Abstract
Objective(s):
Production of a recombinant and immunogenic antigen using dengue virus type-3 envelope protein is a key point in dengue vaccine development and diagnostic researches. The goals of this study were providing a recombinant protein from dengue virus type-3 envelope protein and evaluation of its immunogenicity in mice.
Materials and Methods:
Multiple amino acid sequences of different isolates of dengue virus type-3, corresponding to the envelope protein domain III, were achieved from GenBank. Clustal V alignment tool was used to provide a consensus amino acid sequence. Nucleotide sequence of the coding gene was optimized using "Optimizer". The origami (DE3) strain of Escherichia coli was used as the host in order to express the protein. A commercial affinity chromatography method was used to purify the recombinant protein. Immunogenicity of the recombinant protein was evaluated in mice using ELISA, MTT and cytokine assays.
Results:
A consensus amino acid sequence corresponding to the most important region of dengue virus type-3 envelope protein (domain III) was provided. A high concentration (≥ 20 mg/L culture medium) of soluble recombinant antigen (EDIII3) was achieved. Immunized mice developed specific antibody responses against EDIII3 protein. The splenocytes from EDIII3-immunized mice showed a high proliferation rate in comparison with the negative control. In addition, the concentrations of two measured cytokines (IFN-γ and IL-4) were increased markedly in immunized mice.
Conclusion:
The results showed that the expressed recombinant EDIII3 protein is an immunogenic antigen and can be applied to induce specific immune responses against dengue virus type-3.
Keywords: Dengue virus-3, Envelope protein, Immunogenicity, Recombinant domain III
Introduction
Dengue virus is an emerging mosquito-borne pathogen that causes dengue fever (DF), severe life threatening illnesses and dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS). Dengue infections with high percentage of mortality are prevalent in tropical and subtropical areas of the world (1), where their incidence and geographical spread have greatly increased in recent years (2). In fact, dengue is the most common vector-borne viral disease of humans. All dengue viruses (serotypes 1-4) can infect human. Despite decades of effort, there is still no specific antiviral drug or approved vaccine for human use (3). Hence, there is an urgent need for a dengue vaccine that protects us against virus infection. The envelope protein (E protein) of virus is a large and cysteine-rich protein, which is responsible for a wide range of biological activities, including binding to host cell receptors, fusion and entry into host cells. In addition, the E protein also stimulates host immune system by inducing protective and neutralizing antibodies (4, 5). Therefore, the dengue E protein is an important antigen for vaccine development and is used as a reagent for diagnostic purposes. Many attempts have been made to produce a recombinant dengue E protein in a heterologous expression system (6-11). The dengue E protein consists of three structurally distinct domains (I, II, and III) (12). From a vaccine perspective, the most important properties of E protein are associated with domain III (EDIII) (13-15). This domain appears to play a role in host cell receptor binding for viral entry (13, 16), and in inducing long lasting protective immunity against dengue virus infection (17, 18). Today, most of the efforts to produce a recombinant dengue vaccine are focused on utilization of EDIII protein (19-23). Most often, E. coli-expressed EDIII proteins tend to be insoluble and need to be purified under denaturing conditions, followed by renaturation (24, 25). In this paper, we have presented an efficient system for soluble expression and one step purification of EDIII3 protein. Furthermore, we have evaluated the immunogenicity of this recombinant protein in mice.
Materials and Methods
Design and construction of EDIII3 sequence
GenBank (www.ncbi.nlm.nih.gov/genbank/) was the main reference for retrieval of the amino acid sequences of dengue virus type-3 E protein domain III (amino acid residues 300-400). Briefly, the EDIII amino acid sequences from different isolates of dengue-3 were obtained from GeneBank. Multiple sequence alignments were performed in order to identify an amino acid sequence common to all collected domain III sequences using Clustal X method. In order to increase the purification effectiveness, a 6-HisTag sequence was added to carboxyl terminus of recombinant EDIII proteins. In order to achieve a high level of expression in Escherichia coli, the DNA sequence encoding EDIII3 was optimized according to E. coli codon usage and GC content of the genome, using online Optimizer software (http://genomes.urv.cat/OPTIMIZER/). For cloning purposes, restriction sites for enzymes NdeI and XhoI were introduced at the 5' and the 3' sites of the coding sequence, respectively. The EDIII coding gene was synthesized by Shine Gene Molecular Biotech Inc. (Shanghai, China), and sub-cloned into pET21a expression vector (Novagen, Germany). The expression vector was transformed into E. coli DH5α (as the cloning host) and E. coli origami (DE3) (as the expression host). Resultant transformants were selected on ampicillin plates and subjected to preliminary PCR screening using pET universal primers.
Expression of recombinant EDIII3 protein
The E. coli strain origami (DE3) harboring the designed expression vector was grown overnight at 37°C in 5 ml LB medium (Luria-Bertani medium) containing 50 µg/ml ampicillin, 12.5 µg/ml tetracycline, and 15 µg/ml kanamycin (Sigma, USA). Overnight grown culture was diluted 100-fold in 10 ml medium containing ampicillin and further incubated at 37°C. Culture in logarithmic phase (at OD600 of 0.6) was induced by addition of isopropyl β-D-1-thiogalactopyranoside (IPTG) to the final concentration of 1 mM. After 3 hr, cells were harvested by centrifugation at 5000 g for 10 min, lysed in sample buffer, and analyzed by SDS-PAGE (sodium-dodecylphosphate-polyacryl-amid gel electrophoresis) technique.
Purification of recombinant EDIII protein
According to manufacturer's instruction, soluble EDIII protein that was prepared from origami (DE3) was purified using Nickel-nitrilotriacetic acid (Ni-NTA) resin (Qiagen, Germany) under native condition and monitored on 10% SDS-PAGE. Finally, the purified protein was dialyzed against PBS (phosphate buffered saline) and stored at -20°C for further analysis.
Western blot analysis
Ni-NTA Purified EDIII was run on 10% SDS-PAGE, along with pre-stained protein marker on adjacent lane and transferred onto nitrocellulose membrane using a semidry transfer apparatus. The membrane was incubated in blocking buffer of 5% skimmed milk at 4°C, overnight. Then, the membrane was incubated in the blocking buffer containing primary antibody (anti-HisTag mAb (Abcam)/anti-dengue mAb (Abnova, Taiwan) at a 1:500 dilution) with gentle shaking for 2 hr at 37°C. The membrane was washed by PBST (PBS containing 0.1% Tween 20) three times and then incubated in secondary antibody (a 1:5000 dilution of HRP-conjugated rabbit anti mouse IgG antibody (Abcam) in blocking buffer), with gentle shaking for 1 hr at room temperature (22°C). After washing with PBST for 15 min, detection was performed using DAB (diaminobenzidine) as a substrate.
Animal immunization
The purified recombinant EDIII protein was emulsified (20 µg per dose) in complete Freund's adjuvant (CFA, Sigma, USA) for priming (day 0), and in incomplete Freund's adjuvant (IFA, Sigma, USA) for booster immunizations (days 14 and 28). The total volume of injected mixture that was used per mouse for each immunization was 200 µl. Groups of six BALB/c mice (6-8 weeks of age) were immunized subcutaneously. As the negative control, a group of mice were injected with PBS and adjuvant only (mock). Mice were scarified and blood samples were collected 14 days after the last inoculation. The pooled sera were stored at -70°C for further analyses.
Determination of serum IgG antibody responses to EDIII protein
Specific antibody responses were determined using ELISA assay. Polystyrene 96-well plate (Nunc-Immuno Plate MaxiSorp surface, Nunc, Denmark) was coated with 0.1 µg/well of EDIII protein at 4°C, overnight. The plate was washed three times with PBST (PBS containing 0.05% Tween 20) and the non-specific sites were blocked with 200 µl of blocking buffer (5% skimmed milk in PBS) at 37°C for 1 hr. Mice serum samples were serially diluted in blocking buffer (1:10 to 1:1000,000) in triplicate wells, and incubated for 2 hr at 37°C. In parallel, a similar dilution was made using mouse anti-dengue monoclonal antibody (Abnova, Taiwan), as the positive control. The plate was washed three times with PBST. HRP-conjugated rabbit anti-mouse (IgG) antibody was diluted (1:8000) in blocking buffer, added to wells and incubated for 1 hr at 37°C. The plate was washed four times in PBST and 100 µl of TMB was added as chromogen. Then, the plate was incubated at room temperature for 15 min. Finally, the reaction was stopped with 100 µl of 2N H2SO4 and the absorbance was measured at 450 nm using a micro-plate reader.
Lymphocyte proliferation assay
Splenocytes from each group of mice were collected using a previously described method (26) with minor modifications. Aliquots (100 µl) of each splenocyte suspension were dispensed into each well of a 96-well cell culture plate. Aliquots of the EDIII antigen were then dispensed into the wells containing of cell suspension (in triplicate), at a final concentration of 10 µg/ml of antigen. Wells containing splenocytes derived from mock-immunized mice (as the negative control), as well as ConA (Sigma, USA) stimulated splenocytes derived from unimmunized mice (as the positive control) were also included. Cultures were incubated in incubator (at 37°C; 5% CO2) for 1-3 days. The proliferation assay was performed using colorimetric technique 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyl tetrazolium bromide (MTT) assay. Briefly, after the incubation period (24, 48 and 72 hr), 20 µl of MTT (5 mg/ml) was added into each well and plates were incubated for 4 hr in incubator (at 37°C; 5% CO2). Then, the supernatant was removed and DMSO (100 µl) extraction buffer was added to extract and solubilize formazan crystals. The solution was vigorously mixed to dissolve the reacted dye and was incubated for 20 min in incubator (at 37°C; 5% CO2). Finally, all plates were read at 570 nm wavelength using a microplate reader.
Cytokine assay
To analyze the phenotype of the specific cellular immune response induced by the recombinant EDIII, the splenocyte cultures were prepared in 24-well cell culture plates (5×104 cells per well). Supernatants from all wells were collected 72 hr after stimulation with EDIII3 antigen and frozen at -80°C until the time of analysis. To examine the level of the Th1-type cytokine, IFN-γ and Th2-type cytokine, IL-4 commercially available quantitative ELISA assay kits (mouse IFN-γ and IL-4 kits, Qiagen, USA) were used according to the manufacturer's instructions. Cytokine levels were calculated using standard curves generated with known concentrations of cytokines. Results were expressed in picograms per milliliter (pg/ml).
Statistical analysis
The data are expressed as mean ± standard deviation (SD) (from three independent experiments). P-values of <0.05 were considered statistically significant.
Results
Sequence selection and vector design
Since each serotype has its own level of variation in the E region, in order to address the variation between different isolates of dengue virus type-3, a consensus EDIII peptide sequence was provided and named as EDIII3 (Figure 1). Then, the optimized coding gene sequence was synthesized and cloned in pET21a expression vector.
Figure 1.

The consensus sequence for dengue virus type-3 E protein domain III (EDIII3); two highly conserved cysteine residues (in the positions of 8 and 39) are depicted in red letters.
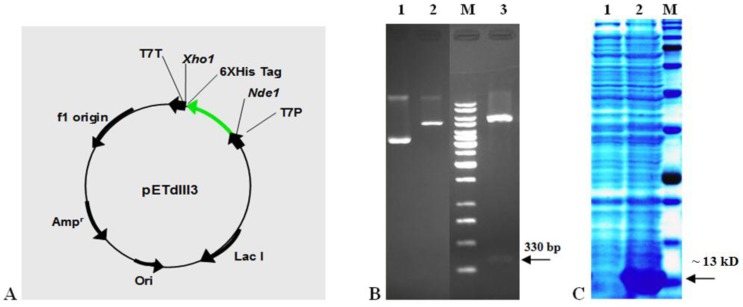
The bacterial vector that was designed to express EDIII3 protein (pETdIII3) is shown in Figure 2A. The pETdIII3 construct is predicted to encode a ~13 kD recombinant protein containing a C-terminal 6x-HisTag, for detection and purification purposes.
Figure 2.
The recombinant EDIII3 expression vector and profile. A Map of pETdIII3 plasmid. In this plasmid the EDIII3 encoding gene is cloned in-frame with the initiator codon in vector and a C-terminal 6x-His-Tag provided by pET21a (thick green arrow). The NdeI and XhoI restriction sites used for cloning are indicated. Phage T7 promoter (T7P) directs expression. Other abbreviations are: T7T, transcriptional terminator; Lac I, Lac repressor gene; Ampr, Ampicilline marker; Ori, replication origin sequences. The arrows indicate direction of transcription. B Analysis of constructed expression vector using restriction enzyme digestion on agarose gel electrophoresis. Lane 1 is undigested vector, lane 2 is digested vector using XhoI restriction enzyme, lane 3 is double digestion of plasmid using NdeI and XhoI restriction enzymes and lane M is 1kb molecular size marker. The arrow on the right hand side indicates the 330 bp fragment after double digestion. C SDS-PAGE analysis of EDIII3 expression in Escherichia coli. The protein derived from the soluble phase of Escherichia coli cell lysates was subjected to 10% SDS-PAGE and stained with Coomassie Brilliant Blue. The profile of Escherichia coli lysates was prepared before (lane 1) and after IPTG induction (lane 2). Lane M is protein molecular weight marker (175, 130, 95, 70, 62, 51, 42, 29, 22, 14 and 10 kD). The arrow on the right hand side indicates the ~13 kD EDIII3 protein band
Expression and purification of recombinant EDIII3 protein
The pETdIII3 vector was transformed into origami (DE3) host cells. Previously, it has been shown that the origami strain of E. coli with glutathione reductase (gor) and thioredoxin reductase (trxB) mutations enhances the formation of disulfide bonds in E. coli cytoplasm (27, 28). Small-scale cultures of the positive clones (selected by PCR) were subjected to IPTG induction. A typical experiment comparing the polypeptide profiles of un-induced and induced cultures is shown in Figure 2C. It is evident that IPTG induction results in the expression of the desired ~13 kD EDIII3 protein. Plasmid DNA were isolated from the EDIII3 expressing cells and verified to be correct using restriction analysis (Figure 2B). We optimized the expression conditions by testing different concentrations of IPTG (1, 1.5 and 2 mM) and various length of induction period (2, 4, 6 and 8 hr). We found that IPTG was equally effective at all tested concentrations, and 4 hr expression showed the best result. Considering the observed results, in all subsequent experiments, the expression was induced with 1 mM IPTG for 2 hr.
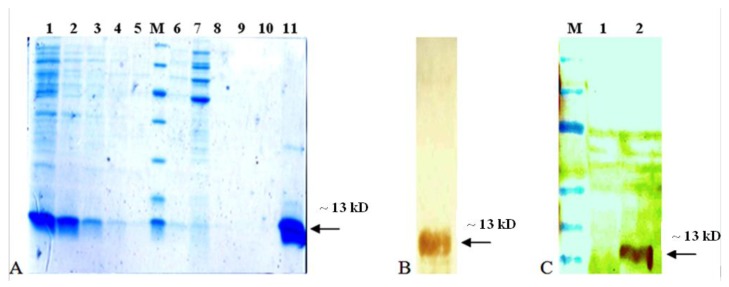
To devise an appropriate purification strategy, we proceeded to examine the relative distribution of the expressed recombinant protein in soluble and insoluble fractions of expressing cell lysate, using a previously described method (24). Virtually, all the expressed recombinant proteins were associated with the supernatant fraction of bacterial lysate, demonstrating soluble expression of EDIII3 protein. Therefore, we attempted to purify the expressed recombinant protein under native condition, using standard protocol of Ni-NTA Fast Start purification kit (Ni-NTA kit, Qiagen, Germany). SDS-PAGE analysis of the sample at different stages of the purification is depicted in Figure 3A. A single major protein band of the expected size (~13 k) was purified to >90% homogeneity as can be seen in Figure 3A lane 11. The faint band in lane 11 (~13 k) presumably represents dimmer species, as described by others (24). The purified protein was subjected to Western blotting analysis using anti-HisTag monoclonal antibody, and a major band was observed in this analysis, corresponding to the 6x-His-Tagged recombinant EDIII3 (Figure 3B). Furthermore, another Western blotting analysis was carried out to investigate the specific reactivity of the expressed recombinant protein with anti-dengue monoclonal antibody (Figure 3C).
Figure 3.
Purification and identification of recombinant EDIII3. A SDS-PAGE analysis of purified EDIII3 protein. Different stages of purification on Ni-NTA affinity column; Lane1: cell lysate, lane 2-5: imidazole elution fractions, lane 6-10: washed fractions, lane 11: the pure EDIII protein after dialyzing against PBS buffer (before injection), lane M: Protein molecular weight markers (116, 66, 45, 35, 25, 18 and 14 kD). B Western blot analysis of purified EDIII3 which reacted with anti-His-Tag Mab. C Specific reactivity of recombinant EDIII3 with a mouse monoclonal anti-dengue antibody in western blotting analysis. Lane 1 is protein fraction from untransformed bacterial lysate (as negative control), lane 2 is protein fraction of EDIII3 expressing bacterial lysate, and lane M is protein molecular weight marker (175, 130, 95, 70, 62, 51, 42, 29, 22, 14 and 10 kD). The arrow indicates the position of the EDIII3 recombinant protein
Taken together, data in Figure 3 suggests that the intended 6x-His-Tagged recombinant domain III of dengue virus type-3 envelope protein was expressed and purified, successfully. The soluble recombinant EDIII3 was subjected to native purification and the yield of purification was ≥20 mg/l culture medium, according to the routine Bradford protein assay method.
Immunogenicity of recombinant EDIII3 protein
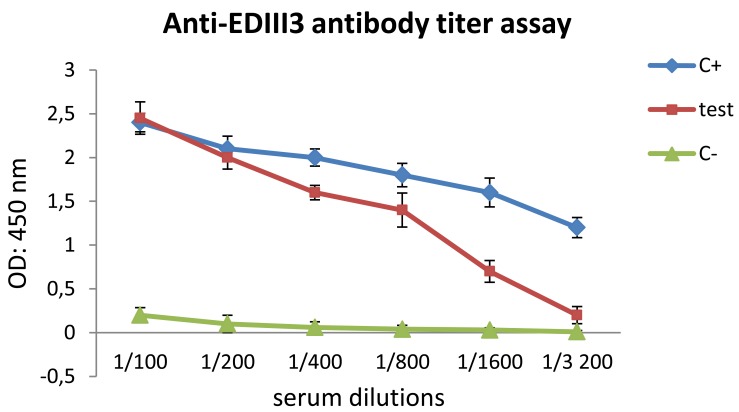
To investigate the immunogenicity of therecombinant EDIII3, groups of BALB/c mice were immunized subcutaneously with purified EDIII3 proteins three times (2 week intervals; 0, 2, and 4). The mice in negative control group were immunized with PBS in combination with adjuvant using the same procedure. Two weeks after the last immunization, sera from immunized mice were analyzed for anti-EDIII3 antibody using ELISA. In parallel, an ELISA reaction was performed using a commercial specific anti-dengue monoclonal antibody against the expressed recombinant EDIII3, as the positive control (Figure 4). In comparison with the positive control, the results showed that the immunized mice developed high-titer antibody responses to recombinant EDIII3 protein. Also, the identity of recombinant antigen was approved by the specific reactivity of recombinant EDIII3 with commercial anti-dengue antibody. These data demonstrated the immunogenic potential of recombinant EDIII3 protein expressed in E. coli using our expression system.
Figure 4.
ELISA test. Titer of serum antibody is increased in mice by Escherichia coli -expressed recombinant EDIII3 protein formulated with Freund's adjuvant sera derived from mice which were immunized with PBS, as negative control group (green triangles), sera derived from mice which were vaccinated with EDIII3, as test group (red squares) and anti-dengue monoclonal antibody (instead of immune sera), as positive control (blue rhombus). These data demonstrated the potential of recombinant EDIII3 protein for inducing a high titer of specific antibody
Cell proliferation and cytokine profiling of splenocytes stimulated by recombinant EDIII3 protein
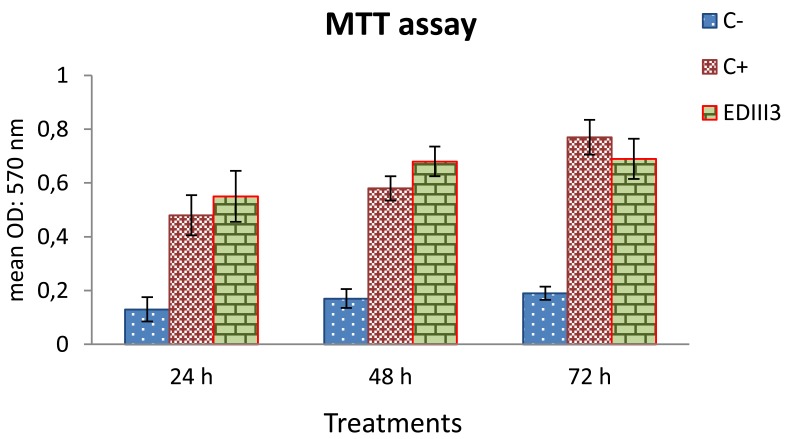
According to the MTT assay, with three day intervals, splenocytes from the EDIII3-immunized mice that were restimulated by EDIII3 protein in vitro, showed high proliferation in comparison to the negative control. Splenocytes from mice in negative control group (mock-immunized) did not show comparable proliferation when stimulated with EDIII3, demonstrating specificity of the proliferation. Splenocytes derived from unimmunized mice responded nonspecifically to stimulation with ConA (as a positive control for monitoring the stimulation level) (Figure 5). The comparison between duration of treatments (24, 48 and 72 hr) did not show any significant differences; except for the positive control group, that showed higher cell proliferation activity on the 3rd day compared to days 1 and 2.
Figure 5.
Lymphocyte proliferation assay. Splenocytes were restimulated with EDIII3 for 24, 48 and 72 hr. This experiment was done using MTT cell proliferation assay. Splenocytes derived from mice which were immunized with adjuvant in combination with PBS, and restimulated with EDIII3 antigen in vitro, were considered as negative control. The splenocytes of the EDIII3-immunized mice showed higher proliferation rate in comparison with the negative control. Splenocytes in negative control did not show comparable proliferation rate when stimulated with EDIII3. Splenocytes derived from unimmunized group responded to ConA nonspecifically
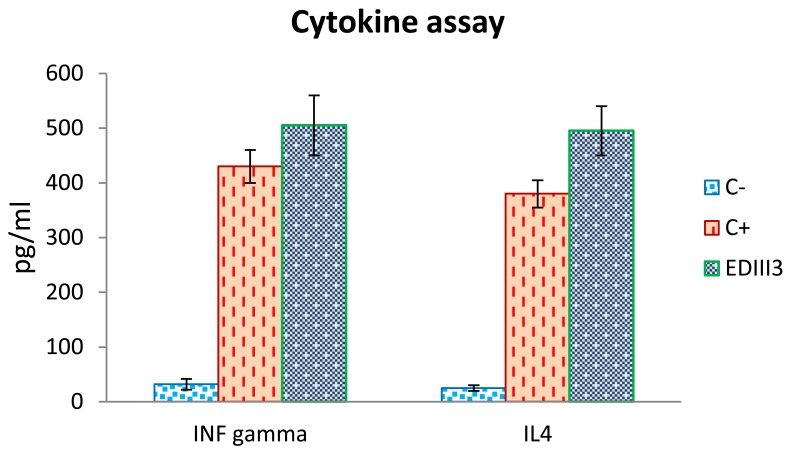
Furthermore, quantitative ELISA assays were used to detect IFN-γ and IL-4 cytokines secreted by splenocytes of the EDIII3-immunized mice upon restimulation with EDIII3 recombinant protein (EDIII3 group) and unimmunized mice upon stimulation with ConA (positive control group). In comparison with the negative control group, concentration of the measured cytokines increased markedly in EDIII3-immunized group and also in ConA-stimulated splenocytes (Figure 6). Since IFN-γ is mark of Th1 response and IL-4 is mark of Th2 response, our results indicate that both Th1- and Th2 immune responses were induced following inoculation.
Figure 6.
Cytokine profile of mice which were immunized with EDIII3. Splenocytes derived from immunized mice were restimulated with antigens thereafter the evaluation of cytokine release was done using commercial quantitative ELISA kits for IFN-γ and IL4. Splenocytes derived from mice which were immunized with adjuvant in combination with PBS and restimulated with EDIII3 antigen in vitro, were considered as negative control. The splenocytes derived from unimmunized mice were stimulated with ConA in vitro as a positive control for cytokines assay
Discussion
Protein subunit vaccines do not have the problem of bio-hazard which is a major problem in live attenuated as well as inactivated viral vaccines. Furthermore, protein subunit vaccines are safe, easy to produce and scale up and also can be inexpensive (29). The dengue E protein is an important antigen for vaccine development and is used as a reagent for diagnostic purposes. On one hand, as large proteins (such as complete dengue E protein) tend to be expressed at lower levels, we have explored the feasibility of expressing a small and biologically critical domain of the E protein (domain III) in E. coli. On the other hand, to enhance the potential of the expression system, the protein to be expressed should be of moderate size (30), and the coding gene should be optimized and expressed in an appropriate host (31). Also, we optimized the EDIII3 coding gene sequence according to E. coli codon usage preference, and origami (DE3) strain was used as the host. This expression host provides efficient expression of soluble proteins containing disulfide bounds. Our results showed that it is possible to express domain III of dengue-3 E protein without any fusion carrier and yet obtain soluble and functional protein under optimized condition. Interestingly, the expressed protein could be extracted and purified under native conditions without any time consuming denaturation/refolding process. In a previous study (24), the EDIII protein of serotype 2 was produced in insoluble fraction of E. coli and processed with denaturation/refolding. In another report, the EDIII2/MBP fusion protein was produced in the soluble form; however the cleavage of EDIII2 from MBP resulted in complete loss of the recognition site for 3H5 Mab (32). Here, we showed the efficiency of the designed expression system in soluble and high level expression of dengue virus type-3 envelope domain III. The soluble forms of these proteins are more effective in production of antibody responses and this is a key point in developing a suitable strategy for both vaccine and diagnostic purposes.
Conclusion
Since the designed expression system uses E. coli as the heterologous host, the process is amenable to inexpensive scale-up. Immunization of mice with this monotypic dengue type-3 protein induced generation of antigen reactive antibodies and enhanced cellular immunity according to conventional assays. In conclusion, the produced recombinant EDIII3 antigen was immunogenic and can be applied to the development of protective immunity against serotype-3 of dengue virus through induction of virus neutralizing antibodies.
Acknowledgment
The results presented in this article were part of doctoral dissertation of a PhD student of Tarbiat Modares University, Tehran, Iran. This work was financially supported by Tarbiat Modares University, Tehran, Iran.
References
- 1.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol . 2002; 10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention. Available at: http://cdc.gov/ncidod/dvbid/dengue .
- 3.Stephenson JR. Understanding dengue pathogenesis: implications for vaccine design. Bull World Health Organ . 2005; 83:308–314. [PMC free article] [PubMed] [Google Scholar]
- 4.Tong GZ, Zhou YJ, Hao XF, Tian ZJ, An TQ, Qiu HJ. Highly pathogenic porcine reproductive and respiratory syndrome, China. Emerg Infect Dis. 2007;13:1434–1436. doi: 10.3201/eid1309.070399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li DJ, Wang HM, Li L. Gene fusion of molecular adjuvant C3d to hCG enhances the anti-hCG antibody response in DNA immunization. J Reprod Immunol. 2003; 60:129–141. doi: 10.1016/j.jri.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Simmons M, Murphy GS, Hayes CG. Short report: Antibody responses of mice immunized with a tetravalent dengue recombinant protein subunit vaccine. Am J Trop Med Hyg. 2001; 65:159–161. doi: 10.4269/ajtmh.2001.65.159. [DOI] [PubMed] [Google Scholar]
- 7.Hermida L, Rodriguez R, Lazo L, Bernardo L, Silva R, Zulueta A, et al. A fragment of the envelope protein from dengue-1 virus, fused in two different sites of the meningococcal P64k protein carrier, induces a functional immune response in mice. Biotechnol Appl Biochem. 2004; 39:107–114. doi: 10.1042/BA20030039. [DOI] [PubMed] [Google Scholar]
- 8.Raja NU, Holman DH, Wang D, Raviprakash K, Juompan LY, Deitz SB, et al. Induction of bivalent immune responses by expression of dengue virus type 1 and type 2 antigens from a single complex adenoviral vector. Am J Trop Med Hyg. 2007; 76:743–751. [PubMed] [Google Scholar]
- 9.Clements DE, Coller BA, Lieberman MM, Ogata S, Wang G, Harada KE, et al. Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys. Vaccine . 2010; 28:2705–2715. doi: 10.1016/j.vaccine.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda ER, Zanchi F, Rios K, Lira E, DeusileneVieira, da Silva LH, et al. Design and heterologous expression of dengue virus envelope protein (E) peptides and their use for serological diagnosis. J Virol Methods . 2012; 186:55–61. doi: 10.1016/j.jviromet.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Clements DE, Coller BA, Lieberman MM, Ogata S, Wang G, Harada KE, et al. Expression of dengue-3 premembrane and envelope polyprotein in lettuce chloroplasts. Plant Mol Biol. 2011; 76:323–333. doi: 10.1007/s11103-011-9766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modis Y, Ogata S, Clements D, Harrison SC. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci USA . 2003;100:6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crill WD, Roehrig JT. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol . 2001; 75:7769–7773. doi: 10.1128/JVI.75.16.7769-7773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung JJ, Hsieh MT, Young MJ, Kao CL, King CC, Chang W. An external loop region of domain III of dengue virus type 2 envelope protein is involved in serotype-specific binding to mosquito but not mammalian cells. J Virol. 2004; 78:378–388. doi: 10.1128/JVI.78.1.378-388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chin JF, Chu JJ, Ng ML. The envelope glycoprotein domain III of dengue virus serotypes 1 and 2 inhibit virus entry. Microbes Infect. 2007; 9:1–6. doi: 10.1016/j.micinf.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, et al. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med . 1997; 3:866–781. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 17.Mune M, Rodriguez R, Ramirez R, Soto Y, Sierra B, Rodriguez Roche R, et al. Carboxy-terminally truncated Dengue 4 virus envelope glycoprotein expressed in Pichia pastoris induced neutralizing antibodies and resistance to Dengue 4 virus challenge in mice. Arch Virol . 2003; 148:2267–2273. doi: 10.1007/s00705-003-0167-9. [DOI] [PubMed] [Google Scholar]
- 18.Apt D, Raviprakash K, Brinkman A, Semyonov A, Yang S, Skinner C, et al. Tetravalent neutralizing antibody response against four dengue serotypes by a single chimeric dengue envelope antigen. Vaccine. 2006; 24:335–344. doi: 10.1016/j.vaccine.2005.07.100. [DOI] [PubMed] [Google Scholar]
- 19.Brandler S, Lucas-Hourani M, Moris A, Frenkiel MP, Combredet C, Fevrier M, et al. Pediatric measles vaccine expressing a dengue antigen induces durable serotype-specific neutralizing antibodies to dengue virus. PLoS Negl Trop Dis. 2007; 1:e96. doi: 10.1371/journal.pntd.0000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leng CH, Liu SJ, Tsai JP, Li YS, Chen MY, Liu HH, et al. A novel dengue vaccine candidate that induces cross-neutralizing antibodies and memory immunity. Microbes Infect . 2009; 11:288–295. doi: 10.1016/j.micinf.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Khanam S, Khanna N, Swaminathan S. Induction of neutralizing antibodies and T cell responses by dengue virus type 2 envelope domain III encoded by plasmid and adenoviral vectors. Vaccine . 2006;24:6513–6525. doi: 10.1016/j.vaccine.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 22.Khanam S, Rajendra P, Khanna N, Swaminathan S. An adenovirus prime/plasmid boost strategy for induction of equipotent immune responses to two dengue virus serotypes. BMC Biotechnol. 2007; 7:10. doi: 10.1186/1472-6750-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etemad B, Batra G, Raut R, Dahiya S, Khanam S, Swaminathan S, et al. An envelope domain III-based chimeric antigen produced in Pichia pastoris elicits neutralizing antibodies against all four dengue virus serotypes. Am J Trop Med Hyg . 2008; 79:353–363. [PubMed] [Google Scholar]
- 24.Jaiswal S, Khanna N, Swaminathan S. High-level expression and one-step purification of recombinant dengue virus type 2 envelope domain III protein in Escherichia coli. Protein Expr Purif. 2004; 33:80–91. doi: 10.1016/j.pep.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Liao M, Kielian M. Domain III from class II fusion proteins functions as a dominant-negative inhibitor of virus membrane fusion. J Cell Biol . 2005; 171:111–120. doi: 10.1083/jcb.200507075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babu JP, Pattnaik P, Gupta N, Shrivastava A, Khan M, Rao PV. Immunogenicity of a recombinant envelope domain III protein of dengue virus type-4 with various adjuvants in mice. Vaccine . 2008; 26: 4655–4663. doi: 10.1016/j.vaccine.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Prinz WA, Aslund F, Holmgren A, Beckwith J. The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm. J Biol Chem . 1997; 272:15661–1567. doi: 10.1074/jbc.272.25.15661. [DOI] [PubMed] [Google Scholar]
- 28.Bessette PH, Aslund F, Beckwith J, Georgiou G. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. PNAS . 1999;96: 13703–13708. doi: 10.1073/pnas.96.24.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swaminathan S, Khanna N. Dengue vaccine current progress and challenges. Current Science, Special Section: Biology and Pathogenesis of Viruses. 2010; 98:3,369–378. [Google Scholar]
- 30.Goeddel DV. Systems for heterologous gene expression. Methods Enzymol. 1990; 185:3–7. doi: 10.1016/0076-6879(90)85003-7. [DOI] [PubMed] [Google Scholar]
- 31.Burgess-Brown NA, Sharma S, Sobott F, Loenarz C, Oppermann U, Gileadi O. Codon optimization can improve expression of human genes in Escherichia coli: A multi-gene study. Protein Expr Purif . 2008;59:94–102. doi: 10.1016/j.pep.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Simmons M, Nelson WM, Wu SJ, Hayes CG. Evaluation of the protective efficacy of a recombinant dengue envelope B domain fusion protein against dengue 2 virus infection in mice. Am J Trop Med Hyg. 1998;58:655–662. doi: 10.4269/ajtmh.1998.58.655. [DOI] [PubMed] [Google Scholar]