Abstract
Demineralized bone matrix has been successfully commercialized as an alternative bone graft material that not only can function as filler but also as an osteoinductive graft. Numerous studies have confirmed its beneficial use in clinical practice. Heterotopic ossification after internal fixation combined with the use of demineralized bone matrix has not been widely reported.
In this paper we describe a 39 year old male who sustained a complex articular fracture that developed clinically significant heterotopic ossification after internal fixation with added demineralized bone matrix.
Although we cannot be sure that there is a cause-and-effect relation between demineralized bone matrix and the excessive heterotopic ossification seen in our patient, it seems that some caution in using demineralised bone matrix in similar cases is warranted. Also, given the known inter- and intraproduct variability, the risks and benefits of these products should be carefully weighed.
Key words: Demineralized bone matrix, Heterotopic ossification, Periarticular, Tibia plateau fracture
Introduction
Since the seminal work of Marshall Urist we know that demineralized bone matrix (DBM) contains osteoinductive factors (1). DBM is bone that is pulverized and then decalcified using acid extraction (2). What remains after loss of the mineralized component is type I collagen and growth factors in varying concentrations. Most important growth factors for its grafting properties are the bone morphogenetic proteins (BMPs) and transforming growth factor beta (TGF-β) (3).
Numerous studies have confirmed its beneficial use in clinical practice, where they most often are used in the spine, long bones and craniomaxillofacial surgery. Of some concern is that performance consistency has been shown to vary widely between different DBM formulations and between production lots of a single DBM product. Most of these reports have measured extracted BMP concentrations in vivo and/or in vitro (3-6). DBM's regulatory status as a medical device (as classified by the Food and Drug Administration) makes it relatively simple and cheap to market DBM with few barriers of entry compared to related products (3). We should realize that there is currently a paucity of Level I or Level II evidence for the use of DBM when used alone in human patients (3).
During the last 5 years, the senior author has used DBM of a single supplier (DBX putty, Synthes BV, Zeist, The Netherlands in partnership with the Musculoskeletal Transplant Foundation (MTF, Edison, NJ, USA)) in over 80 patients for acute fractures and nonunions of the appendicular skeleton. Although in nonunions we generally prefer autologous cancellous bone graft, sometimes the amount that is harvested is not sufficient or bone graft donor sites have already been used prior. We report the occurrence of clinically significant heterotopic ossification (HO) after the use of DBM in the treatment of 1 of these patients. We feel the excessive amount of HO was uncharacteristic for the kind of fracture and its repair. Although we cannot directly relate the excessive HO to the use of DBM, we think its occurrence warrants reporting.
Case presentation
A 39-year old man sustained a Schatzker IV fracture of his right tibia plateau in a motorcycle accident. There were no neurologic injuries. The patient had no known medical history and was not taking any prescribed drugs. The patient was taken to the operating room that same day where a knee spanning external fixator was placed as limb damage control.
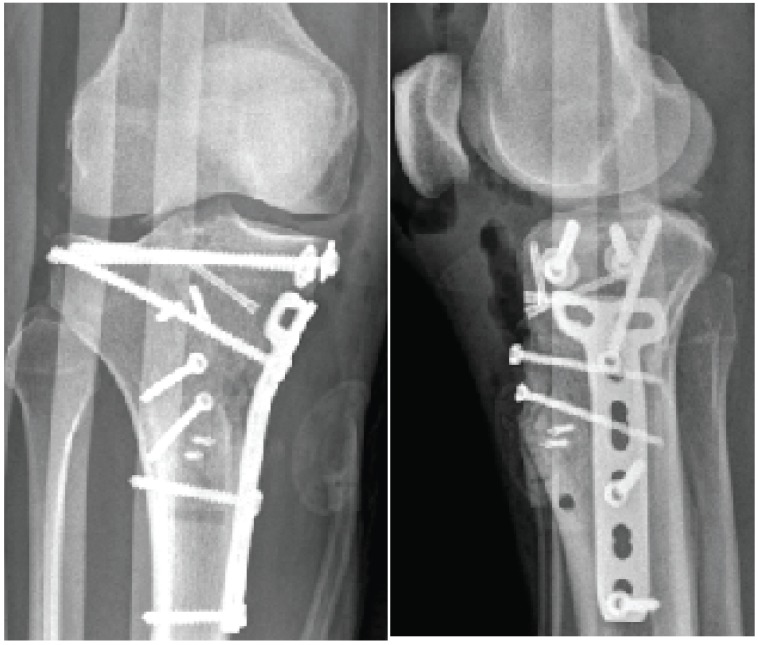
After two weeks, definitive internal fixation of the tibia plateau fracture was done using an anteromedial approach [Figure 1]. After temporarily fixing the plateau with multiple K-wires, two lag screws were placed in the subchondral bone from medial to lateral. A medial 4.5 titanium T-plate was placed as a buttress. The tibial tuberosity was fixed with two bicortical 3.5 mm titanium screws. No autologous bone graft or bone morphogenetic proteins (BMPs) were used. Instead, we used 5cc DBX Putty (Synthes BV, Zeist, The Netherlands) mixed with allograft cancellous bone chips (Bone Bank Allografts, San Antonio, TX, USA) to fill in a corticocancellous defect on the anteromedial aspect of the tibia. Care was given to remove excess DBM from the soft tissues.
Figure 1.
Anteroposterior (a) and lateral (b) radiographs taken 1 day after definitive internal fixation.
Postoperatively the patient was allowed toe-touch weight bearing for 3 months. He was not allowed to actively extend his knee for 6 weeks to protect the fixation of the tibial tuberosity. At 3 months follow up, his knee range of motion was 90 degrees of flexion with full extension. The knee was stable and he was allowed full weight bearing. The radiographs taken at that time showed a healed and well-aligned tibia plateau. Of note, however, were some heterotopic ossification seen in Hoffa's fat pad, patellar tendon and medial to the femoral condyl. We believe the ossification in the medial collateral ligament could possibly be explained as peri-articular ossification after medial collateral ligament (MCL) rupture (Pellegrini-Stieda sign) and may not be interpreted as classic HO.
At the 6 months follow up visit, the patient was full weight bearing, but he was complaining about pain using his right knee during his work as a truck driver. Especially while loading and unloading his truck, he couldn't perform weight bearing activities. His knee range of motion (ROM) was now limited to 90 degrees of flexion and full extension. Plain radiographs demonstrated increased heterotopic ossification in Hoffa's fat pad and the patellar tendon [Figure 2]. Further information on the extent and location of the ossifications was provided by a CT scan [Figure 3]. We offered a removal of ossifications in addition to a release and/or manipulation under anaesthesia if needed. Although there is no clear evidence regarding the need for pre- or postoperative radiation and/or use of indomethacin after removal of HO around the knee, we discussed this option with the patient. The patient declined both the radiation and indomethacin.
Figure 2.
Anteroposterior (a) and lateral (b) radiographs taken 6 months after surgery.
AP radiograph showing ossification of the medial collateral ligament (MCL) that possibly developed after a MCL rupture (Pellegrini- Stieda sign). Lateral view shows heterotopic ossification in Hoffa’s fat pad and the patellar tendon.
Figure 3.
Axial CT-scan taken 6 months after surgery, showing the location of the heterotopic ossification.
The ossifications were surgically removed using the previous incision. Care was given not to injure the patellar tendon as some ossifications were closely related to the under-surface of the tendon. Additionally, a limited quadricepsplasty was done, resulting in an increased ROM to 125 degrees at the end of the procedure. A mersilene cerclage was placed through a horizontal drill hole in the mid portion of the patella and the proximal tibia to protect the patellar tendon in the early post-operative period. Postoperatively an epidural catheter was placed, which helped patient maintain his ROM on a continuous passive machine (CPM) for 3 days in the hospital.
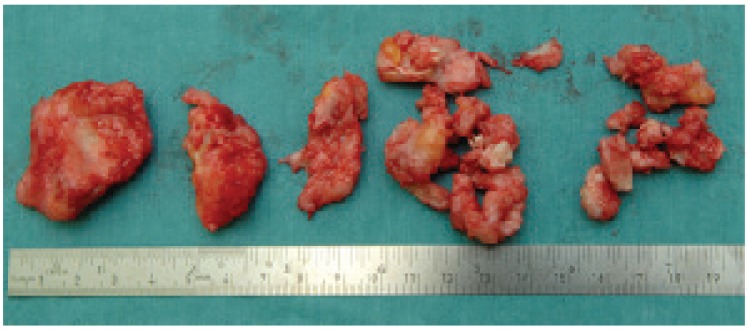
Post-operative radiographs showed near total removal of the ossifications [Figure 4]. The surgically removed tissue was histologically examined and was consistent with heterotopic ossification [Figure 5]. Patient was allowed direct full weight-bearing. At the patient's most recent follow up at 2 year and 8 months after HO removal he denied any pain and had a knee ROM of 0-130 degrees. He was not limited in his activities and had returned to his previous work as a truck driver. Radiographs at this time showed some recurrence of the ossifications [Figure 6].
Figure 4.
Anteroposterior (a) and lateral (b) radiographs taken directly after operative removal of heterotopic ossification showing near total removal of the ossifications.
Figure 5.
The surgically removed heterotopic ossification.
Figure 6.
Anteroposterior (a) and lateral (b) radiographs taken at 2 year and 8 months after operative removal of heterotopic ossification, showing slight recurrence of the ossifications.
Discussion
Demineralized bone matrix (DBM) is often used to augment bone formation. The most frequent site of application seems to be the spine, when fusion success is the goal. There is ample clinical evidence that DBM can induce bone in areas where there has been no bone before (ectopic or heterotopic ossification) and that it -at least partly- can replace autologous bone grafting in fracture and non-union surgery (3,4,7,8).
At the same time, in vivo and in vitro comparison studies have shown significant lot-to-lot variability for a single product, as well as significant differences in osteogenic potential from one DBM product to another. This variability has been a focus of many studies (4).
Heterotopic ossification around the elbow after fractures and surgery is common, with reported incidence ranging between 3% after simple elbow dislocations, 15-20% when the elbow dislocation was combined with fracture (9), to 89% in patients with a closed head injury and elbow trauma (10). From our own database we know that heterotopic ossification after surgery for tibia plateau fractures is very unusual. Our department recently published the long term outcome of 109 tibia plateau fractures. None of these patients were noted to have HO around the knee [Internal communication and ref. 11].
Comparable findings to our patient were recently reported in the literature after the use of bone morhogenetic proteins (BMPs) in periarticular fractures of the elbow and tibia plateau. Boraiah et al. suggested a correlation between the use of BMP-2 in tibia plateau fractures and development of HO (12). Axelrad et al. reported HO after use of commercially available recombinant BMP (OP-1 or rh-BMP-7) in 4 patients (13). Wysocki and Cohen described clinically significant HO in an elbow fracture treated with internal fixation and OP-1 (BMP-7) (14).
In contrast to DBM, bone morphogenetic proteins (BMP-2 (Infuse) and BMP-7 (OP-1)) are approved for use in very specific indications. Despite these restrictions, the use of BMPs has skyrocketed and most use has been "off-label" use. Recently, reports have emerged suggesting that the use of BMPs might be associated with complications. Reported complications with the use of BMP are excessive HO, swelling, bone resorption and remodelling at the grafting site, hematoma, painful seroma around spine and exaggerated inflammatory response forearm and shoulder (15-17).
We acknowledge there are limitations to this report. First, there is no control group. Second, the patient was relatively slow with his postoperative mobilization not only because of our precaution but also because he was relatively apprehensive in doing his exercises. However, in our experience this happens more often after complex fixation of articular fractures.
We are well aware that the reason we used DBM in the first place is to stimulate bone formation and fracture healing. Remarkably, there are few other reports of HO after DBM use, despite the fact that DBM preparations have been in clinical use for over 20 years.
In conclusion, we report a patient who developed clinically significant HO after complex surgery around the knee. Although we cannot be sure that there is a cause-and-effect relation between DBM and the excessive HO seen in our patient, it seems that some caution in using DBM in similar cases is warranted. Also, given the known inter- and intraproduct variability, the risks and benefits of these products should be weighed before using them at all.
References
- 1.Urist MR. Bone: Formation by autoinduction. Science. 1965;150: 893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 2.Kinney RC, Ziran BH, Hirshorn K, Schlatterer D, Ganey T. Demineralized bone matrix for fracture healing: fact or fiction? J Orthop Trauma. 2010;24:52–5. doi: 10.1097/BOT.0b013e3181d07ffa. [DOI] [PubMed] [Google Scholar]
- 3.Bae HW, Zhao L, Kanim LE, Wong P, Delamarter RB, Dawson EG. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine (Phila Pa 1976) 2006; 31:1299–306. doi: 10.1097/01.brs.0000218581.92992.b7. [DOI] [PubMed] [Google Scholar]
- 4.Dinopoulos HT, Giannoudis PV. Safety and efficacy of use of demineralised bone matrix in orthopaedic and trauma surgery. Expert Opin Drug Saf. 2006;5:847–66. doi: 10.1517/14740338.5.6.847. [DOI] [PubMed] [Google Scholar]
- 5.Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007; 81(2):437–42. doi: 10.1002/jbm.a.31085. [DOI] [PubMed] [Google Scholar]
- 6.Bae H, Zhao L, Zhu D, Kanim LE, Wang JC, Delamarter RB. Variability across ten production lots of a single demineralized bone matrix product. J Bone Joint Surg Am. 2010;92(2):427–35. doi: 10.2106/JBJS.H.01400. [DOI] [PubMed] [Google Scholar]
- 7.Hierholzer C, Sama D, Toro JB, Peterson M, Helfet DL. Plate fixation of ununited humeral shaft fractures: effect of type of bone graft on healing . J Bone Joint Surg Am. 2006;88: 1442–7. doi: 10.2106/JBJS.E.00332. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann G, Moghaddam A. Allograft bone matrix versus synthetic bone graft substitutes. Injury. 2011; 42 :16–21. doi: 10.1016/j.injury.2011.06.199. [DOI] [PubMed] [Google Scholar]
- 9.Heyd R, Strassmann G, Schopohl B, Zamboglou N. Radiation therapy for the prevention of heterotopic ossification at the elbow. J Bone Joint Surg Br. 2001;83:332–4. doi: 10.1302/0301-620x.83b3.11428. [DOI] [PubMed] [Google Scholar]
- 10.Hamid N, Ashraf N, Bosse MJ, Connor PM, Kellam JF, Sims SH, et al. Radiation therapy for heterotopic ossification prophylaxis acutely after elbow trauma: a prospective randomized study. J Bone Joint Surg Am. 2010;92: 2032–8. doi: 10.2106/JBJS.I.01435. [DOI] [PubMed] [Google Scholar]
- 11.Rademakers MV, Kerkhoffs GM, Sierevelt IN, Raaymakers EL, Marti RK. Operative treatment of 109 tibial plateau fractures: five- to 27-year followup results. J Orthop Trauma. 2007;21(1):5–10. doi: 10.1097/BOT.0b013e31802c5b51. [DOI] [PubMed] [Google Scholar]
- 12.Boraiah S, Paul O, Hawkes D, Wickham M, Lorich DG. Complications of recombinant human BMP- 2 for treating complex tibial plateau fractures: a preliminary report. Clin Orthop Relat Res. 2009; 467:3257–62. doi: 10.1007/s11999-009-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Axelrad TW, Steen B, Lowenberg DW, Creevy WR, Einhorn TA. Heterotopic ossification after the use of commercially available recombinant human bone morphogenetic proteins in four patients. J Bone Joint Surg Br. 2008;90: 1617–22. doi: 10.1302/0301-620X.90B12.20975. [DOI] [PubMed] [Google Scholar]
- 14.Wysocki RW, Cohen MS. Ectopic ossification of the triceps muscle after application of bone morphogenetic protein-7 to the distal humerus for recalcitrant nonunion: a case report. J Hand Surg Am. 2007;32: 647–50. doi: 10.1016/j.jhsa.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Delimar D, Smoljanovic T, Bojanic I. Could the use of bone morphogenetic proteins in fracture healing do more harm than good to our patients? Int Orthop. 2012;36: 683. doi: 10.1007/s00264-011-1397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritting AW, Weber EW, Lee MC. Exaggerated inflammatory response and bony resorption from BMP-2 use in a pediatric forearm nonunion. J Hand Surg Am. 2012; 37(2):316–21. doi: 10.1016/j.jhsa.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Julka A, Shah AS, Miller BS. Inflammatory response to recombinant human bone morphogenetic protein-2 use in the treatment of a proximal humeral fracture: a case report. J Shoulder Elbow Surg. 2012;21(1):12–6. doi: 10.1016/j.jse.2011.06.006. [DOI] [PubMed] [Google Scholar]