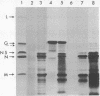
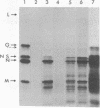
Abstract
The virion transcriptase (nucleosidetriphosphate: RNA nucleotidyltransferase, EC 2.7.7.6) of vesicular stomatitis virus was fully active when ribonucleoprotein cores from purified virions were added to cell-free protein synthesizing systems of eukaryotic origin. Synthesis of mRNA was linear for at least 3 hr and the newly synthesized viral mRNA was efficiently utilized for the synthesis of viral proteins N (nucleoprotein), NS, and M (matrix); small amounts of a putative G (glycoprotein protein precursor and several unidentified polypeptides were regularly synthesized. The ratio of the various newly synthesized viral proteins was identical after different periods of coupled mRNA and protein synthesis. Identical proteins were obtained when the cell-free protein synthesizing systems were programmed with purified VSV mRNA synthesized in vitro. No detectable L protein was synthesized, even though transcripts complementary to the complete viral genome were detectable in the mRNA preparation by hybridization.
Full text
PDF

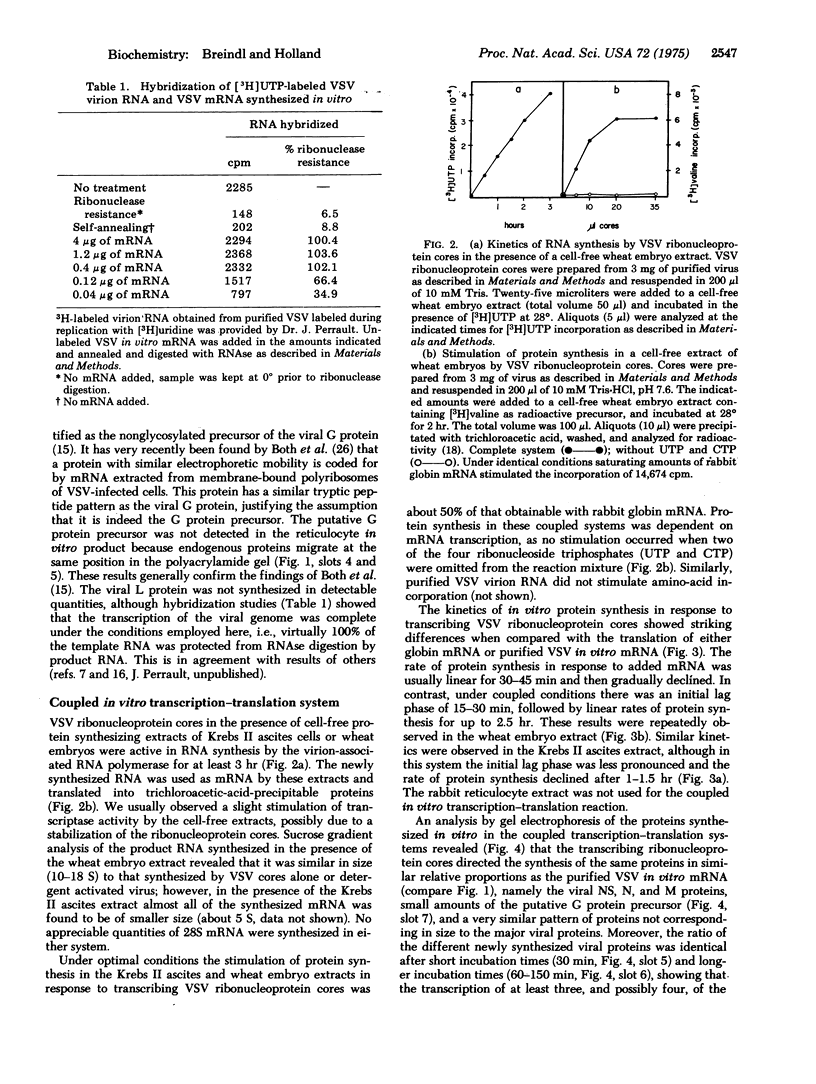
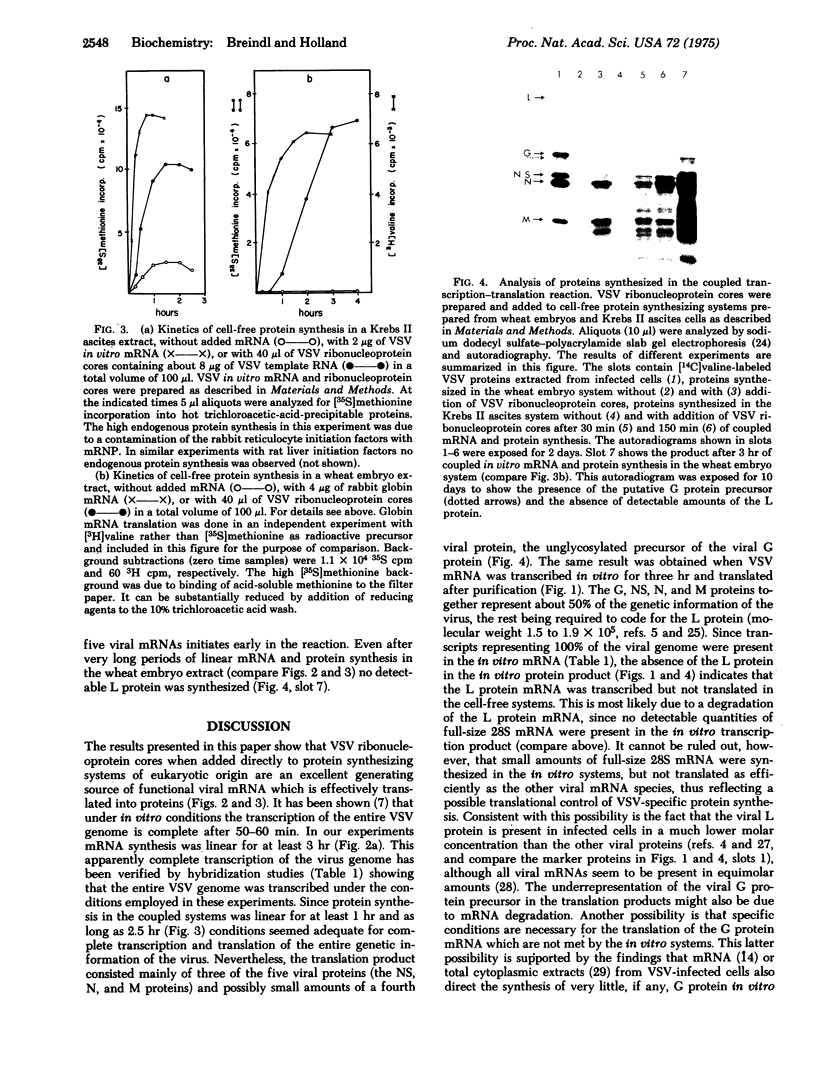

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. K., Rhodes D. P. In vitro synthesis of RNA that contains polyadenylate by virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3566–3570. doi: 10.1073/pnas.70.12.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H. Complete transcription by the transcriptase of vesicular stomatitis virus. J Virol. 1971 Apr;7(4):486–490. doi: 10.1128/jvi.7.4.486-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Kinetics of RNA synthesis by vesicular stomatitis virus particles. J Mol Biol. 1971 May 14;57(3):513–527. doi: 10.1016/0022-2836(71)90106-9. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Properties of the product synthesized by vesicular stomatitis virus particles. J Mol Biol. 1971 Jun 28;58(3):799–814. doi: 10.1016/0022-2836(71)90041-6. [DOI] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the mRNA species synthesized in vitro by the virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1975 Jan;72(1):274–278. doi: 10.1073/pnas.72.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the viral mRNA species isolated from subcellular fractions of vesicular stomatitis virus-infected cells. J Virol. 1975 Apr;15(4):1012–1019. doi: 10.1128/jvi.15.4.1012-1019.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breindl M., Gallwitz D. On the translational control of histone synthesis. Quantitation of biologically active histone mRNA from synchronized HeLa cells and its translation in different cell-free systems. Eur J Biochem. 1974 Jun 1;45(1):91–97. doi: 10.1111/j.1432-1033.1974.tb03533.x. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Summers D. F. In vitro protein-synthesizing activity of vesicular stomatitis virus-infected cell extracts. J Virol. 1973 Aug;12(2):265–274. doi: 10.1128/jvi.12.2.265-274.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Comparative sedimentation coefficients of RNA extracted from plaque-forming and defective particles of vesicular stomatitis virus. J Mol Biol. 1966 Dec 28;22(2):381–384. doi: 10.1016/0022-2836(66)90143-4. [DOI] [PubMed] [Google Scholar]
- JOHNSTON F. B., STERN H. Mass isolation of viable wheat embryos. Nature. 1957 Jan 19;179(4551):160–161. doi: 10.1038/179160b0. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. 3. Intracellular synthesis and extracellular appearance of virus-specific proteins. Virology. 1971 Dec;46(3):678–690. doi: 10.1016/0042-6822(71)90070-5. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. I. Polyacrylamide gel analysis of viral antigens. J Virol. 1969 Apr;3(4):404–413. doi: 10.1128/jvi.3.4.404-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrison T., Stampfer M., Baltimore D., Lodish H. F. Translation of vesicular stomatitis messenger RNA by extracts from mammalian and plant cells. J Virol. 1974 Jan;13(1):62–72. doi: 10.1128/jvi.13.1.62-72.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Polysomal ribonucleic acid of vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Dec;42(4):958–968. doi: 10.1016/0042-6822(70)90344-2. [DOI] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- Obijeski J. F., Marchenko A. T., Bishop D. H., Cann B. W., Murphy F. A. Comparative electrophoretic analysis of the virus proteins of four rhabdoviruses. J Gen Virol. 1974 Jan;22(1):21–33. doi: 10.1099/0022-1317-22-1-21. [DOI] [PubMed] [Google Scholar]
- Reichmann M. E., Villarreal L. P., Kohne D., Lesnaw J., Holland J. J. RNA polymerase activity and poly(A) synthesizing activity in defective T particles of vesicular stomatitis virus. Virology. 1974 Mar;58(1):240–249. doi: 10.1016/0042-6822(74)90158-5. [DOI] [PubMed] [Google Scholar]
- Repik P., Bishop D. H. Determination of the molecular weight of animal RNA viral genomes by nuclease digestions. I. Vesicular stomatitis virus and its defective T particle. J Virol. 1973 Nov;12(5):969–983. doi: 10.1128/jvi.12.5.969-983.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P. Translation of brome mosaic viral ribonucleic acid in a cell-free system derived from wheat embryo. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1799–1803. doi: 10.1073/pnas.70.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamminger G., Lazzarini R. A. Analysis of the RNA of defective VSV particles. Cell. 1974 Sep;3(1):85–93. doi: 10.1016/0092-8674(74)90044-0. [DOI] [PubMed] [Google Scholar]
- Stewart A. G., Gander E. S., Morel C., Luppis B., Scherrer K. Differential translation of duck- and rabbit-globin messenger RNAs in reticulocyte-lysate systems. Eur J Biochem. 1973 Apr;34(2):205–212. doi: 10.1111/j.1432-1033.1973.tb21105.x. [DOI] [PubMed] [Google Scholar]
- Villarreal L. P., Holland J. J. Synthesis of poly(A) in vitro by purified virions of vesicular stomatitis virus. Nat New Biol. 1973 Nov 7;246(149):17–19. doi: 10.1038/newbio246017a0. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]