Abstract
The evolving regulatory landscape has heightened the need for innovative, proactive, efficient and more meaningful solutions for ‘real-world’ post-authorization safety studies (PASS) that not only align with risk management objectives to gather additional safety monitoring information or assess a pattern of drug utilization, but also satisfy key regulatory requirements for marketing authorization holder risk management planning and execution needs. There is a need for data capture across the primary care and secondary care interface, or for exploring use of new medicines in secondary care to support conducting PASS. To fulfil this need, event monitoring has evolved. The Specialist Cohort Event Monitoring (SCEM) study is a new application that enables a cohort of patients prescribed a medicine in the hospital and secondary care settings to be monitored. The method also permits the inclusion of a comparator cohort of patients receiving standard care, or another counterfactual comparator group, to be monitored concurrently, depending on the study question. The approach has been developed in parallel with the new legislative requirement for pharmaceutical companies to undertake a risk management plan as part of post-authorization safety monitoring. SCEM studies recognize that the study population comprises those patients who may have treatment initiated under the care of specialist health care professionals and who are more complex in terms of underlying disease, co-morbidities and concomitant medications than the general disease population treated in primary care. The aims of this paper are to discuss the SCEM new-user study design, rationale and features that aim to address possible bias (such as selection bias) and current applications.
Key Points
| Specialist Cohort Event Monitoring (SCEM) addresses an existing need for safety surveillance of new medicines initiated in the hospital (secondary care) setting. |
| The method can provide insight into the adoption of a new product into clinical practice with inclusion of comparator cohorts receiving standard care, if desired. |
| SCEM can be applied to study medicines irrespective of therapeutic class and has already been incorporated into the risk management plans of some recently marketed products. |
Introduction
Over the last decade, post-marketing safety and risk management has witnessed a fundamental shift towards proactive benefit: risk evaluation. The importance of pharmacovigilance (PV) activities and interventions designed to identify, characterize, prevent or minimize risks relating to medicinal products, including the assessment of the effectiveness of those activities and interventions, has increased dramatically with the evolution of European Union (EU) PV legislation requiring every new medicinal product to have a risk management plan (RMP) in place as part of the approval and licensing processes.
Depending on the nature of the risks (both potential and identified), information considered missing in pre-marketing development (e.g. on special populations or chronic exposure, and the indication for and nature of the therapeutic intervention), additional PV activities are usually required to monitor and study risks in the ‘real-world’. These activities extend beyond routine PV activities, such as spontaneous reporting of suspected adverse drug reactions (ADRs). The evolving regulatory landscape has heightened the need for innovative, proactive, efficient and more meaningful solutions for ‘real-world’ post-authorization safety studies (PASS). Such studies should not only align with risk management objectives to gather additional safety monitoring information or assess a pattern of drug utilization, but also satisfy key regulatory requirements for risk management planning and execution needs.
PASS is defined as “any study relating to an authorized medicinal product conducted with the aim of identifying, characterizing, or quantifying a safety hazard, confirming the safety profile of the medicinal product, or of measuring the effectiveness of risk management measures” [1]. Predominantly non-interventional (however, a PASS may be a clinical trial), their main objective is to gather additional safety monitoring information or to assess a pattern of drug utilization (Fig. 1).
Fig. 1.
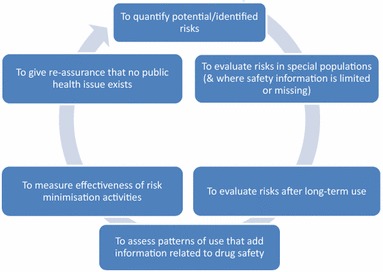
Aims of post-authorization safety studies (PASS) [1]. Within non-interventional PASS conducted in a ‘real-world’ setting, usual clinical course of care applies and receipt of treatment by a patient is not a condition of participation within a PASS. A PASS must address at least one of the aims listed in the figure
There are a number of resources that have been developed to support and strengthen the conduct of PASS in the EU [2]. Whilst opportunities exist for record linkage via the primary care and secondary care interface (http://www.hantshealthrecord.nhs.uk), there remains a need to put in place a proactive resource for studies to systematically obtain the necessary information on the safety and utilization of targeted medicines initiated in hospital (secondary care) settings or at affiliated sites by specialist health care professionals (HCPs) at a national level. Specialist Cohort Event Monitoring (SCEM) studies address this need. The SCEM study design enables a cohort of patients prescribed a medicine in secondary care to be monitored. It also permits the inclusion of a comparator cohort of patients receiving standard care, or another counterfactual comparator group, to be monitored concurrently, depending on the research question. The approach has been developed in parallel with the evolving legislative requirements for PV and RMPs. The aim of this paper is to discuss the SCEM studies’ new-user design, rationale and features that aim to address possible bias (such as selection bias) and current applications.
An Additional Tool for Post-marketing Safety Surveillance
In the UK, a post-marketing event-monitoring system exists on a national scale, which uses dispensed National Health Service (NHS) primary care prescription information derived from primary care physician (GP) practices to identify eligible patients treated with new medicines or treated with existing medications for new indications [3, 4]. In contrast, there is currently no similar system available that has oversight of all prescriptions issued within the secondary care setting across each hospital trust, at the patient level. SCEM studies extend the event-monitoring approach to support the generation or further evaluation of safety signals, describe the exposed patient populations and inform on the incidence of use outside recommended terms of license (off-label) in the secondary care setting.
Observational Research
SCEM studies are, by definition, observational studies to evaluate use of a drug in the naturalistic setting and fulfil the criteria for PASS [1]. SCEM studies are non-interventional because they do not interfere with the prescribing decision process of the practitioner. Data are collected on patients for whom the pharmacotherapeutic treatment decision has been made that the new product is the most appropriate treatment in the secondary care setting, as in everyday ‘real-world’ clinical practice. All specialist HCPs who can prescribe the drug, and thus all patients to whom participating specialist HCPs have access, are eligible for inclusion. A summary of the key characteristics of an SCEM study is presented in Table 1.
Table 1.
Characteristics of Specialist Cohort Event Monitoring (SCEM) design
| Study characteristic | SCEM |
|---|---|
| Setting | Secondary care |
| HCPs | Specialists |
| Design | Observational |
| Treatment | Naturalistic |
| Period of observation | Immediately post-start of treatment; the duration depends on the study question(s) |
| Ethics | Patient consent required |
| Risk level of patient | More likely to capture high-risk patients with more severe disease |
| Data source | Secondary use of data from existing medical records |
| Comparator | Standard care, other or none; the choice depends on the study question |
HCP health care professional
The underlying organizational construct of SCEM is that each study is essentially an organized system that uses observational study methods to collect uniform existing data (clinical and other) to evaluate specified outcomes for a population defined by a particular disease, condition or exposure. Akin to a registry, SCEM studies serve to provide the necessary information on groups of people who possess a particular characteristic. Of major importance is that SCEM studies recognize that the study population comprises those patients who may have treatment initiated under the care of specialists and who are more complex in terms of underlying disease, co-morbidities and concomitant medications than the general disease population treated in primary care. SCEM studies also use a targeted data collection method, which allows exposure to be more effectively defined using actual treatment dates from secondary care medical records with reference to diagnosis of the clinical condition for which the new drug is indicated or another well-defined event with a clearly recognizable onset. This mitigates the risk of immortal time bias [5]. This type of bias can arise when the period between cohort entry and the date of the first exposure (e.g. to a drug), during which an event has not occurred, is either misclassified or simply excluded and not accounted for in the analysis [5]. Commonly observed in classical cohort studies from large health care databases (where a treated group is compared with some reference group) conducted in the primary care setting, immortal time can bias results in favour of the treated group because the period of immortality is misclassified with regard to treatment, and the outcome cannot occur (Fig. 2).
Fig. 2.
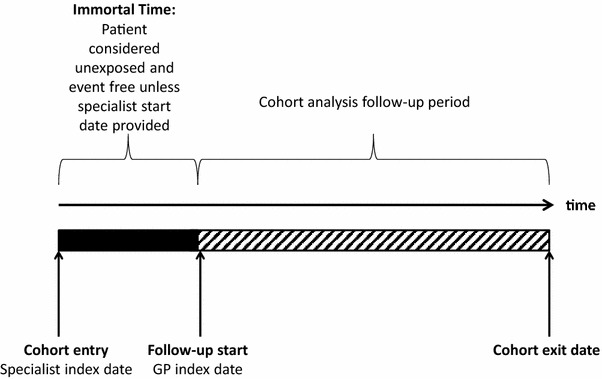
Possible immortal time in observational studies using primary care medical records data. Immortal time bias is introduced in cohort studies using primary care medical records when the period of immortal time is excluded from the analysis because the start of follow-up for new users whose treatment is initiated in secondary care is defined by the start of treatment in primary care, not by the start of treatment in secondary care. GP primary care physician
Study Setup
SCEM studies are conducted in accordance with national and international guidelines [6–9]. Following the principles of good pharmacoepidemiology practice [10], a full protocol is written for each SCEM study in accordance with the EU PASS guidelines. Patient information security is assured through strict measures guided by Drug Safety Research Unit (DSRU) policies. A single UK-wide ethical opinion is applied for from the National Research Ethics Service. Local Research and Development (R&D) approval is also required at each participating trust. Consent is required for access to information from existing secondary care hospital charts, and also (for some SCEM studies) to enable contact with patients’ GPs to access information from existing primary care charts.
All SCEM protocols are now reviewed by the Pharmacovigilance Risk Assessment Committee (PRAC). Once the RMP is approved, SCEM studies are registered on the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCEPP) e-register of studies (http://www.encepp.eu). They may also be registered on clinicaltrials.gov, where appropriate.
The DSRU works collaboratively with the UK National Institute of Health Research (NIHR) Clinical Research Networks (CRNs), which are associated with undertaking research with health service providers (trusts) at a national level. SCEM studies are evaluated against specific eligibility criteria before being adopted into the NIHR portfolio [11]. These eligibility criteria include confirmation that the study is for research and a requirement for high-quality peer review of the protocol and other study documents. Accordingly, relevant subject matter CRN study officers are able to link the DSRU with specialist HCPs within hospital and/or secondary care settings. Thus, the SCEM study population is recruited through an active research network of specialist HCPs relevant to the clinical condition for which the new product may be prescribed.
Two SCEM studies—the Observational Assessment of Safety of Seroquel (OASIS) [12] and the Observational Safety Evaluation of Asenapine (OBSERVA) [13]—have used the Mental Health Research Network, whilst a third—the Rivaroxaban Observational Safety Evaluation (ROSE) [14]—has used the Non-malignant Haematology Speciality Group, the Cardiovascular Speciality Group and the Stroke Research Network. Specialist HCPs within these networks are invited to express interest prior to the start of the study after adoption into the NIHR portfolio. In particular, they are informed that they will be participating in an observational study, which will monitor the use of a new product in accordance with the requirements set out within an RMP.
Data Collection
Once consent has been obtained, patients are observed. The length of the patient observation period depends on the questions sought to be answered by the particular SCEM study. Information is provided by specialist HCPs, and, where necessary, further information is obtained from the patients’ GPs. The study design utilizes a multiphase approach for secondary data collection from medical records, which is intended to facilitate cooperation with data reporting, as well as spreading the workload (Fig. 3).
Fig. 3.
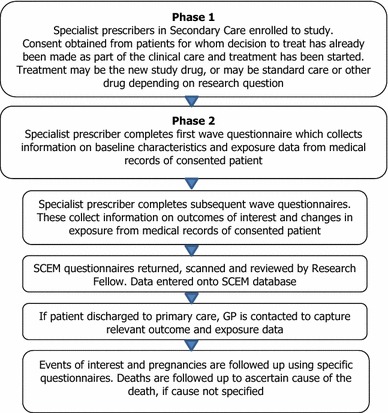
Process flow of a Specialist Cohort Event Monitoring (SCEM) study. GP primary care physician
The first phase of an SCEM study involves collecting specialist HCP demographic and specialist expertise data. Such data is a combination of self-reported information (upon registration) and may also be supplemented from publically available information from relevant professional bodies during the course of the study. In the second phase, since the principle of event monitoring applies, and data are abstracted from existing medical records, data collection is regarded as secondary use in accordance with EU regulatory guidelines [1]. Information on baseline characteristics and exposure at the start of the observation (the index date) is obtained from the first-wave questionnaire, whilst information on outcomes and changes in exposure are collected from subsequent waves during observation. Reported data are examined for events of interest, which have been defined according to the research objective and may include known, potential or missing risks. All clinical events of medical interest (targeted/non-targeted) and serious adverse event reports [15] may undergo further evaluation and follow-up as appropriate. This process continues until the target final study milestone is reached and the final cohort of evaluable1 patients is confirmed.
A number of SCEM studies are ongoing, and an overview is presented in Table 2 to illustrate the potential applications of SCEM in the context of PV and risk management [12–14]. The common aim is to examine the safety and use of the study drug. They may include a comparator group of patients receiving standard care, or another counterfactual group, to be monitored concurrently. An SCEM study may also follow a single exposure cohort design, depending on the research question being addressed. These studies have been designed to address specific research questions, including characterization of real-life drug use, evaluation of particular sub-populations, adherence to prescribing recommendations or guidelines, and targeted surveillance or analysis of specific events, including those considered to require special monitoring by regulatory authorities. Each of these studies has been undertaken as part of a post-approval commitment within the RMP.
Table 2.
Examples of applications of Specialist Cohort Event Monitoring (SCEM) methodology
| ENCEPP e-register of PASS | Rationale and background | Research question | Study design | Setting, timeframe and size | Subject sampling | Data analysis applications |
|---|---|---|---|---|---|---|
| Observational Assessment of Safety in Seroquel (OASIS) [12] | Post-approval RMP commitment for further understanding of safety during titration and at higher doses (>600 mg/day) of the XL formulation of quetiapine fumarate (Seroquel XLTM) | To examine short-term (up to 12-week) safety and use of Seroquel XL™ newly initiated in patients with a clinical diagnosis of schizophrenia or manic episodes associated with bipolar disorder in the mental health care trust setting |
Observational, population-based cohort design Two waves of data collection (at baseline and 12 weeks post-index date) Quetiapine IR used in referent cohort |
Mental health trust setting in secondary care; patients recruited from February 2010 to December 2012; evaluable patients with baseline data (n = 869) and with event data (n = 845) |
Accessible target patient population was treated by specialist HCPs in psychiatry Sampling supported by MHRN CRN |
Univariate analysis to explore impact of formulation and dose on identified risks and for general surveillance Time-to-event analysis to characterize pattern of hazard over time for important identified risks |
| Observational Safety Evaluation of Asenapine (OBSERVA) [13] | Post-authorization commitment requested by the CHMP to further investigate the safety profile of asenapine (Sycrest™) in clinical practice | To monitor short-term (12-week) use and safety of asenapine prescribed to asenapine-naïve (new-user) patients for treatment of moderate to severe manic episodes associated with bipolar I disorder and other psychiatric disorders |
Observational, population-based, single-exposure cohort design Two waves of data collection (at baseline and 12 weeks post-index date) |
Mental health trust setting in secondary care; patients recruited over 3 years from December 2012 Per-protocol sample size (n = 1,000) |
Accessible target patient population was treated by specialist HCPs in psychiatry Sampling supported by MHRN CRN |
Association between oral events (oral and pharyngeal hypoaesthesia, oropharyngeal swelling) and starting treatment will be examined using the self-controlled case series method [16] |
| Rivaroxaban Observational Safety Evaluation (ROSE) study [14] | Post-authorization commitment requested by the CHMP to further investigate the safety profile of rivaroxaban (Xarelto™) in clinical practice | To monitor the short-term (12-week) use and safety profile of rivaroxaban prescribed to new-user (rivaroxaban-naïve) adult patients (who may or may not be antithrombotic therapy naïve) for prevention of stroke and systemic embolism in adult patients with non-valvular AF, treatment of DVT and PE, and prevention of recurrent DVT and PE in adult patients requiring anticoagulationa |
Observational, population-based, single-exposure cohort design Two waves of data collection (at baseline and 12 weeks post-index date) Additional follow-up with GP for patients discharged within the 12-week observation period Warfarin standard care used in referent cohort |
All clinical practice specialities requiring anticoagulation in secondary care; patients recruited over 2 years from September 2013 Per-protocol sample size (n = 1,700) |
Accessible target patient population was treated by specialist HCPs in anticoagulation Sampling supported by CRN specialist groups |
Contextual cohort of patients receiving alternative anticoagulant therapy will inform on the adoption of rivaroxaban into clinical practice and variation in determinants of treatment choices; case definition of haemorrhagic outcomes is according to ISTH criteria [24] |
AF atrial fibrillation, CHMP Committee for Medicinal Products for Human Use, CRN Clinical Research Network, DVT deep vein thrombosis, ENCEPP European Network of Centres for Pharmacoepidemiology and Pharmacovigilance, HCP health care professional, IR immediate release, ISTH International Society on Thrombosis and Haemostasis, MHRN Mental Health Research Network, PASS post-authorization safety studies, PE pulmonary embolism, RMP risk management plan, XL prolonged-release
aRivaroxaban is now also indicated for secondary prevention following an acute coronary syndrome in combination with aspirin alone or with aspirin plus clopidogrel or ticlopidine
Data Analysis for Signal Detection
Signal detection and evaluation is one of the primary concerns of PV to try and distinguish possible ADRs from adverse events that are unrelated to use of the study drug. Several methods are undertaken using SCEM study data for signal detection, both qualitative and quantitative. The framework for how the data are analysed is based on the cohort design. After a defined point in time (starting a new treatment), patients are observed to see if they develop a particular endpoint or outcome. The longitudinal nature of the data permits examination of temporal relationships between outcomes and new exposures. Information is gathered on the numerator (the number of reports of an event) and denominators in terms of the number of patients and also the number of patient-weeks of exposure. Calculating and ranking crude incidence density (ID) rates is one of a number of standard quantitative evaluations used in event monitoring methodology for signal generation purposes as part of initial inspection of all event data for general safety surveillance. It is used as a means of identifying early potential signals as priorities for further evaluation. Medical judgment, however, is also part of this evaluation and prioritization process. Calculation of the crude incidence (risk) of incident events by week or IDs (or rates) of incident events, expressed usually in counts per 1,000 patient-weeks of treatment (or observation), provide estimates of the ‘real-world’ frequency of reported events. These are descriptive methods in which one may observe disproportionately higher counts than expected from the summary of product characteristics or clinical trial data.
Within-group comparisons of crude ID rate differences or ratios according to different time periods after the start of treatment can identify signals of events that occur significantly more frequently after the study drug is started. The null hypothesis is that the incidence rates are constant between the two time periods being compared; the alternative hypothesis is that the incidence rates are different. A 95 % confidence interval (CI) is applied to the rate difference or ratio (based on the normal approximation). Thus, if the rate of events in the first month (ID1) is being compared with the rate in the second month and the third month combined (ID2–3), where the confidence limits around the rate difference estimate or the rate ratio point estimate exclude the null value (0 or 1, respectively), the null hypothesis is rejected. These results can be considered to be a signal of an event associated with starting treatment with the study drug if the estimates are positive, or correspondingly a signal of a delayed-onset event if the events are negative. In comparing these two time periods, the assumption is made that, given an event, its reporting is equivalent in both periods in a fixed cohort.
As this approach may sometimes mask significant signals in specific risk groups, the subgroups defined by specific characteristics (e.g. previous history of a condition, previous/concurrent use of selected medications, off-label indication groups) will have risks and IDs calculated and compared according to strata for relevant events, where appropriate. An example of this approach is seen in the OASIS study [12]. Since it was anticipated that dosing regimens would be individualized, it was necessary to account for variation of the dose over time, since some events may be dose related. For the exposure group of interest (patients receiving Seroquel XL™) and the internal comparator group (patients receiving quetiapine immediate release [IR]), for each patient, six periods of person-time fixed at 2-week intervals were created and the modal dose was calculated according to the dose recorded for more than 50 % of each period. This approach does introduce an artificial time structure; however, group-level analysis of person-period data permits simple univariate exploration of associations between the dose, events and selected risk factors in defined periods for signal detection purposes.
For SCEM studies in particular, the relatively short timeframe can result in low counts for some events. As such, the statistical assumptions that underpin such comparisons may no longer apply. Thus, other methodological approaches need to be considered, such as the self-controlled case series approach to explore changes in the risk of oral events over time in users of asenapine in the OBSERVA study [13, 16]. Complementary quantitative analyses may also be undertaken. These include capturing information on, and ranking by frequency, the reasons for stopping the study drug and comparisons with ranked IDs. These values can be used to compare and contrast groups descriptively. Regardless of which method is used, all signals then require confirmation or refutation by further study. A qualitative assessment of cases contributing data to a signal may be undertaken to include evaluation of patient demographic characteristics, treatment details, detection and clinical features and management of those events of interest, resolution, relevant investigations prior to and during therapy, the patient’s relevant medical history and concurrent medication, and any sequelae. Data are derived from the SCEM, and follow-up questionnaires are sent to gather other relevant essential information for construction of a case-series summary descriptive table.
Evaluation of the Specialist Cohort Event Monitoring Design
The strengths and limitations of the SCEM design are summarized in Table 3. One key feature distinguishes the SCEM design from other data resources for PASS. This relates to the goal to obtain a representative distribution of a cohort of patients exposed to treatment within the secondary care setting in the early post-marketing period. As with other observational epidemiological studies, there remains the possibility that bias may affect the external validity of the SCEM study results. This bias may be introduced from a number of sources associated with the study design, such as coverage, the sampling process and non-response [17].
Table 3.
Evaluation of the Specialist Cohort Event Monitoring (SCEM) design: strengths and limitations
| Limitations | PASS requirement affected | SCEM design features | Strengths | Distinguishing attributes for PASS |
|---|---|---|---|---|
| Selection bias from incomplete coverage, sampling process and non-response | Representativeness |
Open label in secondary care setting Minimal exclusion criteria |
Study off-label use Identify at-risk groups |
Unique system that systematically collects good-quality information at a national scale on hospital initiation, as well as early complications and cessation, of treatment Responsive design; study fieldwork activities maintain and enhance engagement of specialist HCPs |
| Depletion of susceptibles—loss of sub-groups of high risk patients during follow-up | New-user/inception cohort |
Targeted data collection to characterize the new-user study population Exposure data collected from dispensed prescription charts |
Mitigates confounding by disease severity and immortal time bias, which can result in underestimation of risk of early-onset events |
Indication for use reported from medical records and exposure from prescription chart; no assumptions made on surrogate markers Consent obtained to contact the GP for censored patients |
| Misclassification and underreporting | Safety outcomes | Use of well-defined case definitions based on acceptable agreed clinical standards | Expertise of the specialist provides more accurate information (through original medical records review) than that provided from hospital episode/ discharge statistics or free-text review of hospital letters sent to GPs | Can address specific regulatory questions in the context of the RMP for the product |
| Confounding and effect modification | Adjustment for measured confounders in statistical analysis |
Relevant data on prognostic and potential confounding factors can be collected as recorded in medical charts before treatment was started Rigorous data quality review of returned questionnaires |
Deeper level of granularity of relevant variables from secondary care medical records |
Improved quality of information on important confounding variables relevant to the targeted outcomes and population of the study GP records may suffer from significant proportions of missing data that may be relevant for the study but not of clinical relevance |
GP primary care physician, HCP health care professional, PASS post-authorization safety studies, RMP risk management plan
With regard to coverage and the sampling process, the ultimate desire is for wide national coverage with simple random sampling to ensure that every eligible patient has an equal chance of selection. However, there are circumstances when it is not feasible, practical or theoretically sensible to undertake simple random sampling in the post-authorization setting [18]. This is because of the requirements that such sampling imposes, such as the need to identify every single patient in advance, and cost. Nevertheless, the principle of random sampling of study patients can still apply because the SCEM approach relies on nested phases of sampling, where eligible patients are identified from the accessible population who present at random to participating specialist HCPs within the established national framework identified from the first phase.
Non-response and the levels of non-response have often been taken as an indicator of selection bias. This is because the underlying assumption is that the non-responses are correlated with the variables being measured (i.e. non-ignorable non-response). The mechanisms of non-response may be considered analogous to mechanisms of missing data [19]. Assessing the non-response mechanism in SCEM is a particular challenge because of the hierarchical data structure. Potential causes of non-response in the first phase, where specialist HCPs are asked to participate, may be at the institutional or specialist level, whilst causes of non-response in the second phase may be patient specific. In each phase, the response propensity is unknown in advance. Thus, the a priori beliefs are that responders (specialist HCPs or patients) are a random sample of the eligible population, and that study participation is not correlated with the variables of interest (such that the non-response error is ignorable) [20, 21].
Contribution of Specialist Cohort Event Monitoring to Pharmacovigilance: Lessons Learned
The SCEM registry design offers a new direction in the post-marketing surveillance of newly marketed drugs in the ‘real-world’ conditions of clinical practice. Through careful consideration of ongoing methodological enhancements in the field of pharmacoepidemiology, the SCEM design has evolved to address some of the limitations associated with conduct of PASS in the primary care setting.
A particular lesson learned from the implementation of SCEM, which is relevant to any PASS, is the need to understand how the widespread application of clinical guidelines (national/regional), pharmacoeconomic policies and policies for reimbursement determine treatment choices. These activities may introduce possible selection biases that are beyond the capabilities of existing pharmacoepidemiological methods to handle. It is important to note that in the UK, the choice of drugs that can be prescribed is guided by both clinical experience and recommendations from therapeutic committees and experts [22]. Initially, those accessible secondary care settings that respond within the SCEM research framework may only be those for which recommendations for prescribing the study drug have been adopted. This may result in a study population that is weighted initially in favour of those individuals who are more readily accessible, because specialist HCPs may respond only at sites where product adoption has occurred. Such knowledge can be utilized to ensure that an appropriate feature is built into the design. For example, for the ROSE study, a counterfactual comparator was unlikely to be identified, because of national treatment guidelines. Therefore, a contextual cohort was chosen as a referent group with which to investigate use of multi-level modelling of the extent to which variance in prescribing can be attributed to the patient, the prescriber or the health care setting [23].
A second lesson learned is to ensure that for a PASS that relies on contribution of patient data by experts, an appropriate strategy exists to cope with non-response and enhance response rates. In this regard, the SCEM design has been shown to be responsive. In the first phase, the goal of the collaborative fieldwork arrangement between CRNs and DSRU research staff has been to maximize initial contacts with specialist HCPs. Thereafter, the desire has been to achieve high rates of conversion to positive engagement within the study by specialist HCPs who initially refused to participate in the study.
A third lesson is the need for a PASS to be adaptive. The SCEM design offers a real-time framework that not only continuously monitors the adoption of the product and sources of variation in prescribing, but also monitors trust, specialist HCP and patient accrual. Responsive use of these real-time metrics can guide interim study decisions that may be necessary to enhance response.
Conclusions
SCEM studies combine the advantages of event monitoring (of general safety and identification of unexpected risks of a medicine) with that of a more targeted safety study that addresses specific questions (to better understand known or partially known risks with a medicine). A key strength of the SCEM is that through identification of cohorts of patients treated in secondary care, the design facilitates surveillance of a diverse patient population in hospital or under the care of specialists. By design, SCEM studies have the capacity to achieve a more comprehensive assessment of medical and other potential determinants of specific early-onset outcomes. The method enables the identification of comparator cohorts prescribed standard care or other medication concurrently. Thus, PASS using SCEM may also lend themselves to exploring related objectives that are equally as important in understanding risk, such as defining and exploring current care patterns, identifying barriers to initial use and adherence, and monitoring off-label use. These help inform on the value of the product within clinical practice. These PASS may extend beyond the immediate post-marketing period and national boundaries; therefore, a key to success is to establish and maintain relationships with key stakeholders, both nationally and internationally. The approach offers opportunities for a number of additional research applications, including areas of specific regulatory concerns and the possibility of extension into other EU countries, with modifications. Thus, SCEM should be considered a valuable tool when developing an RMP for the evaluation of the safety of a new medicine.
Funding and conflicts of interest
The Drug Safety Research Unit (DSRU) is an independent charity (No. 327206), which works in association with the University of Portsmouth. It receives unconditional donations from pharmaceutical companies. The companies have no control on the conduct or the publication of the studies conducted by the DSRU. The DSRU has received unconditional support from pharmaceutical manufacturers. The has DSRU retained its full academic independence in respect of the conduct of the SCEM registry and external communications of any results. Saad A. W. Shakir has received lecturing fees (and related travel expenses) from Lilly and has acted as an expert witness for an unrelated product manufactured by the company Sanofi. Deborah Layton has received money for development of an educational module on ADR reporting for the Royal Pharmaceutical Society and as a guest lecturer to undergraduate pharmacy students.
Footnotes
Evaluable patients exclude eligible consented patients who withdraw during the study or for whom insufficient clinical information is provided on the questionnaires. There is no means of evaluating events reported for patients who have withdrawn their consent.
References
- 1.European Medicines Agency (EMA). Guideline on good pharmacovigilance practices (GVP) module viii—post-authorisation safety studies (rev 1). EMA/813938/2011. London: EMA, 19 April 2013.
- 2.European Network of Centres of Excellence for Pharmacoepidemiology and Pharmacovigilance (ENCEPP). Guide on methodological standards in pharmacoepidemiology (revision 2). EMA/95098/2010. London: ENCEPP, 18 June 2013.
- 3.Layton D, Hazell L, Shakir SA. Modified prescription-event monitoring studies: a tool for pharmacovigilance and risk management. Drug Saf. 2011. [DOI] [PubMed]
- 4.Layton D, Shakir SAW. Prescription-event monitoring. In: Strom BL, Kimmel SE, Hennessy S, editors. Pharmacoepidemiology, 5th edn. Chichester, UK: John Wiley & Sons Ltd; 2011. p. 301–330.
- 5.Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16(3):241–249. doi: 10.1002/pds.1357. [DOI] [PubMed] [Google Scholar]
- 6.Royal College of Physicians of London. Guidelines on the practice of Ethical Committees in Medical Research involving Human Subjects, 4th edn. 2007. https://www.rcplondon.ac.uk/sites/default/files/documents/guidelines-practice-ethics-committees-medical-research.pdf. Accessed 30 Dec 2014. [PMC free article] [PubMed]
- 7.General Medical Council. Good practice in prescribing and managing medicines and devices. 2013. http://www.gmc-uk.org/Prescribing_Guidance__2013__50955425.pdf. Accessed 23 Dec 2013.
- 8.Council for International Organizations of Medical Sciences (CIOMS) and World Health Organization (WHO), Geneva. International Ethical Guidelines for Biomedical Research Involving Human Subjects. 2002. http://www.cioms.ch/publications/layout_guide2002.pdf. Accessed 10 Dec 2013. [PubMed]
- 9.British Medical Association Board of Science, British Medical Association Science & Education. Reporting adverse drug reactions. A Guide for healthcare professionals. London: British Medical Association Board of Science, British Medical Association Science & Education; 2006. http://bmaopac.hosted.exlibrisgroup.com/exlibris/aleph/a21_1/apache_media/GYVFNJ1RT2PFNDUJ8IUKPUNTFVRF8G.pdf. Accessed 10 Dec 2013.
- 10.Epstein M. Guidelines for good pharmacoepidemiology practices (GPP) Pharmacoepidemiol Drug Saf. 2005;14(8):589–595. doi: 10.1002/pds.1082. [DOI] [PubMed] [Google Scholar]
- 11.Department of Health. Eligibility criteria for NIHR Clinical Research Network support. London: Department of Health; 2013. http://www.crn.nihr.ac.uk/wp-content/uploads/About%20the%20CRN/Eligibility%20Criteria%20for%20NIHRCRN%20support.pdf. Accessed 17 Nov 2014.
- 12.European Network of Centres of Excellence for Pharmacoepidemiology and Pharmacovigilance (ENCEPP). E-register of studies. A cohort study to monitor the safety and use of prolonged-release quetiapine. 2014. http://www.encepp.eu/encepp/viewResource.htm?id=5412. Accessed 20 Jan 2014.
- 13.European Network of Centres of Excellence for Pharmacoepidemiology and Pharmacovigilance (ENCEPP). E-register of studies. An observational post-authorisation safety specialist cohort monitoring study (SCEM) to monitor the safety and utilisation of asenapine (Sycrest) in the mental health trust setting in England. 2014. http://www.encepp.eu/encepp/viewResource.htm?id=5323. Accessed 30 Dec 2014.
- 14.European Network of Centres of Excellence for Pharmacoepidemiology and Pharmacovigilance (ENCEPP). E-register of studies. An observational post-authorization safety specialist cohort event monitoring study (SCEM) to monitor the safety and utilization of rivaroxaban (Xarelto®) for the prevention of stroke in patients with AF, treatment of DVT and PE, and the prevention of recurrent DVT and PE in the secondary care hospital setting in England and Wales. 2014. http://www.encepp.eu/encepp/viewResource.htm?id=5320. Accessed 30 Dec 2014.
- 15.Establishing requirements for the use of terms for reporting adverse drug reactions (ADR). Consultant, Council for International Organizations of Medical Sciences (CIOMS), World Health Organization. Mater Med Pol 1993;25(1):45–6. [PubMed]
- 16.Farrington CP. Control without separate controls: evaluation of vaccine safety using case-only methods. Vaccine. 2004;22(15–16):2064–2070. doi: 10.1016/j.vaccine.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Groves R. Non response rates and non-response bias in household surveys. Public Opin Q Spec Issue. 2006;70(5):646–675. doi: 10.1093/poq/nfl033. [DOI] [Google Scholar]
- 18.Chapter 3. Registry Design. p. 53-72. In: Gliklich RE, Dreyer NA, editors. Registries for evaluating patient Ooutcomes: a user’s guide. 2nd edition (Prepared by Outcome DEcIDE Center [Outcome Sciences, Inc. d/b/a Outcome] under Contract No. HHSA29020050035I TO3.). AHRQ Publication No.10-EHC049. Rockville, MD: Agency for Healthcare Research and Quality; 2010. http://www.effectivehealthcare.ahrq.gov/ehc/products/74/531/Registries%202nd%20ed%20final%20to%20Eisenberg%209-15-10.pdf. Accessed 01 Dec 2013.
- 19.Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581–592. doi: 10.1093/biomet/63.3.581. [DOI] [Google Scholar]
- 20.Schouten B, Cobben F, Bethlehem J. Indicators for the representativeness of survey response. Survey Methodol. 2009;35(1):101–113. [Google Scholar]
- 21.Groves R, Peytcheva E. The impact of non-response rates on non-response bias: a meta-analysis. Public Opin Q. 2008;72(2):167–189. doi: 10.1093/poq/nfn011. [DOI] [Google Scholar]
- 22.National Institute of Health and Clinical Excellence. Medicines and prescribing support from NICE. 2014. http://www.nice.org.uk/about/nice-communities/medicines-and-prescribing. Accessed 9 April 2014.
- 23.Groenewegen PP, Leufkens HG, Spreeuwenberg P, Worm W. Neighbourhood characteristics and use of benzodiazepines in the Netherlands. Soc Sci Med. 1999;48(12):1701–1711. doi: 10.1016/S0277-9536(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 24.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]


