Table 5). Factor 2 also correlated positively with the TG to HDL ratio in females; factors 9 and 14 correlated negatively with the TG to HDL ratio in males.
Leptin
Leptin levels were significantly higher in females than males, even after adjusting for age, BMI z-score, and factor 15. After adjusting for age and sex (Table 4), leptin correlated positively with BMI z-score and negatively with factor 15. These associations were also significant in sex-stratified models. Interestingly, age had significant effects in sex-stratified models in opposite directions: positively associated in females but negatively associated in males.
Adjustment for pubertal status and height z-scores
Tanner staging information was available for 53 subjects (32 males and 21 females). The distribution of Tanner stages (II/III/IV/V) across males (6/6/11/9) and females (0/2/10/9) was comparable based on the Fisher's exact test (P = .1116). The selected models were reestimated by controlling for Tanner stage in addition to sex, age, and BMI z-score. Even when adjusted for puberty, the models were reproducible (Supplemental Table 3).
To investigate the possible effects of height on insulin resistance, we included height z-score in the selected regression models along with age, sex, BMI z-score, and PCA factors. We did not find height z-score to be statistically significant in any of the models.
Discussion
Comprehensive metabolic profiling has provided new insights into the mechanisms underlying insulin resistance in adult populations (12–17). In contrast, only a few studies have used metabolomics to characterize the metabolic status of obese children and adolescents (18–20). In this study we used targeted metabolic profiling to identify markers of insulin resistance in obese adolescents. Our findings include three important observations. First, the fasting levels of BCAA and products of BCAA catabolism (C3 and C5 acylcarnitines and glutamate/glutamine) are significantly higher in obese teenage boys than obese girls of a similar age and BMI z-score. Second, BCAA-related metabolites and byproducts of FAO are associated with IR in adolescents. Finally, the relationships among surrogate measures of IR (HOMA-IR, adiponectin, and the TG to HDL ratio) and amino acid and fatty acid metabolism differ strikingly according to sex.
Our study used HOMA-IR, adiponectin, and the TG to HDL ratio as surrogate markers of IR. HOMA-IR is largely a measure of hepatic insulin sensitivity because it reflects fasting insulin and glucose levels (4, 21, 22). Adiponectin functions as an insulin sensitizer by decreasing hepatic glucose output and increasing glucose uptake and fatty acid oxidation in muscles (23–25). The ratio of TG to HDL has been associated previously with IR in obese white boys and girls (26).
We used a series of statistical analyses to assess the relationships between these surrogate markers of IR and the levels of various amino acids and amino acid and fatty acid metabolites. In the initial bivariate analyses, HOMA-IR in females correlated negatively with HDL and positively with TGs. In males, HOMA-IR correlated positively with BMI z-score and TGs and negatively with HDL, adiponectin, and the ratio of C2 carnitine to the sum of C3 and C5 acylcarnitines. Adiponectin in males correlated positively with HDL and negatively with insulin and uric acid. The ratio of TG to HDL correlated positively with insulin and uric acid in males and females, negatively with adiponectin in males, and positively with levels of BCAA in females.
We then used PCA to reduce the large number of correlated metabolites into clusters of fewer components that were not correlated with each other. The metabolites that compose a factor were biologically related, providing a biological description for each factor.
Of 17 factors identified in our analysis, factors 1 and 2 were of particular interest because they relate to FAO and BCAA catabolism, respectively. Fatty acids and fatty acid-derived metabolites have been implicated in the development of insulin resistance and T2D, whereas BCAA and related metabolites are associated with insulin resistance in adults (12–17) and, in one study (20), children. It is proposed that BCAA synergizes with hyperlipidemia to facilitate progression from the obese, insulin-resistant state to overt T2D (27).
In our study, HOMA-IR correlated positively with a BCAA-related metabolic signature and negatively with byproducts of complete FAO. Furthermore, the ratio of C2 to (C3 + C5) also correlated negatively with HOMA-IR. C2 (acetyl) carnitine is an end product of complete FAO, whereas C3 and C5 (propionyl, α-methylbutyryl, and isovalerylcarnitine species) carnitines are byproducts of BCAA and methionine catabolism. Thus, increased BCAA, increased BCAA catabolism, and decreased complete FAO were associated with higher HOMA-IR. Adiponectin, a marker of insulin sensitivity, correlated negatively with the BCAA-related metabolic signature but did not associate with byproducts of FAO (factor 1). The TG to HDL ratio associated with the BCAA signature but not with factor 1.
The BCAA-related metabolic signature included the glutamate/glutamine as well as C3 and C5 acylcarnitines. Glutamate is produced in the first step of BCAA catabolism. Accumulation of glutamate may increase transamination of pyruvate to alanine, which may promote gluconeogenesis and thereby contribute to development of glucose intolerance in obesity (27). Consistent with this hypothesis, the glucogenic amino acids proline and alanine also correlated positively with HOMA-IR and the TG to HDL ratio.
In contrast to BCAA catabolites, the byproducts of FAO (factor 1) correlated negatively with HOMA-IR independent of age, sex, and BMI z-score. Muoio and Newgard (28) suggest that high-fat feeding promotes an adaptive increase in the enzymes of FAO in muscle that is not matched in sedentary animals or humans by a parallel increase in enzymes of the tricarboxylic acid cycle. This disconnect results in the accumulation of incompletely oxidized lipid species in mitochondria; the latter causes mitochondrial stress and impairs insulin action (29, 30). Thus, we hypothesize that obese adolescents who are able to oxidize fatty acids completely are protected against developing insulin resistance.
Our findings differ in some respects from previous studies in children. Michaliszyn et al (19) profiled amino acids, β-cell function, and insulin sensitivity in normal-weight, obese, and T2D adolescents. In contrast to studies in adults and to our findings, they found that the disposition index was positively associated with BCAA, neutrally transported amino acids, basic amino acids, glycine, and C3, C4, and C5 acylcarnitines. However, they did not analyze their results according to sex. The contrasting observations between adults and youth were explained by developmental differences along the life span. Our results do not support this hypothesis. The inclusion of patients who had already progressed to diabetes might have influenced their results (31, 32).
Mihalik et al (18) compared acylcarnitine species, common amino acids, FAO byproducts, and plasma amino acids in normal-weight, obese, and T2D adolescents. They found no differences in long-chain acylcarnitines, but medium- to short-chain acylcarnitines were significantly lower in T2D patients. They explained the inconsistency with findings in adults with early adaptive metabolic plasticity in youth. However, they did not use PCA or explore possible differences between males and females. McCormack et al (20) found that sex did not predict HOMA-IR in a longitudinal study of obese children.
In contrast, we identified striking differences in metabolism in adolescent girls and boys (Tables 1, 2, 4, 5 and Supplemental Table 4). In sex-stratified multivariate models, HOMA-IR levels were higher in females than males after adjusting for age, BMI z-score, and factors 1, 2, 11, 12, and 14. The associations between HOMA-IR, the BCAA-related metabolic signature, and the byproducts of FAO were statistically significant in males but not females. In contrast, HOMA-IR in females was related most closely to BMI z-score and a factor consisting of C22 and uric acid. Likewise, adiponectin correlated negatively with the BCAA-related metabolic signature only in males. Interestingly, the TG to HDL ratio correlated with the BCAA signature in females but not males. These findings suggest that sex differences play diverse roles in the pathogenesis of pubertal IR.
The pathogenesis of sex-dependent differences in amino acid and fatty acid metabolism are poorly understood. Possible factors include differences in sex steroid and/or GH production and action and variations in body fat content and distribution. Studies in genetically engineered mouse models demonstrate that estrogen increases insulin sensitivity and limits body fat deposition; these effects are opposed by progesterone (33, 34). Yet a study in teenage boys and girls found no relationship between sex steroid levels and measures of carbohydrate metabolism during puberty (35). Likewise, a longitudinal investigation in boys and girls found no correlation between pubertal changes in insulin sensitivity and changes in levels of estrogens and androgens (36). GH secretory responses are higher in pubertal girls than boys; however, the relationship between GH secretion and the metabolic differences we observed will require additional study. Finally, body fat content and sc fat deposition increase in girls during puberty, whereas fat percentage declines and lean body mass increases in teenage boys (37). Increases in body (particularly abdominal and visceral) fat and reductions in lean body mass are associated with IR in adolescents as well as adults.
Although not emphasized previously, other studies have found sex differences in metabolic predictors of insulin sensitivity and disposition index in adults (38). More importantly, the Type 2 Diabetes in Adolescents and Youth study showed that T2D is more common in adolescent females than males despite comparable BMI z-scores (39). Moreover, treatment with metformin plus rosiglitazone was far more effective in diabetic girls than boys (40). It is unclear whether the sex differences in amino acid and fatty acid metabolism that we observed contribute to differential rates of T2D in teenage girls and boys and/or differences in their responses to pharmacological agents.
Our study has several limitations. It was a cross-sectional investigation with a relatively small sample size, limiting the ability to detect ancestry-specific effects. Normal-weight children and patients with T2D were not included. Female subjects were not studied at standard phases of the menstrual cycle. We did not measure sex steroid levels or GH secretory responses. Body composition and distribution were not analyzed. Moreover, we used surrogate measures of IR; alternative methods including insulin and glucose clamps and iv and oral glucose tolerance tests might have provided useful information.
Finally, multiple statistical comparisons and associations may in some cases lead to spurious conclusions. To ensure the validity of the various associations, we selected a conservative threshold of 0.01 for significance tests for bivariate correlations; moreover, nearly all of the significant values in our regression analyses were P < .01. However, the exploratory nature of our study requires that our findings be interpreted with caution.
Nevertheless, we have identified striking sex-dependent differences in biomarkers associated with insulin resistance in obese adolescents. In males, HOMA-IR correlates positively, and adiponectin negatively, with a metabolic signature containing BCAA, glucogenic amino acids, and uric acid. HOMA-IR also associates negatively with byproducts of FAO. In contrast, HOMA-IR in females associates primarily with BMI z-score, whereas the TG to HDL ratio correlates with BCAA metabolism in females but not males. Our findings underscore the roles of sex differences in metabolic function and outcomes in pediatric obesity. Future prospective studies will be needed to determine whether metabolic profiling can predict the development of glucose intolerance and T2D in high-risk children.
Acknowledgments
This work was supported by a grant from the Duke Children's Miracle Network (to D.D. and D.N.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BCAA
- branched-chain amino acid
- BF%
- body fat percentage
- BMI
- body mass index
- CRP
- C-reactive protein
- FAO
- fatty acid oxidation
- HDL
- high-density lipoprotein
- HOMA-IR
- homeostasis model assessment index of insulin resistance
- IR
- insulin resistance
- PCA
- principal components analysis
- T2D
- type 2 diabetes mellitus
- TG
- triglyceride.
References
- 1. Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. [DOI] [PubMed] [Google Scholar]
- 2. Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116:473–480. [DOI] [PubMed] [Google Scholar]
- 3. Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among US adolescents: NHANES 2005–2006. Diabetes Care. 2009;32:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levy-Marchal C, Arslanian S, Cutfield W, et al. ESPE-LWPES- ISPAD-APPES-APEG-SLEP-JSPE; Insulin Resistance in Children Consensus Conference Group. Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab. 2010;95:5189–5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 6. An J, Muoio DM, Shiota M, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole animal insulin resistance. Nat Med. 2004;10:268–274. [DOI] [PubMed] [Google Scholar]
- 7. Millington DS, Kodo N, Norwood DL, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis. 1990;13:321–324. [DOI] [PubMed] [Google Scholar]
- 8. Wu JY, Kao HJ, Li SC, et al. ENU mutagenesis identifies mice with mitochondrial branched chain aminotransferase deficiency resembling human maple syrup urine disease. J Clin Invest. 2004;113:434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chace DH, Hillman SL, Millington DS, Kahler SG, Roe CR, Naylor EW. Rapid diagnosis of maple syrup urine disease in blood spots from newborns by tandem mass spectrometry. Clin Chem. 1995;41:62–68. [PubMed] [Google Scholar]
- 10. Ferrara CT, Wang P, Neto EC, et al. Genetic network of liver metabolism revealed by integration of metabolic and transcriptional profiling. PLoS Genet. 2008;4(3):e1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Rourke N, Hatcher L. A Step-by-Step Approach to Using SAS for Factor Analysis and Structural Equation Modeling. 2nd ed Cary, NC: SAS Publishing; 2014:1–42. [Google Scholar]
- 12. Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. Metabolomics applied to diabetes research: moving from information to knowledge. Diabetes. 2009;58:2429–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Batch BC, Shah SH, Newgard CB, et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism. 2013;62(7):961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah SH, Bain JR, Muehlbauer MJ, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3(2):207–214. [DOI] [PubMed] [Google Scholar]
- 17. Bain JR. Targeted metabolomics finds its mark in diabetes research. Diabetes. 2013;62(2):349–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mihalik SJ, Michaliszyn SF, de las Heras J, et al. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: evidence for enhanced mitochondrial oxidation. Diabetes Care. 2012;35(3):605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michaliszyn SF, Sjaarda LA, Mihalik SJ, et al. Metabolomic profiling of amino acids and β-cell function relative to insulin sensitivity in youth. J Clin Endocrinol Metab. 2012;97(11):E2119–E2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCormack SE, Shaham O, McCarthy MA, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013;8:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–E26. [DOI] [PubMed] [Google Scholar]
- 22. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. [DOI] [PubMed] [Google Scholar]
- 23. Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27(7):762–778. [DOI] [PubMed] [Google Scholar]
- 24. Trujillo ME, Scherer PE. Adiponectin—journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257(2):167–175. [DOI] [PubMed] [Google Scholar]
- 25. Winer JC, Zern TL, Taksali SE, et al. Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:4415–4423. [DOI] [PubMed] [Google Scholar]
- 26. Giannini C, Santoro N, Caprio S, et al. The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care. 2011;34(8):1869–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Newgard CB. Interplay between lipids and branched chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. [DOI] [PubMed] [Google Scholar]
- 29. Koves TR, Li P, An J, et al. Peroxisomal proliferator-activated receptor-γ co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. [DOI] [PubMed] [Google Scholar]
- 30. Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. [DOI] [PubMed] [Google Scholar]
- 31. Lowe WL, Jr, Bain JR. “Prediction is very hard, especially about the future”: new biomarkers for type 2 diabetes? Diabetes. 2013;62(5):1384–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferrannini E, Natali A, Camastra S, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA. 2000. July;97(23):12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bryzgalova G1, Gao H, Ahren B, et al. Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49(3):588–597. [DOI] [PubMed] [Google Scholar]
- 35. Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res. 2006;60(6):759–763. [DOI] [PubMed] [Google Scholar]
- 36. Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444–2450. [DOI] [PubMed] [Google Scholar]
- 37. Brufani C, Tozzi A, Fintini D, et al. Sexual dimorphism of body composition and insulin sensitivity across pubertal development in obese Caucasian subjects. Eur J Endocrinol. 2009;160(5):769–775. [DOI] [PubMed] [Google Scholar]
- 38. Huffman KM, Shah SH, Stevens RD, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32(9):1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Copeland KC, Zeitler P, Geffner M, et al. TODAY Study Group. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96(1):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]