Abstract
Objective(s):
Cardiomyocytes have small potentials for renovation and proliferation in adult life. The most challenging goal in the field of cardiovascular tissue engineering is the creation of an engineered heart muscle. Tissue engineering with a combination of stem cells and nanofibrous scaffolds has attracted interest with regard to Cardiomyocyte creation applications. Human adipose-derived stem cells (ASCs) are good candidate for use in stem cell-based clinical therapies. They could be cultured and differentiated into several lineages such as cartilage, bone, muscle, neuronal cells, etc.
Materials and Methods:
In the present study, human ASCs were cultured on random and aligned polycaprolactone (PCL) nanofibers. The capacity of random and aligned PCL nanofibrous scaffolds to support stem cells for the proliferation was studied by MTT assay. The cardiomyocyte phenotype was first identified by morphological studies and Immunocytochemistry (ICC) staining, and then confirmed with evaluation of specific cardiac related gene markers expression by real-time RT-PCR.
Results:
The proliferation rate of ASCs on aligned nanofibrous PCL was significantly higher than random nanofibrous PCL. ICC and morphological studies results confirmed cardiomyocyte differentiation of ASCs on the nanofibrous scaffolds. In addition, the expression rate of cardiovascular related gene markers such as GATA-4, α-MHC and Myo-D was significantly increased in aligned nanofibrous PCL compared with random nanofibrous PCL.
Conclusion:
Our results show that the aligned PCL nanofibers are suitable physical properties as polymeric artificial scaffold in cardiovascular tissue engineering application.
Keywords: Adipose-derived stem cells, Cardiomyocyte differentiation, PCL nanofibers, Tissue engineering
Introduction
Cardiovascular diseases pose a major public health problem in both industrialized and so-called 'emerging' countries. In Iran cardiovascular diseases are responsible for close to 102.45 deaths from 100000 deaths per year (24.14% of all deaths) (1).
The heart is the center of the circulation system and cannot regenerate significantly after injury, since adult cardiac myocytes are terminally differentiated and cannot renew after any injury (2). The most challenging goal in tissue engineering is to create, repair or replace tissues and organs by using combinations of cells, scaffolds, and biologically active molecules. So cardiac tissue engineering strategies promise to revolutionize current therapies for irreversible myocardial damage, heart failure, and significantly improve the quality of life for millions of patients.
There are three strategies in repairing heart damaged tissues. The first one is the therapies that prompt the heart to renew damage tissues. The second approach is the injection of viable cells directly into the damaged environments of the tissue. The third one is the tissue engineering techniques, in which cells are grown in 3D scaffolds and then implanted to damaged site (3).
New findings in stem cell biology imply that stem cells are a good candidate to create an engineered heart muscle and can be used by clinicians to rebuild or replace damaged heart tissue.
The optimal cell source to create engineered cardiomyocyte should be easy to harvest with high proliferation rate and no immunogenic effects. It should also be able to differentiate into mature functional cardiomyocyte(4). Adipose tissue as a stem cell source can easily be harvested in large quantities and has several advantages compared to other sources (5). Its harvesting procedure is easy with minimal invasive and isolation of adipose-derived mesenchymal stromal/stem cells yields a high amount of stem cells and has the capacity to differentiate into several cell types including cardiomyocyte, which is essential for stem-cell-based therapies and tissue engineering (6).
Nanofibers are the important part of tissue-engineering and have advantages over traditional scaffolds because of increased surface/volume ratio, which increases cell-scaffold interactions. The size and 3-dimensionality of these fibers mimic the extracellular matrix (ECM), thereby increase the adherence of cells on scaffolds, promote migration and proliferation of cells and as a result improve this approach function (7). Cardiomyocytes are highly aligned in the heart. The unique physical and electrical properties of myocardium make its structure unbeatable. Mimicking this unique property of myocardium may advance cardiac tissue engineering.
PCL is a FDA approved polymer which has a long history of safe use in humans. This biomaterial is biodegradable and biocompatible with high range of hydrophobicity (8). The surface hydrophobicity of these polymeric nanofibers scans decrease with certain surface treatment methods such as plasma treatment (9). Plasma treatment is one of the best techniques to increase the surface hydrophilicity of nano-scaffolds; thereby improve cell attachment, expansion, and proliferation on them (10-12).
Several studies have shown the differentiation of animal ASCs toward cardiomyocytes after treatment with chemical substances such as 5-azacytidin. Furthermore, in vivo animal ASCs studies have shown that when ASCs were transplanted after myocardial infarction (MI), the differentiation of ASCs towards cardiomyocytes increased and cardiac function was improved (13,14). Spontaneous differentiation of ASCs towards cardiomyocytes has also been described. However, studies on human ASCs are limited. In recent years, several studies demonstrated differentiation of human ASCs into cardiomyocytes without using any scaffolds (4, 15).
In the present study, human ASCs were cultured on random and aligned PCL nanofibrous scaffolds and then their biological behavior and cardiomyocyte differentiation were investigated in vitro.
Materials and Methods
Electrospun nanofibers
PCL nanofibers were produced by an electrospinning method in our laboratory using procedures reported earlier (10). Briefly, a PCL solution (5% w/w) was prepared in chloroform and dimethylformamide (DMF) in7.5:2.5 ratios. The polymer solutions fed into a blunted needle attached to a pump on a vertical mount. A rotating cylindrical drum was used as a collector and placed at a distance of 18 cm from the needle. For random scaffolds, the collecting rate was at 250 rpm and for aligned scaffolds at 2800 rpm. Voltage was 25 kV and flow rate was 0.5 mL/h. The copper plate was a plate with 10″ diameter and 30″ length. To increase the hydrophilicity of the nanofibers, surface modification of the PCL scaffold was performed by plasma treatment. A low frequency plasma generator (40 kHz) with a cylindrical quartz reactor (Diener, Electronics, Ebhausen, Germany) was used. The pressure of pure oxygen gas was at a 0.4 mbar, and the glow discharge was ignited for 2.5 min. Plasma-treated sheets were punched with a device of 1 cm diameter.
Characterization of nanofibers
Morphology study:
The morphology of the random and aligned PCL nanofibers was studied by scanning electron microscopy (SEM; XL30; Philips, Eindhoven, Netherlands).
Mechanical properties:
The tensile properties were performed on the nanofibrous webs using Galdabini testing equipment. Prepared scaffolds were cut into 10 mm × 60 mm × 0.11 mm specimens and tensile test was conducted at 50 mm/min crosshead speed at room temperature.
Porosity:
For porosity determination, four randomized circular samples with the diameter of 20 mm were used and the estimated porosity of each sample was calculated by the following equation: Porosity=1-(calculated scaffold density/known material density) × 100
Contact angle measurement:
The hydrophilicity of the nanofibrous after surface modification was measured by contact angle goniometer (Kruss, Hamburg, Germany), at room temperature. A water droplet was dropped on surface of the nanofibers, and the contact angle was measured after 10 seconds.
Isolation and characterization of ASCs
Human ASCs Isolation:
Human subcutaneous adipose tissue samples were obtained as waste materials after liposuction surgery from the abdomen region (Erfan hospital in Tehran, Iran) and donated after informed consent according to guidelines of the Medical Ethics Committee, Ministry of Health IR Iran. Seven healthy non-obese female donors (age range: 35-45 years) were included in this study. Adipose tissue was stored in sterile phosphate-buffered saline containing penicillin and streptomycin 3X and amphotericin 3X at 4°C and processed within 24 hours after surgery as described previously. In brief, after three times washes with PBS, the tissue was digested with 0.2% collagenase I (Roche Diagnostics, Mannheim, Germany) under intermittent shaking for 45 min at 37°C. Then, the material was centrifuged for 15 min at 1200 rpm and supernatant was removed and the cell pellet was treated with RBC lysis buffer (Dako, Glostrup, Denmark) at room temperature for 5 min. After centrifuged of samples at 1200 rpm for 5 min, the cell pellet was resuspended on the 75 cm2 culture flask (Nunk) under Dulbecco's Modified Eagle's Medium (DMEM, Invitrogen Co, Carlsbad, CA, USA) with 10% Fetal Bovine Serum (FBS, Invitrogen Co., Carlsbad, CA, USA) incubated with 95% air and 5% CO2 at 37°C (16). After reaching confluence (about 80-85%) during ten days, cells were detached using trypsin and replated.
Human ASCs characterization:
Cells were characterized by flow cytometric analysis of the expression of cell surface markers according to protocol reported by Ardeshirylajimi et al(17). In briefly, the expression of mesenchymal cell surface markers were evaluated using monoclonal antibodies including fluorescent isothiocyanate (FITC)-conjugated mouse anti-human CD45 (leukocyte common antigen), CD 44, CD 73 (ecto-5′- nucleotidase), CD 34, phycoerythrin (PE)-conjugated CD105 (Endoglin or SH2) and CD90.
MTT assay
The proliferation rate of human ASCs on random and aligned PCL nanofibrous scaffolds was measured by MTT assay. Aligned and random PCL nanofibers after being sterile were placed in a 24-well culture plate and seeded with a ASCs density of 4×103 cells per cm2 and incubated at 37°C, 5% CO2. After 1, 2, 3, 4 and 5 days of cell seeding, 50 µI of MTT solution (5 mg/ml in DMEM) was added to each well. For conversion of MTT to formazan crystals by mitochondrial dehydrogenases of living cells, the plates were incubated at 37°C for 4 hr. Then, supernatant was removed and constant amount of an appropriate solvent was added. The optical density was read at a wavelength of 570 nm in a micro plate reader (BioTek Instruments, USA). The same procedure was performed for cultured cells in tissue culture polystyrene (TCPS) as control.
Scaffold seeding and cardiomyogenic differentiation
Isolated stem cells were seeded at 1×105 cells/cm2 and cultured for several passages in DMEM supplemented with 10% FBS, (all from Invitrogen Co., Carlsbad, CA, USA), in a humidified atmosphere of 5% CO2 at 37°C. Media were changed twice a week. Once 80%-90% confluency has been reached, cells were detached with 0.5 mM EDTA trypsin (Invitrogen Co, Carlsbad, CA, USA), for 5 min at 37°C and replated. To assess the ASCs differentiation towards cardiomyocytes, culture-expanded cells (p3) were plated into 6-well culture dishes at a density of 20,000 cells per well, each well having normal culture medium. The cells were then cultured in DMEM containing 15% FBS and when the confluency has been reached 50-60%, stimulated with 5-aza-2-deoxycytidine for 24 hr (9 µM; Fluka, Sigma Aldrich, St. Louis, Mo. USA) in DMEM supplemented with 15% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM L-glutamine and 1% ITS + premix (BD Biosciences, Bedford, Mass., USA). Culture stem cells with this condition of 5-aza-deoxycytidine has been shown to induce differentiation of rabbit ASCs towards cardiomyocytes on uncoated culture plates (18). The cells were observed daily, and the medium changed three times per week until the experiment was terminated.
Immunocytochemistry analysis
Cells were fixed with 4% paraformaldehyde for 20 minute at room temperature, then permeabilized with Triton X-100 (0.3%) for 20 min and processed for immunocytochemistry using primary antibodies to βTubulin III 1:50 (mouse monoclonal; Sigma Aldrich, St. Louis, Mo. USA), and Neurofilament-M (NFM) 1:500 (mouse monoclonal; Sigma Aldrich, St. Louis, Mo. USA). For fluorescence, FITC conjugated (Sigma Aldrich, St. Louis, Mo. USA) anti-mouse secondary antibody 1:500 was applied. After washing with PBS, cells were incubated with DAPI (4′, 6-diamidino-2-phenylindole; 1:1000) for nuclear staining.
Real-time reverse transcription-polymerase chain reaction
For relative quantification of the gene expression, total cellular RNA was extracted using TRI-reagent (Sigma Aldrich, St. Louis, Mo. USA). Synthesis of cDNA was carried out with M-MuLV reverse transcriptase (RT) and random hexamer as primer, according to the manufacturer's instructions (Fermentas, Burlington, Canada). PCR amplification was performed using a standard procedure with Taq DNA Polymerase (Fermentas, Burlington, Canada) with denaturation at 94°C for 15 second, annealing at 55°C or 60°C for 30 sec according to the primers, and extension at 72°C for 45 sec. Depending on the abundance of particular mRNA, the number of cycles varied between 30 and 40. The primers sequences and product lengths are listed in Table 1.
Table 1.
Primers used in real-time RT-PCR
| Gene | Primer sequence (F, R, 5'→3') | Product length (base pair) |
|---|---|---|
| TroponinT | CCAGGGCAGAAGAAGATG | 135 |
| CCACTCTCTCTCCATCGG | ||
| α-MHC | GCTTCACTTCAGAGGAGAAAG | 135 |
| CCGACTTGTCAGCATCTTC | ||
| GATA-4 | CACCAGCAGCTCCTTCAG | 138 |
| GCCCGTAGTGAGATGACAG | ||
| MYOD | ACGGCATGATGGACTACAG | 218 |
| CGGAGGCGACTCAGAAG | ||
| β-Actin | CTTCCTTCCTGGGCATG | 85 |
| GTCTTTGCGGATGTCCAC | ||
Statistical analysis
The real-time RT-PCR data were analyzed by REST 2009 software (Qiagen Inc., Hilden, Germany). Each experiment was repeated independently at least three times in vitro. Data were reported as the mean ± SD. One-way analysis of variance (ANOVA) was used to compare the results. All analyses were performed by using SPSS 17.0 software (SPSS, Chicago, IL, USA). P-values of less than 0.05 were considered as statistically significant.
Results
Characterization of adipose tissue derived mesenchymalstem cells
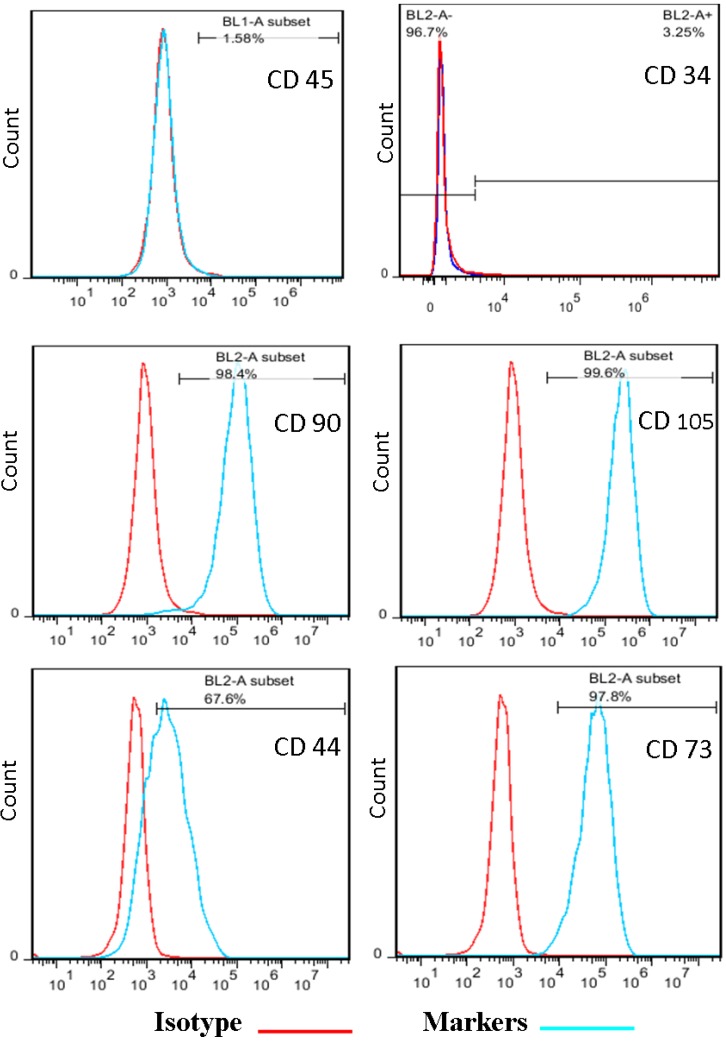
Isolated stem cells from adipose tissue were characterized based on their surface markers. ATMSCs were negative for CD45 and CD34 and were positive for CD90, CD 44, CD73 and CD105 (Figure 1).
Figure 1.
Flow cytometry analysis of human ASCs. Data showed that the expression cell surface markers such as CD45, CD34 was negative, but CD90, CD105, CD 44 and CD 73 was positive
Characterization of nanofibers
Morphology study
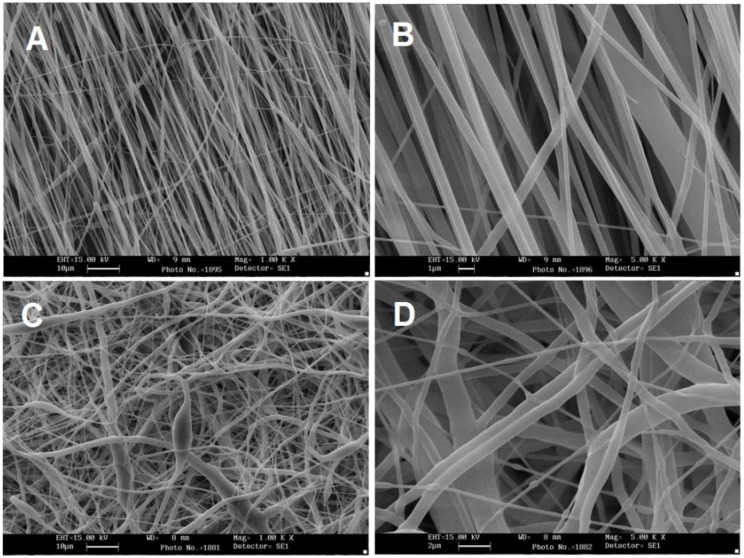
SEM images of random and aligned PCL scaffolds are shown in Figure 2 (A and C). Obviously the PCL nanofibrous scaffolds were highly porous with a uniform and smooth morphology. The diameters distribution is in the range of 450-800 nm with an average of 565±30 nm. The extra cellular matrix of the cells in human tissues is nanostructured and is composed of protein fibers which make a 3D matrix for cells to attach, differentiate and prolife rate. As it shown in the SEM images, the electrospun PCL nanofibers are nano-structured and highly porous which makes it a good candidate to be used in tissue engineering. The tensile strength of random PCL nanofibers was 3.83±0.58 MPa and elongation at break of 63.45±1.28%. And for aligned PCL nanofibers was 2.53±0.58 MPaand elongation at break of 42.72±2.46%. The porosity of random PCL scaffolds was calculated as 72.27%±1.24% and for aligned PCL scaffolds was 84.56%±0.94%.
Figure 2.
SEM images of nanofibrous PCL scaffolds. Aligned PCL scaffold without (a) and with (b) stem cells and random PCL scaffold without (c) and with (d) stem cells
Contact angle measurement
The contact angle of PCL nanofibers decreased from 132° to 0° after surface treatments. It shows that the hydrophilicity of the surfaces have been increased after surface modification. After plasma treatment the results prove the role of hydrophilic groups (OH and COOH) to increase the hydrophilicity of the surface.
Biocompatibility
Biocompatibility of the scaffolds was evaluated using MTT assay and SEM analysis. After 21 days cultured stem cells under basal medium (DMEM/FBS10%) on random and aligned PCL nanofibrous scaffolds, the samples were fixed and then investigated by SEM (Figure 2 B and D).
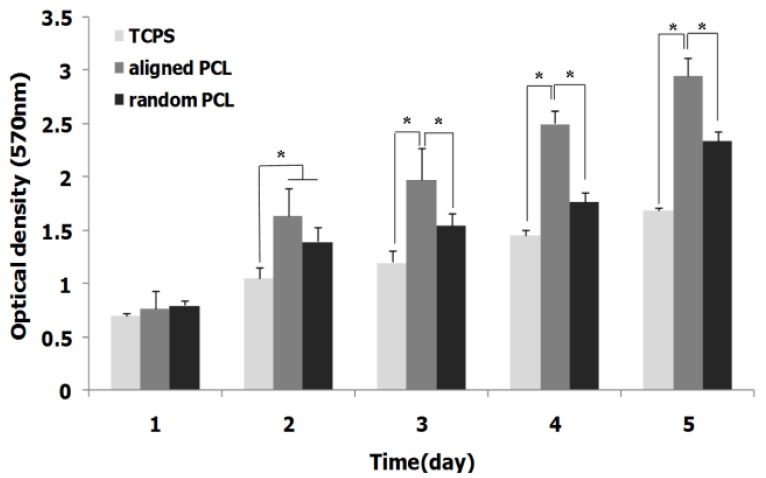
Results of MTT assay have demonstrated that the viability and proliferation rate of ASCs increased in PCL nanofibrous scaffold compared with TCPS. As it shown in Figure 3, also, significant differences were observed in proliferation rate of cells in aligned PCL compared with random PCL.
Figure 3.
Proliferation rate of ASCs on aligned and random nanofibrous PCL scaffolds during a 5 day cell culture period, asterisk shows significant difference with P<0.05
Evaluation of cardiogenic differentiation microscopicstudy
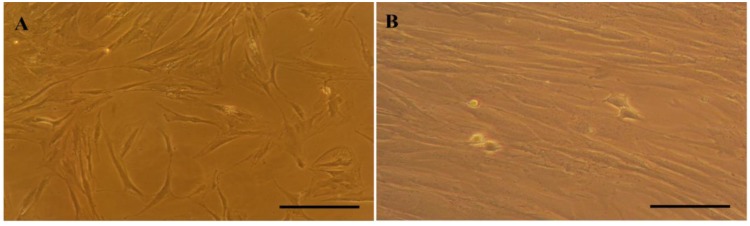
Isolated ASCs from human adipose tissue displayed a fibroblast and spindle-like morphology after 2 passages (Figure 4A). As showed in Figure 4B, the morphology of the cells was changed after 3 weeks incubation under cardiomyocyte differentiationmedium.
Figure 4.
The morphology of stem cells under basal medium after 2 passages (A) and after a 21 days culture under cardiogenic induction medium (B), scale bars (100 µm)
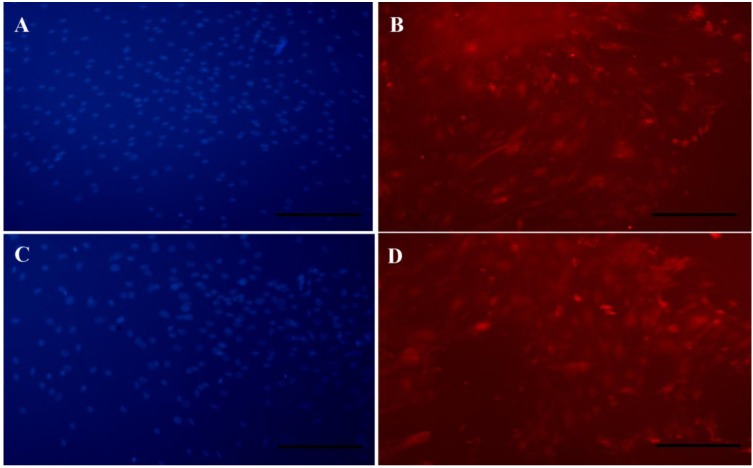
Immunocytochemistry
ICC analyze was used to investigate the βTubulin III as an intracellular protein and proved its expression in the cells cultured on random and aligned PCL scaffolds under differentiation medium. The result showed the presence of βTubulin III in cells which cultured on both scaffolds (Figures 5B and D). The appropriate nuclear localization of these markers was confirmed after merging by DAPI (Figures 5 A and C).
Figure 5.
Immunofluorescent staining of differentiated stem cells on PCL nanofibers.Staining of DAPI in random (A) and aligned (C) PCL nanofibers andβTubulin III in random (B) and aligned (D) PCL nanofibers.scale bars (100 µm)
Gene expression
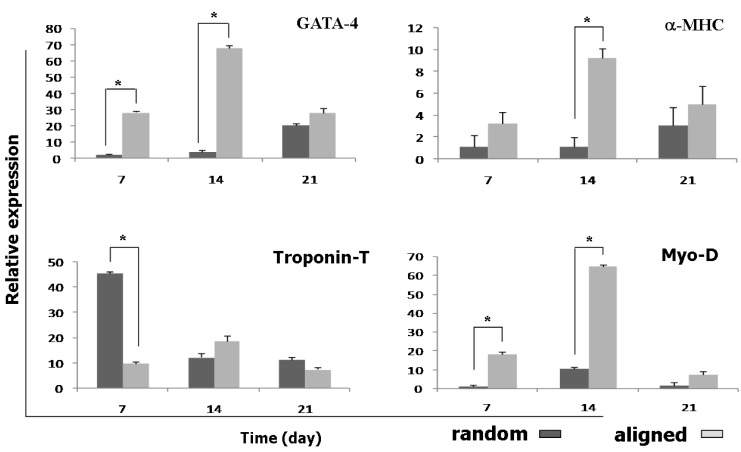
To investigate the effects of random and aligned PCL scaffolds on cardiomyocyte differentiation, relative expression of four important cardiac-related genes was investigated in three time points during cardiomyocyte differentiation of ASCs (Figure 6). All experiments were performed in three groups including ASCs seeded on aligned and random PCL and TCPS as control. The expression of all genes increased significantly in scaffolds compared with TCPS.
Figure 6.
Relative expression of troponin T, GATA-4, Myo-D and α-MHC on days 7, 14 and 21 in the human ASCs during cardiogenic differentiation, asterisk shows significant difference between two groups on each day at P<0.05
The expression of troponin-T as an early cardiac gene marker in random scaffolds at day 7 was significantly increased compared with aligned scaffolds, but at day 14 the higher expression of troponin-T was observed in aligned scaffold. Three weeks after treatment, the expression of troponin-T was decreased in both scaffolds. The expression of GATA-4 as another early cardiac gene marker, after 7 and 14 days, significantly increased in aligned scaffolds compared with random scaffolds and GATA-4 expression was down-regulated on day 21 in both scaffolds.
At day 14 the highest amounts of α-MHC was observed in aligned scaffolds. Myo-D was expressed in a significantly higher level on day 7 and 14 in aligned scaffolds compared with random scaffolds.
Discussion
Many studies have been reported the application of tissue engineering toalleviate many diseases (10, 19, 20). As we know stem cells have very potentials for cell therapy of various diseases (21). Artificial scaffolds and stem cells are utilized to regenerate cardiac tissue and restore heart functions after any heart injuries. However, the injection of cells directly into the infracted area involves the problems of low cell retention and engraftment rate (22, 23). A bioactive, biocompatible scaffold mimicking native tissue biochemical and biomechanical environment are needed for successful cardiac tissue regeneration (24, 25). Nanofibrous PCL scaffolds, because of their native tissue like properties are widely used as the cell delivery carriers and supporting matrices for cardiac tissue regeneration. It would seem that due to their characteristics, such as capacity for multi-linage differentiation and self-renewal potential, among all human adult stem cells, the ASCs exhibit the greatest potential (13).
Some of the studies have been reported the in vitro differentiation of animal ASCs towards cardiomyocytes after treatment with chemical agents such as 5-azacytidine (7, 14, 26). Neofytou et al described the differentiation ability of mouse adipose derived stem cells into cardiomyocyte (7). Rangappaet al demonstrated the appearance of beating cells on treatment of mesenchymal stem cells isolated from rabbit adipose tissue (18) and also Planat-Benard et al showed the ASCs towards cardiomyocytes can differentiate spontaneously (2). However, not many studies reported cardiomyocyte differentiation of human ASCs.
In recent years, several approaches to modify cardiomyogenic differentiation of human ASCs were reported. Van Dijket al showed that use of laminin as ECM molecules during cell culture lead to higher rate of differentiation (27). Choi et al demonstrated that co-culture the human ASCs with contracting cardiomyocyteswas a key inducer for cardiomyogenic differentiation (4). Metzeleet al found that human ASCs exhibit both stem cell and cardiomyocyte properties after fusion with rat cardiomyocytes (15).
RT-PCR analyses of cardiac related gene markers including troponin-T, GATA4, α-MHC and Myo-D showed that the expression of cardiac markers were increased on nanofibrous scaffolds compared to TCPS because of the presence of these physicochemical structures. Moreover, it can also be observed that GATA4, α-MHC and Myo-D gene markers were highly expressed in aligned scaffolds compared with random ones.
Troponin-T is one of the essential proteins for contractile function and an indicator of differentiation in cardiomyocytes(28). GATA4 is known as a key transcription factor for commitment to cardiac lineage (29). Expression of Myo-D and α- MHC as secondary gene markers follow the same pattern. Expression of all four genes increased significantly when the cells cultured on scaffolds compared with the cells which cultured on TCPS. This result confirmed the positive effects of nanofibrous scaffolds on proliferation and differentiation of stem cells. As we know GATA4 and troponin-T genes are early cardiac related gene markers and it is expected that their expression decreases during differentiation. Although we expected to have increased expression of two other genes (α-MHC and Myo-D) during differentiation, but not like this. However, these genes showed higher expression compared to the control samples. They also had higher expression in stem cells cultured on aligned nanofibers. In addition, the expression of GATA4, Myo-D and α-MHC increased more significantly in aligned nanofibers compared with random nanofibers.
However, the expression of troponin-T in random PCL was higher than aligned PCL at day 7; this may be done via different signaling pathways with GATA4 as another early cardiomyocyte gene marker. In another hand, random fibers may act through activation of Troponin-T and aligned may act through GATA4 (30).This proper supporting matrix can hold cells at the infracted area initially and further provide support for cell survival and functioning. We hypothesized that in the presence of certain physical and chemical cues, ASCs differentiate into cells that resemble cardiac myocytes and may be applicable for cardiac regeneration. With this approach cells would remain adhered to the nanofibrous scaffold preventing cell loss and providing a more site directed repair mechanism. It is accepted that physical cues play a key role in cell growth and tissue assembly (31). These signals are important in stem cells during selfrenewal, proliferation, and differentiation. A softer substrate and the ability to tune the mechanical properties within a given range could be advantageous as cell differentiation was shown to be affected by substrate stiffness. Additionally, it has been estimated that a cell number on the order of one billion would need to be replaced in patients with heart failure (3).
Conclusion
In this study, cardiomyocyte differentiation of ASCs on the random and aligned PCL nanofibrous scaffolds surface demonstrated that aligned PCL nanofibrous scaffold more appropriate to guide cardiac regeneration than random PCL. In addition, aligned PCL nanofibers hold promising potential as efficient cardio-inductive implants for cardiac tissue engineering application.
Acknowledgment
This study was supported by Stem Cell Technology Research Center, Tehran, Iran. The results described in this paper were part of student thesis.
References
- 1.Khosravi A, Aghamohamadi S, Kazemi E, Pour Malek F, Shariati M. Mortality Profile in Iran (29 provinces) over the Years 2206 to 2010. Tehran: Ministry of Health and Medical Education; 2013. [Google Scholar]
- 2.Planat-Benard V, Menard C, Andr M, Puceat M, Perez A, Garcia-Verdugo J-M, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res . 2004;94:223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 3.Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Ther . 2005;105:151–163. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Choi YS, Dusting GJ, Stubbs S, Arunothayaraj S, Han XL, Collas P, et al. Differentiation of human adiposederived stem cells into beating cardiomyocytes. J Cell Mol Med . 2010;14:878–889. doi: 10.1111/j.1582-4934.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mardani M, Kabiri A, Esfandiari E, Esmaeili A, Pourazar A, Ansar M, et al. The Effect of platelet rich plasma on chondrogenic differentiation of human adipose derived stem cells in transwell culture. Iran JBasic Med Sci . 2013;16:1163–1169. [PMC free article] [PubMed] [Google Scholar]
- 6.Kim U, Shin DG. Hayat M.A. , editor. Stem Cells and Cancer Stem Cells. Therapeutic Applications in Disease and Injury. Differentiation of human adiposederived stem cells into cardiomyocytes. 2012. pp. 95–102.
- 7.Neofytou EA, Chang E, Patlola B, Joubert LM, Rajadas J, Gambhir SS, et al. Adipose tissue‐derived stem cells display a proangiogenic phenotype on 3D scaffolds. J Biomed Mater Res A . 2011; 98:383–393. doi: 10.1002/jbm.a.33113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed CR, Han L, Andrady A, Caballero M, Jack MC, Collins JB, et al. Composite tissue engineering on polycaprolactone nanofiber scaffolds. Ann Plast Surg. 2009; 62:505–512. doi: 10.1097/SAP.0b013e31818e48bf. [DOI] [PubMed] [Google Scholar]
- 9.Shabani I, Haddadi-Asl V, Seyedjafari E, Babaeijandaghi F, Soleimani M. Improved infiltration of stem cells on electrospun nanofibers. Biochem Biophys Res Commun. 2009; 382:129–133. doi: 10.1016/j.bbrc.2009.02.150. [DOI] [PubMed] [Google Scholar]
- 10.Ardeshirylajimi A, Dinarvand P, Seyedjafari E, Langroudi L, Adegani FJ, Soleimani M. Enhanced reconstruction of rat calvarial defects achieved by plasma-treated electrospun scaffolds and induced pluripotent stem cells. Cell Tissue Res. 2013;354:849–860. doi: 10.1007/s00441-013-1693-8. [DOI] [PubMed] [Google Scholar]
- 11.Park H, Lee KY , Lee SJ, Park KE, Park WH. Plasmatreated poly (lactic-co-glycolic acid) nanofibers for tissue engineering. Macromol Res . 2007; 15:238–243. [Google Scholar]
- 12.Mohammadi Y, Soleimani M, Fallahi-Sichani M, Gazme A, Haddadi-Asl V, Arefian E, et al. Nanofibrous poly (epsilon-caprolactone)/poly (vinyl alcohol)/chitosan hybrid scaffolds for bone tissue engineering using mesenchymal stem cells. Int J Artif Organs . 2007; 30:204–211. doi: 10.1177/039139880703000305. [DOI] [PubMed] [Google Scholar]
- 13.Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med . 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 14.Nassiri SM, Khaki Z, Soleimani M, Ahmadi SH, Jahanzad I, Rabbani S, et al. The similar effect of transplantation of marrow-derived mesenchymal stem cells with or without prior differentiation induction in experimental myocardial infarction. J Biomed Sci. 2007; 14:745–755. doi: 10.1007/s11373-007-9188-9. [DOI] [PubMed] [Google Scholar]
- 15.Metzele R, Alt C, Bai X, Yan Y, Zhang Z, Pan Z, et al. Human adipose tissue-derived stem cells exhibit proliferation potential and spontaneous rhythmic contraction after fusion with neonatal rat cardiomyocytes. FASEB J . 2011; 25:830–839. doi: 10.1096/fj.09-153221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mardani M, Hashemibeni B, Ansar MM, Esfahani SHZ, Kazemi M, Goharian V, et al. Comparison between chondrogenic markers of differentiated chondrocytes from adipose derived stem cells and articular chondrocytes in vitro. Iran JBasic Med Sci. 2013;16:763–773. [PMC free article] [PubMed] [Google Scholar]
- 17.Ardeshirylajimi A, Sleimani M, Hosseinkhani S, Parivar K, Yaghmaei P. A comparative study of osteogenic differentiation of human induced pluripotent stem cells and adipose tissue derived mesenchymal stem cells. Cell J . 2013;16:5. [PMC free article] [PubMed] [Google Scholar]
- 18.Rangappa S, Fen C, Lee EH, Bongso A, Sim EK. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg . 2003; 75:775–779. doi: 10.1016/s0003-4975(02)04568-x. [DOI] [PubMed] [Google Scholar]
- 19.Ardeshirylajimi A, Hosseinkhani S, Parivar K, Yaghmaie P, Soleimani M. Nanofiber-based polyethersulfone scaffold and efficient differentiation of human induced pluripotent stem cells into osteoblastic lineage. Mol Biol Rep . 2013; 40:4287–4294. doi: 10.1007/s11033-013-2515-5. [DOI] [PubMed] [Google Scholar]
- 20.Soleimani M, Mohammadi Y, Ahmadbeigi N, Tafti HA, Nassiri SM, Boroumand MA, et al. Tissue cardiomyoplasty using multi-layer cell-seeded nanostructural scaffolds to repair damaged myocardium: An experimental pilot study. Arch Med Sci. 2008;4:364–370. [Google Scholar]
- 21.Ardeshirylajimi A, Hagh MF, Saki N, Mortaz E, Soleimani M, Rahim F. Feasibility of cell therapy in multiplesclerosis: A systematic review of 83 studies. Int J Hematol Oncol Stem Cell Res . 2013; 7:15–33. [PMC free article] [PubMed] [Google Scholar]
- 22.Murtuza B, Nichol JW, Khademhosseini A. Microand nanoscale control of the cardiac stem cell niche for tissue fabrication. Tissue Eng Part B Rev. 2009; 15:443–454. doi: 10.1089/ten.teb.2009.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubinov , Sykov E. Nanotechnologies in regenerative medicine. Minim Invasive Ther Alied Technol . 2010; 19:144–156. doi: 10.3109/13645706.2010.481398. [DOI] [PubMed] [Google Scholar]
- 24.Yucel D, Kenar H, Ndreu A, Endogan T, Hasirci N, Hasirci V. Nanotechnology in biomaterials: nanofibers in tissue engineering. biotechnology Global prospects II CRC Press Taylor and Francis group. 2012. pp. 227–246.
- 25.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng . 2005; 11:101–109. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 26.Gaustad KG, Boquest AC, Anderson BE, Gerdes AM, Collas P. Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochem Biophys Res Commun . 2004; 314:420–427. doi: 10.1016/j.bbrc.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 27.Van Dijk A, Niessen HW, Zandieh Doulabi B, Visser FC, van Milligen FJ. Differentiation of human adiposederived stem cells towards cardiomyocytes is facilitated by laminin. Cell Tissue Res . 2008; 334:457–467. doi: 10.1007/s00441-008-0713-6. [DOI] [PubMed] [Google Scholar]
- 28.Xing Y, Huang PJ, Zhang KM. Cardiac troponin T and I: application in myocardial injury and forensic medicine. Fa Yi Xue Za Zhi . 2003; 19:242–244. [PubMed] [Google Scholar]
- 29.Rajala K, Pekkanen-Mattila M, Aalto-Setala K. Cardiac differentiation of pluripotent stem cells. Stem Cells International . 2011; 2011 doi: 10.4061/2011/383709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai B, Li J, Wang J, Luo X, Ai J, Liu Y, et al. micro- RNA‐124 regulates cardiomyocyte differentiation of bone marrow‐derived mesenchymal stem cells via targeting STAT3 signaling. Stem Cells . 2012; 30:1746– 1755. doi: 10.1002/stem.1154. [DOI] [PubMed] [Google Scholar]
- 31.Baker BM, Mauck RL. The effectof nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials . 2007;28:1967–977. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]