Abstract
Purpose
We retrospectively examined whether or not initial responses of first low-dose 131I-meta-iodo-benzyl-guanidine radiotherapy (131I-MIBG therapy) in patients with malignant pheochromocytoma and paraganglioma had prognostic values.
Materials and methods
This study included 26 patients with malignant pheochromocytoma (n = 18) and paraganglioma (n = 8) who underwent the first 131I-MIBG therapy between October 2001 and September 2007. Based on the initial subjective, hormonal, scintigraphic, and objective responses to 131I-MIBG therapy, the responses were divided into progression disease (PD) and non-PD. We examined the following factors for prognostic significance: sex, age, disease, initial diagnosis (benign or malignant pheochromocytoma), hypertension, diabetes mellitus, palpitations, symptoms related to bone metastases, and number of low-dose 131I-MIBG therapy. Univariate Cox proportional regression analysis was used to identify prognostic factors for overall survival. Overall survival was analyzed by Kaplan–Meier method and the curves were compared using the log-rank test.
Results
The median survival time was 56 months. In the follow-up period, 16 patients died from exacerbation of their diseases. Univariate analysis showed that the hormonal PD [hazard ratio (HR) 3.20, P = 0.034, confidence interval (CI) 1.09–9.93], objective PD (HR 11.89, P = 0.0068, CI 2.14–65.85), single-time 131I-MIBG therapy (HR 3.22, P = 0.020, CI 1.21–8.79), hypertension (HR 2.93, P = 0.044, CI 1.02–10.50), and symptoms related to bone metastases (HR 3.54, P = 0.023, CI 1.18–13.04) were bad prognostic factors for overall survival. Kaplan–Meier analysis demonstrated that the hormonal non-PD (P = 0.026), objective non-PD (P = 0.0002), multiple-time 131I-MIBG therapy (P = 0.013), and no symptom related to bone metastases (P = 0.024) were significantly associated with good prognosis. Overall survival rate was 70 and 50 % at 5 years from the initial diagnosis and from the first 131I-MIBG therapy, respectively.
Conclusion
The hormonal and objective responses to the first low-dose 131I-MIBG therapy as well as complication of hypertension and symptoms related to bone metastases may be prognostic factors in patients with malignant pheochromocytoma and paraganglioma.
Keywords: Malignant pheochromocytoma, Malignant paraganglioma, Low-dose of 131I-MIBG, Initial response, Radiotherapy
Introduction
Radioisotope 131I-meta-iodo-benzyl-guanidine radiotherapy (131I-MIBG therapy) has been widely used for patients with malignant pheochromocytoma and paraganglioma to cure or control inoperable tumors since the 1980s [1]. The treatment protocols can be categorized into three types based on the dose: low-dose (2.96–7.4 GBq/person/session), intermediate-dose (up to 18.5 GBq/person/session), and high-dose therapy (0.44–0.67 GBq/kg/session) [2]. For the high-dose 131I-MIBG therapy, the risk-adapted side effect of bone marrow suppression is usually rescued by autologous stem cell rescue.
In high-dose therapy, relatively good therapeutic effect has been reported in patients with malignant pheochromocytoma and paraganglioma. Gonias et al. [3] reported that the overall complete remissions (CR) plus partial response (PR) rate was 22 % in 49 patients. In 30 patients, Fitzgerald et al. [4] reported that 4 sustained CR, 15 sustained PR, 1 sustained stable disease (SD), 5 progressive disease (PD), and 5 initial PR or SD but relapsed to PD. In contrast, the low-dose 131I-MIBG therapy rarely resulted in CR [5, 6].
The laws concerning the prevention from radiation hazards due to radioisotopes and others restrict the maximum dose of radioisotope in Japan. The high-dose 131I-MIBG therapy for adult patients is not permitted in Japan. Therefore, the repeated low-dose therapy has been adopted in our hospital. In clinical practices, we perform the low-dose 131I-MIBG therapy aiming at symptom palliation, tumor arrest, and/or tumor reduction in patients with advanced malignant pheochromocytoma and paraganglioma. We attempt to lead patients clinically into “stabilization of disease” [7]. Ultimately, we aspire to total eradication of tumor not only by repeated low-dose 131I-MIBG therapy but also by combination of the therapy and surgery for the patients with less advanced cases.
The low-dose 131I-MIBG therapy requires multiple courses, but there was no consensus as to how many times it should be repeated nor as to as how and when it should be assessed. Loh et al. [5] reviewed that the responders to first low-dose 131I-MIBG therapy had a longer mean period of survival time in 116 patients at 24 centers in 10 countries. Buscombe et al. [8] showed that patients with PD at 6 months after the last low-dose 131I-MIBG therapy had poor prognosis compared to those with stability and response in patients with neuroendocrine tumors, which mainly consisted of carcinoids. However, no study has shown whether or not initial responses to the first low-dose 131I-MIBG therapy had prognostic value during follow-up exceeding 5 years in patients with malignant pheochromocytoma and paraganglioma in a single institute.
In the present study, we examined (up to 9 years of) long-term follow-up data to determine whether or not the responses to first low-dose 131I-MIBG therapy had prognostic values in patients with malignant pheochromocytoma and paraganglioma.
Materials and methods
Patients
The present study consisted of 27 patients with malignant pheochromocytoma and paraganglioma who were referred for low-dose 131I-MIBG therapy without autologous stem cell rescue in our hospital by their primary physicians between October 2001 and September 2007. We analyzed 26 patients, because 1 patient was lost during follow-up soon after 131I-MIBG therapy.
The extent of metastatic disease was assessed by computed tomography (CT) or magnetic resonance imaging (MRI), and 123I-MIBG diagnostic scintigraphy. Malignant status was determined by the presence of metastatic disease. For all patients, 123I-MIBG tracer study was examined to confirm the uptakes of 123I-MIBG to metastatic lesions before 131I-MIBG therapy. Sites of metastases were classified as follows: lung, bone, liver, local site, and others. Local site included invasion into neighboring tissue, recurrence at the site where the primary tumor had been excised, dissemination into the abdominal cavity, and regional lymph nodes. The 24-h sampling of urine catecholamine and their metabolites was assessed by measuring each concentration of adrenaline (AD), noradrenaline (NA), dopamine (DO), homovanillic acid (HVA), vanillylmandelic acid (VMA), metanephrine (MN), and normetanephrine (NMN). Normal ranges were 1–23 μg/day for AD, 29–120 μg/day for NA, 100–1,000 μg/day for DO, 1.6–5.5 mg/day for HVA, 1.4–4.9 mg/day for VMA, 0.05–0.20 mg/day for MN, and 0.10–0.28 mg/day for NMN. Our study defined hypertension as use of antihypertensive drugs, diabetes mellitus as use of anti-diabetic medication, and symptoms of bone metastases as pain and/or numbness related to bone metastases from medical records.
The thyroid gland was blocked by daily oral administration of 250 mg potassium iodide during 2 weeks, starting on the day before infusion of 131I-MIBG. 131I-MIBG with a high specific activity was obtained from a commercial source (Daiichi Radioisotopes, Tokyo, Japan, from October 2001 to June 2005 and Izotope, Budapest, Hungary, from July 2005 to September 2007). A fixed dose of 3.7 or 7.4 GBq of 131I-MIBG was administered intravenously at the first 131I-MIBG therapy. Blood pressure was monitored hourly until bedtime. Electrocardiogram was monitored for 3 days after the infusion. Hydration was maintained during and following treatment to facilitate the reduction of radiation exposure to the bladder. Patients stayed in the isolation room until the radiation dose rate decreased to less than 30 μSv per hour at 1 m on the government regulation.
After 3–6 months of 131I-MIBG therapy, 24-h sampling of urine catecholamine was performed. 123I-MIBG scintigraphy and objective measurement (CT and/or MRI) were performed to compare to their previous images. Evaluation of initial response and determination of the subsequent treatment strategy were done based on the 123I-MIBG scintigraphy, objective measurement of tumors, catecholamine excretions, symptoms, and complicating diseases.
131I-MIBG therapy was approved by the Institutional Review Board. This study was a retrospective observational study, and the clinical data were obtained in routine examinations.
Evaluation methods
Hormonal response was classified as CR in complete normalization of catecholamine levels, PR in more than 50 % decrease but failure to achieve normal levels, SD in less than 50 % decrease and less than 25 % increase, and PD in more than 25 % increase [5].
Subjective response of symptoms and/or complicating diseases was classified as follows: CR, complete disappearance of the symptoms and/or no need for medication; PR, improvement of the symptoms and/or reduced need for medications; SD, no change; and PD, exacerbation of the symptoms and/or increased need for medications. Their attending doctors adjusted medication dosage for hypertension, diabetes mellitus, palpitations, and symptoms of bone metastases.
123I-MIBG scintigraphy was assessed by 2 board-certified nuclear medicine physicians who were blinded to clinical data as follows: CR, disappearance of all lesions; PR, a decrease in the number and/or intensity of the lesions; SD, no discernible change; and PD, the appearance of a new lesion. The 123I-MIBG whole body scan images were acquired using a dual-head gamma camera equipped with low-middle-energy general-purpose collimators (e.cam signature, Siemens Medical Solutions, from October 2001 to April 2007 and SymbiaT6, Siemens Medical Solutions, from May 2007 to September 2007). In the whole body scan, both anterior and posterior images were acquired at a speed of 15 cm/min and a 159-keV photopeak with 15 % window.
Objective response was assessed based on the findings of the CT/MRI. One-dimensional percent changes were taken into consideration to define the response to treatment according to the following Response Evaluation Criteria in Solid Tumors guideline (version 1.1): CR is disappearance of all target lesions; PR is at least a 30 % decrease in the sum of diameters of target lesions; PD is at least a 20 % increase in the sum of diameters of targeted lesion and the sum must also demonstrate an absolute increase at of least 5 mm; and SD is neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD [9].
Evaluation of bone marrow status after the low-dose 131I-MIBG therapy was graded with Common Terminology Criteria for Adverse Events version 4.0 (CTCAE). Anemia was classified as follows (hemoglobin range): grade 0, within normal limits; grade 1, <11.2 to 10.0 g/dL; grade2, <10.0 to 8.0 g/dL, grade 3, <8.0 g/dL; transfusion indicated: grade 4, life-threatening consequences, urgent intervention indicated; and grade 5, death. White blood cell decrease was classified as follows (white blood cell range): grade 0, within normal limits; grade 1, <3,300 to 3,000/mm3; grade2, <3,000 to 2,000/mm3; grade 3, <2,000 to 1,000/mm3; and grade 4, <1,000/mm3. Platelet cell decrease was classified as follows (platelet cell range): grade 0, within normal limits; grade 1, <163,000 to 75,000/mm3; grade2, <75,000 to 50,000/mm3; grade 3, <50,000 to 25,000/mm3; and grade 4, <25,000/mm3.
Statistical analysis
For statistical analysis, we used a statistical software package (JMP® SAS Institute Inc., Cary, NC, USA.).
Univariate Cox proportional hazards regression analyses were used to identify prognostic factors for overall survival from the first 131I-MIBG therapy. Overall survival was analyzed by the Kaplan–Meier method to clarify the time-dependent cumulative survival rate, and the curves were compared using the 2-sample log-rank test. P < 0.05 was considered statistically significant. Their survival periods were measured from two different time-zero points: the time of initial diagnosis of malignancy and first 131I-MIBG therapy. The last follow-up was December 2011.
We examined the following factors for prognostic significance: sex, age, disease, initial diagnosis (benign or malignant pheochromocytoma), hypertension, diabetes mellitus, palpitations, symptoms of bone metastases, number of low-dose 131I-MIBG therapy, and initial responses (hormonal, subjective, scintigraphic, and objective responses).
Chi-squared test was applied to identify the difference of the frequency of bone marrow suppressions (anemia, white blood cell decrease, and platelet count decrease) between the patients with and without chemotherapy before 131I-MIBG therapy.
Results
Patient characteristics are given in Table 1. Seven patients had undergone excision of their primary tumors or partial resection of metastasis including emergency decompression operation after diagnosis of malignant pheochromocytoma or paraganglioma. The median survival time from the first 131I-MIBG therapy was 56 months (range 8–117 months) for the entire population. During follow-up time, 16 patients died from exacerbation of their diseases.
Table 1.
Patient characteristics
| Parameters | |
|---|---|
| Age (median, range) | 54, 19–78 years |
| Sex (male/female) | 12/14 |
| Malignant pheochromocytoma | 18 |
| Malignant paraganglioma | 8 |
| Time to 131I-MIBG therapy from initial diagnosis (median, range) | 22, 2–108 months |
| Before 131I-MIBG therapy (number) | |
| Chemotherapy | 11 |
| External radiation | 3 |
| Chemotherapy and external radiation | 2 |
| Chemotherapy, radiation, and radiofrequency ablation | 1 |
The median time from diagnosis of metastases to the therapy with 131I-MIBG was 22 months (range 2–108 months). At the initial visit, 20 patients had metastases at 2 or more organ sites and 6 patients had a single metastatic site. Bone was the most common site of the metastases (22 out of 26 patients). As for symptoms and/or complicating diseases, 21 patients were included at the initial visit. There were symptoms of bone metastases (12 out of 21 patients), hypertension (16 out of 21 patients), palpitations (9 out of 21 patients) and diabetes mellitus (6 out of 21 patients).
The median dose of the first 131I-MIBG therapy was 7.4 GBq (range 70–215 MBq/kg). Through the recorded review, initial subjective response was observed in 11/21 patients (52 %). There were 11 PR: 9 patients had improvement of symptoms of bone metastases, 2 patients had improvement of palpitations, and medication for hypertension was reduced in 2 patients. In initial scintigraphic response, 21 patients were judged as non-PD (3 PR, 18 SD) (84 %) and 4 as PD (16 %), respectively. In initial hormonal response, 15 patients showed non-PD (9 PR and 6 SD) (60 %) and 10 patients showed PD (40 %), respectively. One patient did not undergo either initial 123I-MIBG scintigraphy or 24-h urinary examination in our hospital. In initial objective response, 17 patients were judged as non-PD (17SD) (85 %) and 3 patients as PD (15 %). One patient did not have evaluable tumor with RECIST. As for the other 5 patients, we could not refer their CT/MRI. Figures 1 and 2 show representative cases of objective responses.
Fig. 1.
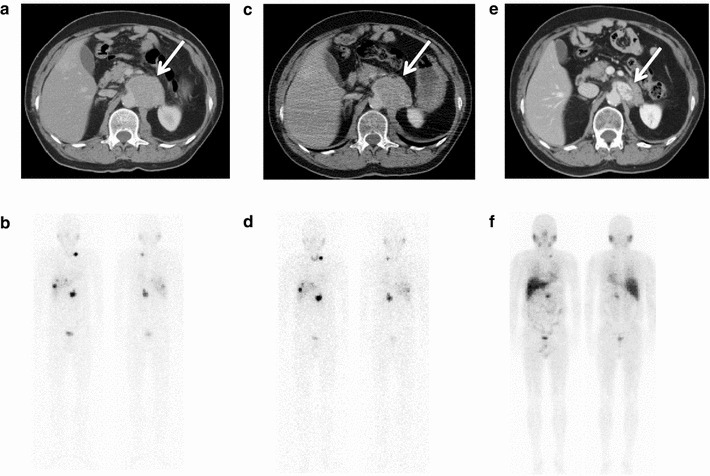
A 45-year-old patient with malignant pheochromocytoma had abdominal mass adjacent to aorta (white arrow): a, b before and c, d after first 131I-MIBG therapy (7.4 GBq). The objective and scintigraphic responses were not observed after the first 131I-MIBG therapy (non-PD). e, f Repeated 131I-MIBG therapy (total 66.0 GBq) decreased the size of mass (white arrow) and uptake of metastases. He is still alive 74 months after first 131I-MIBG therapy without progression of disease. The scintigraphy was obtained at 24 h after injection of 123I-MIBG [b (111 MBq), d (111 MBq), and f (222 MBq)]
Fig. 2.
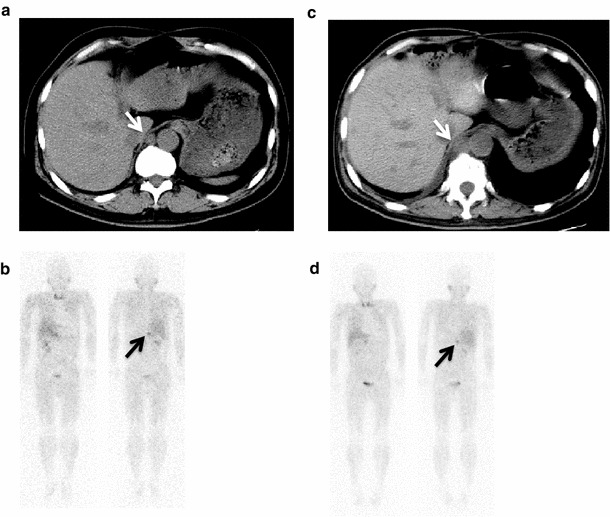
A 52-year-old patient with malignant pheochromocytoma had metastases of retrocrural nodules (white arrow), lung, liver, and bone: a, b before and c, d after first 131I-MIBG therapy (7.4 GBq). The retrocrural nodules (white arrow) were progressed after first 131I-MIBG therapy. The scintigraphic response was not observed after the first 131I-MIBG therapy (black arrow). He died 8 months after first 131I-MIBG therapy with progression of disease unfortunately. The scintigraphy was obtained at 24 h after injection of 123I-MIBG [b (111 MBq) and d (111 MBq)]
After the first 131I-MIBG therapy, 16 patients repeated low-dose 131I-MIBG therapy (median treatment times 2, range 1–6). Additional therapies were carried out in 7 patients: surgical decompression (n = 1), chemotherapy (n = 2), external radiation (n = 5), and radiofrequency ablation (n = 1).
Table 2 shows results of univariate analysis. The analysis showed that the hormonal PD [hazard ratio (HR) 3.20, P = 0.034, confidence interval (CI) 1.09–9.93], objective PD (HR 11.89, P = 0.0068, CI 2.14–65.85), single-time 131I-MIBG therapy (HR 3.22, P = 0.020, CI 1.21–8.79), hypertension (HR 2.93, P = 0.044, CI 1.02–10.50), and symptoms related to bone metastases (HR 3.54, P = 0.023, CI 1.18–13.04) were bad prognostic factors for overall survival. Other variables were not significant prognostic factors.
Table 2.
Overall survival estimated by univariate analysis
| Variable | HR (CI) | P value |
|---|---|---|
| Sex (M/F) | 1.10 (0.41–1.31) | ns |
| Age (over 50 years) | 2.09 (0.75–6.75) | ns |
| Disease (paraganglioma/pheochromocytoma) | 0.92 (0.29–2.56) | ns |
| Initial diagnosis (benign/malignant pheochromocytoma) | 0.53 (0.15–2.50) | ns |
| Hypertension | 2.93 (1.02–10.50) | 0.044 |
| Diabetes mellitus | 1.87 (0.52–5.41) | ns |
| Palpitations | 0.94 (0.29–2.62) | ns |
| Symptoms of bone metastases | 3.54 (1.18–13.04) | 0.023 |
| Number of 131I-MIBG therapy (single/multiple 131I-MIBG therapy) |
3.22 (1.21–8.79) | 0.020 |
| Initial responses | ||
| Hormonal response (PD/non-PD) | 3.20 (1.09–9.93) | 0.034 |
| Subjective response (PD/non-PD) | 2.31 (0.82–7.06) | ns |
| Scintigraphic response (PD/non-PD) | 2.28 (0.51–7.33) | ns |
| Objective response (PD/non-PD) | 11.89 (2.14–65.85) | 0.0068 |
HR hazard ratio, CI 95 % confidence interval, ns not significant
Figure 3 shows Kaplan–Meier curves. Kaplan–Meier analysis demonstrated that the hormonal non-PD (P = 0.026), objective non-PD (P = 0.0002), and multiple-time 131I-MIBG therapy (P = 0.013) were significantly associated with good prognosis. No symptom related to bone metastases (P = 0.024) was significantly associated with good prognosis.
Fig. 3.
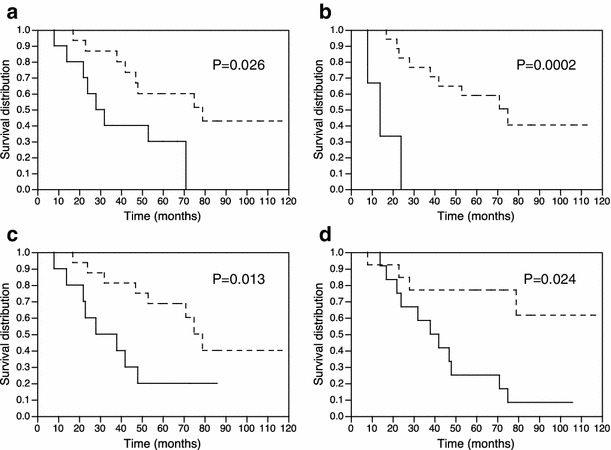
Kaplan–Meier curves of overall survival after first 131I-MIBG therapy: a hormonal non-PD group (dashed line) (P = 0.026), b objective non-PD (dashed line) (P = 0.0002), c multiple-time 131I-MIBG therapy (dashed line) (P = 0.013), and d no symptom related to bone metastases (dashed line) (P = 0.024) are associated with good prognosis
Overall survival rate was 70 and 50 % at 5 years from initial diagnosis of malignancy and from the first 131I-MIBG therapy, respectively.
Table 3 represents the numbers of patients with bone marrow suppressions through the first low-dose 131I-MIBG therapy in terms of anemia (11/25), white blood cell decrease (13/25), and platelet count decrease (7/25) according to the CTCAE grading. The bone marrow suppressions through the first low-dose 131I-MIBG therapy did not correlate with chemotherapy before 131I-MIBG therapy.
Table 3.
Change of patients’ bone marrow suppression through the first 131I-MIBG therapy
| Grade change (before→after 131I-MIBG therapy) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0→0 | 0→1 | 0→2 | 0→3 | 1→1 | 1→2 | 2→2 | 0→No data | |
| Anemia | 14 (6) | 4 (3) | 0 | 1 | 3 (3) | 1 (1) | 2 (1) | 1 |
| White blood cell decreased | 12 (6) | 2 | 4 (4) | 6 (4) | 1 | 0 | 0 | 1 |
| Platelet count decreased | 18 (8) | 4 (4) | 2 (1) | 1 (1) | 0 | 0 | 0 | 1 |
The number of patients with chemotherapy before 131I-MIBG therapy is given in parentheses
Discussion
131I-MIBG therapy has been used mainly in patients with disseminated and unresectable malignant pheochromocytoma and paraganglioma [10]. In 26 such patients the current study revealed that the hormonal PD, objective PD, single-time 131I-MIBG therapy, hypertension, and symptoms related to bone metastases were bad prognostic factors for overall survival.
A representative review of 116 patients with malignant pheochromocytoma and paraganglioma from 24 centers in 10 countries evaluated the effect of the repeated low-dose 131I-MIBG therapy. Their individual dose ranged from 3.7 to 7.4 GBq and cumulative dose of 3.6–85.9 GBq. Initial symptomatic response was observed in 76 %, tumor response in 30 %, and hormonal response in 45 % of the patients. The responders had a longer mean period of survival of 23.2 ± 8.1 months (median 22 months) compared to the non-responders with 14.3 ± 8.3 months (median 13 months) [5]. In our study, the hormonal and objective non-PD were significantly associated with good prognosis. Symptom responses were observed in half of the patients, but those were not predictive for prognosis.
Although SD was included in non-responders in several previous studies, we considered SD as non-PD. Buscombe et al. [8] considered PD as a poor prognostic sign of progression-free and overall survival compared with SD, PR, and CR in patients with neuroendocrine tumor at 6 months after the last low-dose 131I-MIBG therapy. We focused on the PD of the first 131I-MIBG therapy in this study, because PD within 6 months of the therapy was likely to have a bad prognosis in clinical practice. In our study, there were 10 in hormonal PD (40 %) and 3 in objective PD (15 %), respectively. Therefore, we recommend that hormonal and objective response to first 131I-MIBG therapy be assessed closely as a routine follow-up, because hormonal and objective PD are prognostic factors of poor outcome.
Relevant to this finding, hormonal and objective PD patients with no response to first 131I-MIBG therapy should be given chemotherapy. Only a small prospective study has performed 131I-MIBG therapy combined with CVD (cyclophosphamide, vincristine, and dacarbazine) chemotherapy on patients with malignant pheochromocytoma. They were treated with three times low- to intermediate-dose 131I-MIBG therapy followed by a year of chemotherapy. Of 6 patients, 2 patients who completed their protocols were considered to have partial but not complete responses. Both patients exhibited further reduction of the abnormal uptake on 131I-MIBG scintigraphy after the chemotherapy [11]. For the CVD chemotherapy, Huang, et al. reported that it produced a complete response rate of 11 % and a partial response rate of 44 % in 18 patients with advanced malignant pheochromocytoma and paraganglioma. Median survival from starting the therapy was 3.8 years for patients whose tumors responded to the therapy and 1.8 years for patients whose tumors did not respond (not significant) [12]. Nomura et al. [13] reported that the CVD chemotherapy was not shown to extend survival in 25 patients with malignant pheochromocytoma and paraganglioma. The interaction between 131I-MIBG therapy and CVD chemotherapy is unknown, but they certainly warrant further study of the effects of the combination therapy in patients with malignant pheochromocytoma and paraganglioma.
Malignant pheochromocytoma and paraganglioma sometimes progress rapidly within a few months or years after initial diagnosis. In contrast, some patients can live more than 10 years in a status of stabilization [14]. In our study, 5-year survival rate was 70 % after initial diagnosis of malignancy and 50 % after the first 131I-MIBG therapy, respectively. Additionally, median time from diagnosis of metastases to the first 131I-MIBG therapy was 22 months (range 2–108 months). Six patients took more than 5 years before the first 131I-MIBG therapy. The longer the interval from diagnosis to 131I-MIBG therapy, the more activated tumor function would be a concern. We should start the first MIBG therapy as early as possible, because hypertension and symptoms related to bone metastases were bad prognostic factors of overall survival. The repeated low-dose 131I-MIBG therapy can extend overall survival in selected patients.
There were some limitations in the current study. The study was a retrospective study with a small population. Objective responses could not be evaluated in 6 out of 26 patients. The criteria for reducing medications were not standardized. To resolve these problems, prospective studies are needed to confirm these points.
Conclusion
The hormonal and objective responses to the first low-dose 131I-MIBG therapy may be prognostic factors of overall survival in patients with malignant pheochromocytoma and paraganglioma. Complication of hypertension and symptoms related to bone metastases were bad prognostic factors of overall survival; therefore, early induction of low-dose 131I-MIBG therapy should be considered.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Sisson JC, Shapiro B, Beierwaltes WH, Glowniak JV, Nakajo M, Mangner TJ, et al. Radiopharmaceutical treatment of malignant pheochromocytoma. J Nucl Med. 1984;25:197–206. [PubMed] [Google Scholar]
- 2.Carrasquillo JA, Pandit-Taskar N, Chen CC. Radionuclide therapy of adrenal tumors. J Surg Oncol. 2012;106:632–642. doi: 10.1002/jso.23196. [DOI] [PubMed] [Google Scholar]
- 3.Gonias S, Goldsby R, Matthay KK, Hawkins R, Price D, Huberty J, et al. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J Clin Oncol. 2009;27:4162–4168. doi: 10.1200/JCO.2008.21.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald PA, Goldsby RE, Huberty JP, Price DC, Hawkins RA, Veatch JJ, et al. Malignant pheochromocytomas and paragangliomas a phase II study of therapy with high-dose 131I-metaiodobenzylguanidine (131I-MIBG) Ann N Y Acad Sci. 2006;1073:465–490. doi: 10.1196/annals.1353.050. [DOI] [PubMed] [Google Scholar]
- 5.Loh KC, Fitzgerald PA, Matthay KK, Yeo PP, Price DC. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients. J Endocrinol Invest. 1997;20:648–658. doi: 10.1007/BF03348026. [DOI] [PubMed] [Google Scholar]
- 6.Shilkrut M, Bar-Deroma R, Bar-Sela G, Berniger A, Kuten A. Low-dose iodine-131 metaiodobenzylguanidine therapy for patients with malignant pheochromocytoma and paraganglioma: single center experience. Am J Clin Oncol. 2010;33:79–82. doi: 10.1097/COC.0b013e31819e2c28. [DOI] [PubMed] [Google Scholar]
- 7.Castellani MR, Seghezzi S, Chiesa C, Aliberti GL, Maccauro M, Seregni E, et al. (131)I-MIBG treatment of pheochromocytoma: low versus intermediate activity regimens of therapy. Q J Nucl Med Mol Imaging. 2010;54:100–113. [PubMed] [Google Scholar]
- 8.Buscombe JR, Cwikla JB, Caplin ME, Hilson AJ. Long-term efficacy of low activity meta-[131I]iodobenzylguanidine therapy in patients with disseminated neuroendocrine tumours depends on initial response. Nucl Med Commun. 2005;26:969–976. doi: 10.1097/01.mnm.0000184941.06123.b9. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Kopf D, Goretzki PE, Lehnert H. Clinical management of malignant adrenal tumors. J Cancer Res Clin Oncol. 2001;127:143–155. doi: 10.1007/s004320000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sisson JC, Shapiro B, Shulkin BL, Urba S, Zempel S, Spaulding S. Treatment of malignant pheochromocytomas with 131-I metaiodobenzylguanidine and chemotherapy. Am J Clin Oncol. 1999;22:364–370. doi: 10.1097/00000421-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Abraham J, Hung E, Averbuch S, Merino M, Steinberg SM, et al. Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine recommendation from a 22-year follow-up of 18 patients. Cancer. 2008;113:2020–2028. doi: 10.1002/cncr.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nomura K, Kimura H, Shimizu S, Kodama H, Okamoto T, Obara T, et al. Survival of patients with metastatic malignant pheochromocytoma and efficacy of combined cyclophosphamide, vincristine, and dacarbazine chemotherapy. J Clin Endocrinol Metab. 2009;94:2850–2856. doi: 10.1210/jc.2008-2697. [DOI] [PubMed] [Google Scholar]
- 14.Sisson JC, Shulkin BL, Esfandiari NH. Courses of malignant pheochromocytoma implications for therapy. Ann N Y Acad Sci. 2006;1073:505–511. doi: 10.1196/annals.1353.053. [DOI] [PubMed] [Google Scholar]


