Abstract
Invasive candidiasis is a leading infectious cause of morbidity and mortality in premature infants. Improved recognition of modifiable risk factors and antifungal prophylaxis have contributed to the recent decline in the incidence of this infection among infants. Invasive candidiasis typically occurs in the first six weeks of life and presents with non-specific signs of sepsis. Definitive diagnosis relies on growth of Candida in blood culture or cultures from other normally sterile sites, but this may identify fewer than half of cases. Improved diagnostics are needed to guide initiation of antifungal therapy in premature infants.
Keywords: neonatal candidiasis, Candida, premature infants, risk factors
Background
Invasive candidiasis is a leading infectious cause of morbidity and mortality in extremely premature infants. It affects 4–8% of extremely low birth weight (ELBW; birth weight <1000 g) infants, and is associated with 30% mortality.1–8 Infants with invasive candidiasis who survive frequently have long-term neurological impairment including cerebral palsy, blindness, hearing impairment, cognitive deficits, and periventricular leukomalacia.2, 5, 9–11
The incidence of neonatal candidiasis rose rapidly in the 1980’s and 1990’s with the improved survival of ELBW infants and the increased use of central venous catheters.12 However, this trend has reversed, with the incidence of invasive candidiasis among premature infants declining substantially over the past 15 years.13–15 In one study that included data from 322 neonatal intensive care units (NICUs), the incidence of invasive candidiasis decreased from 3.6 episodes per 1000 infants in 1997 to 1.4 episodes per 1000 infants in 2010.15 Fluconazole prophylaxis, reduced use of broad-spectrum antibacterial antibiotics, empirical antifungal therapy, and improved care of central venous catheters have contributed to the declining incidence of invasive candidiasis.13, 15
Pathogenesis
Candida species are yeast that frequently colonize skin, the gastrointestinal (GI) tract, and the female genitourinary tract.16 Infants admitted to the NICU are colonized by Candida rapidly after birth, with the GI and respiratory tracts being the most frequent sites during the first two weeks of life.17–21 Colonization during this age period may be related to the birthing process; infants delivered vaginally have higher rates of colonization than infants born by Caesarean section and the colonizing Candida species are identical to those isolated from the maternal genitourinary tract in the majority of cases.17, 20–22 Colonization of infants >2 weeks of age frequently occurs on the skin and may be related to contact with maternal skin or the hands of health care providers.20 In particular, health care workers may be the primary source of Candida parapsilosis colonization in the NICU environment.22, 23
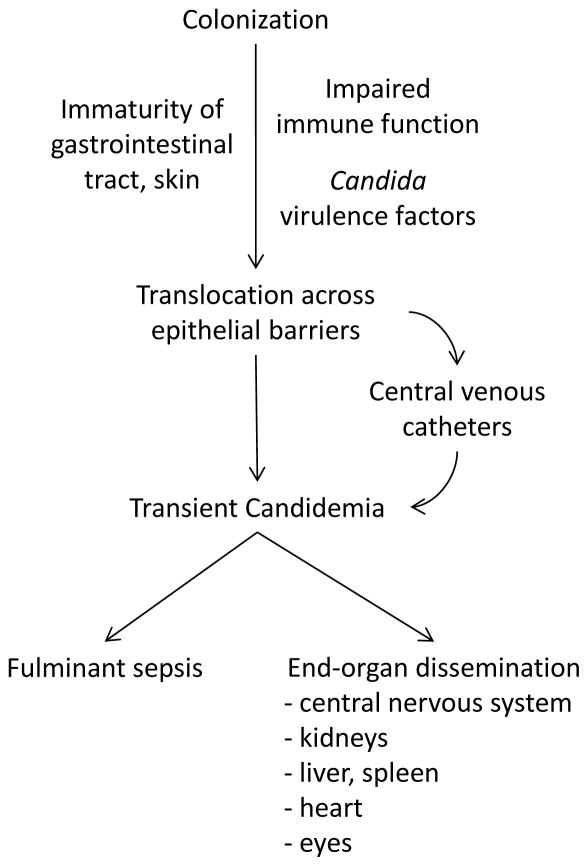
Colonization of infants by Candida species is not sufficient for the development of invasive candidiasis (Figure 1), although up to 5–10% of very low birth weight (VLBW; birth weight <1500 g) infants colonized by Candida develop invasive disease.18, 20, 24, 25 Premature infants are predisposed to invasive candidiasis for several reasons. First, the typical barriers to invasion by Candida species are not fully developed in premature infants. The epidermis of the infant born at <30 weeks gestational age is thin and poorly formed compared with the skin of term infants.26 Moreover, immaturity of the barrier and immune functions of the GI tract predispose to translocation by Candida.27 Cellular immunity is also impaired; premature infants have fewer neutrophils and T lymphocytes than term infants, and both groups have altered neutrophil chemotaxis and phagocytosis compared with older children and adults.28–30 Finally, virulence factors of the colonizing yeast isolate also appear to be important in determining risk of progression to invasive disease. Bliss et al. observed enhanced virulence characteristics among more than half of Candida isolates from infants with invasive candidiasis.31
Figure 1.
Pathophysiology of invasive candidiasis in premature neonates.
Once Candida species have invaded across mucosal surfaces or entered the bloodstream, they have a predilection for tissue invasion in the central nervous system, kidneys, liver, spleen, heart, and retina. Within the central nervous system, Candida can cause a meningoencephalitits, cerebral abscesses, and ventriculitis with obstructive hydrocephalus.32, 33 Candida can also infiltrate with or without abscess formation in the liver, spleen, and (most commonly) the kidneys.32, 34 Finally, endocarditis and endogenous endophthalmitis may result from seeding of the heart valves or eyes during fungemia.
Risk Factors
Neonatal candidiasis generally occurs after the first two weeks of life in the setting of extreme prematurity or among infants of any gestational age with GI pathology.35 Over the past decade, investigators have identified risk factors for invasive candidiasis in several large cohorts of infants.
Prematurity & Birth Weight
Extreme prematurity is the strongest risk factor for the development of invasive candidiasis.2, 4, 15 The incidence of invasive candidiasis is low (0.06%) among infants admitted to the NICU with birth weight >1500 g.36 In comparison, invasive candidiasis develops in 2–5% of VLBW infants, while 4–16% of ELBW infants have historically been affected.2, 6, 15, 37–39 The incidence of invasive candidiasis is inversely related to birth weight even among ELBW infants, with infants born at <750 g being at least twice as likely to develop invasive candidiasis as infants with birth weights between 751 and 1000 g.2, 15 Mortality from invasive candidiasis is also inversely related to birth weight, approaching 50% for infants <750 g.10
NICU site
NICU site is also strongly related to risk of invasive candidiasis.8 In a cohort of ELBW infants admitted to 12 NICUs, the incidence of invasive candidiasis ranged from 2.4% to 20.4%.2 Empirical use of third-generation cephalosporins correlated with the center-specific incidence observed in this study.2 Use of antifungal prophylaxis might also contribute to the differing incidence by center. However, substantial variation in the incidence of invasive candidiasis was still observed among infants receiving placebo in several recent trials of antifungal prophylaxis (Table 1).24, 25, 40–42
Table 1.
Variation in the cumulative incidence of definite invasive candidiasis among placebo recipients in recent clinical trials of antifungal prophylaxis among premature infants.
| Study | Year | Patient Population (Birth Weight, Age) | Patients (N) | Cumulative Incidence of Invasive Candidiasis | Patients, Birth Weight <750 g (n) | Cumulative Incidence of Invasive Candidiasis, Birth Weight <750 g |
|---|---|---|---|---|---|---|
| Kicklighter et al.24 | 2001 | <1500 g, 0–28 days | N=50 | 0% | n=10 | 0% |
| Kaufman et al.25 | 2001 | <1000 g, 0–42 days | N=50 | 20% | n=24 | 25% |
| Manzoni et al.45 | 2007 | 1000–1500 g, 0–30 days <1000 g, 0–45 days |
N=106 | 13% | n=18 | 17% |
| Parikh et al.46 | 2007 | <1500 g, 0–28 days | N=60 | 25% | n=0 | - |
| Benjamin et al.44 | 2014 | <750 g, 0–42 days | N=173 | 7% | n=173 | 7% |
Broad-spectrum antibiotics
The strongest modifiable risk factor is antibiotic exposure and, more importantly, the choice of antibiotics for routine empiric therapy. Antibacterial therapy increases the density of Candida colonization by reducing the competitive pressure exerted by commensal bacteria, and receipt of broad-spectrum antibacterial antibiotics (e.g., third-generation cephalosporins) is among the most consistently identified risk factors for neonatal candidiasis.2, 4, 8, 17, 23, 37, 43 Studies suggest that exposure to third-generation cephalosporins is associated with an approximate doubling of the risk of invasive candidiasis among ELBW infants.2, 38 Carbapenems are likely to be increasingly used in NICUs with the emergence of multi-drug resistant Gram-negative bacteria.44 In one study of VLBW infants, receipt of a carbapenem or third-generation cephalosporin in the prior seven days was associated with invasive candidiasis, although no studies have assessed risk specifically associated with carbapenem use.4
Central venous catheters
Central venous catheters are indispensable in the treatment of critically ill premature infants, minimizing the need for venipuncture and facilitating the administration of parenteral nutrition, blood products, and inotropic therapy. However, these devices also play a critical role in the pathogenesis of invasive candidiasis, providing a portal of entry for Candida as well as a foreign surface for adhesion and biofilm formation.1, 3, 8, 43 The portion of central venous catheters that is within the vessel lumen frequently becomes covered by a fibrin sheath.45 Candida species can grow within this fibrin matrix while remaining protected from host immune defenses and antifungal therapy.46 As a result, central venous catheter removal is often necessary to clear candidemia, while delayed catheter removal (>1 day after initiation of antifungal therapy) is associated with an increased risk of death or neurodevelopmental impairment from invasive candidiasis.38
Other risk factors
Translocation across the GI tract is generally thought to be the most frequent source of invasive candidiasis.47 Necrotizing enterocolitis, congenital GI anomalies (e.g. gastroschisis),3, 32 spontaneous intestinal perforation,48 and prior abdominal surgery43, 49 are all associated with an increased risk of invasive candidiasis among premature infants. Histamine-2-receptor antagonists (H2 antagonists) encourage overgrowth of Candida in the GI tract through suppression of gastric acid production, and may facilitate invasion by Candida species through inhibition of the neutrophil oxidative burst.50, 51 In a study conducted among infants in six NICUs, H2 antagonists more than doubled the risk of invasive candidiasis.1 Data also suggest that corticosteroid treatment increases the risk of invasive candidiasis among premature infants.52, 53 Corticosteroids alter number and function of T lymphocytes and result in hyperglycemia, which facilitates growth and inhibits phagocytosis by Candida species in vitro.54, 55 In a placebo-controlled trial, dexamethasone increased the risk of sepsis and meningitis among VLBW infants, with Candida species accounting for roughly one-quarter of these infections.53 Finally, in a prospective cohort study of more than 1500 infants, presence of an endotracheal tube increased the risk of invasive candidiasis by more than 50%.8 Although the mechanism for this association has not been defined, endotracheal intubation can result in abrasion of the respiratory mucosa, which may enable invasion by Candida species.56
Microbiology
Although there are more than 150 species of Candida, the majority of cases of invasive candidiasis among infants are caused by a relatively small number of species. Candida albicans is generally the most commonly isolated species, accounting for 45–55% of episodes of invasive candidiasis among infants.2, 3, 8, 13, 57 In the majority of cohorts, C. parapsilosis is the most frequent non-albicans Candida species (20–35%), followed by Candida tropicalis (1–6%).3, 13, 39, 58 Non-albicans species may be responsible for a growing proportion of neonatal candidiasis.13, 14, 57 Candida krusei and Candida glabrata warrant special consideration given their inherent or potential resistance to fluconazole.57 However, these species still account for a relatively small proportion (<5%) of neonatal candidiasis, and no increase in disease caused by these species was observed in recent cohorts.8, 13, 14, 39
C. albicans is also the most pathogenic species of Candida. In a number of studies, mortality associated with invasive candidiasis caused by C. albicans was higher than for disease caused by C. parapsilosis.2, 12, 22, 59, 60 Moreover, the mortality differences in several of these studies were substantial, as in a cohort where the case fatality rates for invasive candidiasis caused by C. albicans and C. parapsilosis were 24% and 3%, respectively.59 However, this mortality difference was not observed in some studies, and a recent meta-analysis concluded that invasive candidiasis caused by C. parapsilosis is associated with a mortality rate of approximately 10% among premature infants.37, 58
Diagnosis
Delayed initiation of appropriate antifungal therapy is associated with increased mortality from invasive candidiasis.61, 62 However, identification of infants with candidiasis is challenging as infants typically have non-specific symptoms and diagnostic capabilities are currently limited.
Clinical Findings
Infants with invasive candidiasis frequently present with features suggestive of sepsis, including lethargy or apnea, feeding intolerance, cardiorespiratory instability, and hyperbilirubinemia.46 Hyperthermia, a generally unreliable marker for infection in infants, is present in only half of infants with invasive candidiasis.35, 39 Thrombocytopenia lacks specificity for a diagnosis of invasive candidiasis, and studies reporting the sensitivity of this finding yielded conflicting results.4, 63, 64 Glucose intolerance and leukopenia or leukocytosis are also common findings, although white blood cell count is normal in 40% of infants with fungal sepsis.39 Finally, C-reactive protein and procalcitonin are often elevated in infants with fungal sepsis, but the specificity of these results is poor.65, 66
Clinical judgment in determining risk of invasive candidiasis among ELBW infants was evaluated in one study.8 At the time that blood cultures were obtained for sepsis, the bedside clinician was asked to estimate the probability of invasive candidiasis. Of the sepsis episodes resulting in a diagnosis of invasive candidiasis, only 25% were deemed to have been “probably” or “highly likely” to be caused by Candida by the treating clinician.8 Moreover, the accuracy of clinical judgment was similar across levels of medical training (resident, fellow, and attending).8
Culture-Based Methods
Blood culture remains the gold standard for diagnosis of neonatal candidiasis. However, autopsy studies suggest that the sensitivity of blood culture for invasive candidiasis is <50% even when an optimal volume of blood (7.5–10 mL) is obtained for culture.67, 68 Blood culture yield varies based on the number of organs that are involved, ranging from 28% when one vital organ is involved to 78% when at least four vital organs are involved.67 Blood culture sensitivity may be even lower in premature infants because of the small volumes that are typically used to inoculate blood culture bottles in this patient population. Substantial improvements in blood culture technology were made since these original autopsy studies. The precise impact of these advances on the yield of blood cultures for invasive candidiasis is unknown, but blood culture likely remains an insensitive test for invasive candidiasis.69
Once growth of Candida in blood culture occurs, a lengthy process to identify the species generally ensues. Over the past decade, a number of techniques became available that can reduce the time needed for identification of yeast species from positive blood cultures. Matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) emerged as a powerful technique for the rapid identification of bacteria and fungi from growth on solid media. MALDI-TOF MS uses mass spectrometry to identify bacterial and fungal species based on the ribosomal protein patterns, often providing results in less than one hour.70 Several studies confirm that the MALDI-TOF identifies Candida species from solid growth with 90–95% accuracy, effectively reducing the time needed for species identification following blood culture positivity.70–72
The peptide nucleic acid fluorescent in situ hybridization (PNA-FISH) Yeast Traffic Light Assay (AdvanDx, Inc., Woburn, MA) enables rapid detection of Candida directly from liquid media, including positive blood cultures.73, 74 This assay uses species-specific fluorescent probes and is capable of identifying the five most commonly isolated species of Candida within 90 minutes.73 When viewed under a fluorescent microscope, green fluorescence is seen in the presence of C. albicans or C. parapsilosis, yellow fluorescence with C. tropicalis, and red fluorescence in the presence of C. glabrata or C. krusei.73, 74 For each of the probes, the sensitivity and specificity are above 90%, and this assay generally identifies blood culture isolates more quickly than the MALDI-TOF as it does not require growth on solid media.73, 74
Polymerase chain reaction (PCR) holds great promise for earlier identification of Candida species from positive blood cultures. Several PCR-based assays are commercially available that can identify yeast species from positive blood cultures with sensitivity and specificity >98%.75–77 Moreover, like the PNA-FISH Yeast Traffic Light Assay, PCR does not require growth on solid media, and the total time needed for this technique is generally <4 hours.75 Given its ability to detect small quantities of fungal DNA, PCR is also being evaluated for the direct detection of Candida species from whole blood.78–80 Among the most studied assays for this purpose is the LightCycler SeptiFast (Roche Diagnostics), which can detect Candida species from whole blood in approximately 60% of patients with culture-confirmed candidemia.78
Fungal Antigens
There are a number of fungal antigens that may be detectable in the blood of patients with invasive candidiasis. These include mannan, a component of the outer cell wall of Candida species, and 1-3-β-D-glucan, found in the middle layers of the cell wall.81 The Platelia Candida Antigen Plus assay (Bio-Rad, Marnes-la-Coquette, France) is most frequently used for detection of mannan antigen in blood. The available data indicate that the specificity of this assay for invasive candidiasis in adults is excellent (>90%), but sensitivity is poor (30–60%).81–84 Few studies have been conducted in infants, although mannan antigen was positive in 11 of 12 infants with proven invasive candidiasis in one small study.85 Mannan is poorly expressed by C. parapsilosis and the sensitivity of mannan antigen for invasive candidiasis caused by this species is likely to be lower.82, 83
There are a number of commercially available kits for the detection of 1-3-β-D-glucan from clinical specimens, including the Fungitell assay (Associates of Cape Cod, Inc., Falmouth, MA) and the Fungitec G-test (Seikagaku Corporation, Tokyo, Japan). Several reports suggest that 1-3-β-D-glucan may be a useful screening test for invasive fungal infection in certain populations.86–88 A recent meta-analysis including 19 studies concluded that 1-3-β-D-glucan assays have a sensitivity of 81% and a specificity of 81% for diagnosis of invasive candidiasis, although most of the included data were from adults.89 Goudjil et al. retrospectively examined serum 1-3-β-D-glucan levels in 61 infants with clinical suspicion of fungal infection. Among 18 infants who were diagnosed with invasive candidiasis, the mean 1-3-β-D-glucan level was 364 pg/mL (range 131–976) compared with 89 pg/mL (range 30–127) among non-infected control infants.87 However, healthy children and infants have higher 1-3-β-D-glucan levels than adults, suggesting that age-specific cutoffs may be necessary, and larger prospective studies are needed before use of 1-3-β-D-glucan assays can be recommended for the diagnosis of neonatal candidiasis.90, 91
Treatment
Indications and neonatal dosing for specific antifungal agents are discussed in detail elsewhere in this issue. However, for the bedside clinician, two aspects of invasive candidiasis warrant special consideration: 1) involvement of the kidneys should guide choice of antifungal therapy, and 2) central nervous system involvement should be presumed in the infant with invasive candidiasis. More specifically, liposomal formulations of amphotericin B should not be used for infants with renal candidiasis given the sub-optimal penetration of these agents into the renal parenchyma.92 Central nervous system involvement should be presumed because the incidence of meningoencephalitis exceeds 15% in neonates with invasive candidiasis,33 cerebrospinal fluid parameters (white blood cells, glucose, protein) and culture unreliably detect disease, and imaging is not sufficiently sensitive to exclude central nervous system involvement.
The optimal duration of antifungal therapy for neonatal candidiasis has not been defined, but guidelines are available from the Infectious Diseases Society of America.92 Candidemia without evidence of end-organ dissemination should be treated with 3 weeks of antifungal therapy from clearance of blood cultures and resolution of signs of infection. Infants with Candida meningoencephalitis should receive antifungal therapy until these conditions are met and cerebrospinal fluid abnormalities have completely resolved. Native valve endocarditis should be treated with ≥6 weeks of antifungal therapy and may require valve replacement. Central venous catheters should be promptly removed or replaced in the setting of bloodstream infection as this reduces duration of candidemia, the rate of end-organ dissemination, and mortality.32, 38, 59
Conclusions
Although improved recognition of risk factors led to a substantial reduction in neonatal candidiasis over the past decade, this infection remains a barrier to achieving further reductions in the morbidity and mortality associated with extreme prematurity. The diagnosis of invasive candidiasis continues to rely on clinical suspicion and the detection of candidemia. Several methods were recently developed that can shorten the duration of time needed for identification of yeast from positive blood cultures. However, improved diagnostics that can rapidly identify infants with invasive candidiasis and permit initiation of prompt antifungal therapy are still needed.
Key Points.
Invasive candidiasis occurs primarily in extremely premature infants and is associated with substantial morbidity and mortality.
The incidence of invasive candidiasis is strongly related to gestational age and birth weight, but most cases are preventable.
The diagnosis of invasive candidiasis relies on clinical suspicion and detection of Candida in blood culture or cultures from other normally sterile sites.
Several methods were recently developed that can shorten the time needed for identification of yeast from a positive culture, but improved diagnostics are still needed.
Best Practices Box.
What is the current practice?
Blood cultures are sent routinely from premature infants with signs of sepsis.
The decision of whether to start empirical antifungal therapy is determined by the clinician’s suspicion for invasive candidiasis based on local epidemiology and infant- specific risk factors.
Widespread variation exists in the practices of antifungal prophylaxis and empirical antifungal therapy for premature infants with sepsis.
What changes in current practice are likely to improve outcomes?
Further research to determine what impacts the variation in the incidence of invasive candidiasis across NICUs.
Improved molecular or fungal antigen-based diagnostics that can rapidly identify Candida species from blood or other normally sterile sites.
Determining optimal duration of antifungal therapy for invasive candidiasis among premature infants.
Is there a clinical algorithm?
Major Recommendations
Minimize exposure to modifiable risk factors for invasive candidiasis (broad-spectrum antibacterials, central venous catheters) in caring for premature infants.
Consider empirical antifungal therapy for premature infants with signs of sepsis, particularly in the setting of established infant risk factors.
Infants with invasive candidiasis should be treated presumptively for central nervous system disease.
Footnotes
Conflicts of Interest:
Dr. Benjamin receives support from the United States government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-05, 1K24HD058735-05, UL1TR001117, and NICHD contract HHSN275201000003I) and the nonprofit organization Thrasher Research Fund for his work in neonatal candidiasis (www.thrasherresearch.org); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saiman L, Ludington E, Pfaller M, Rangel-Frausto S, Wiblin RT, Dawson J, et al. Risk factors for candidemia in Neonatal Intensive Care Unit patients. The National Epidemiology of Mycosis Survey study group. The Pediatric infectious disease journal. 2000;19(4):319–24. doi: 10.1097/00006454-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK., Jr The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics. 2006;118(2):717–22. doi: 10.1542/peds.2005-2677. [DOI] [PubMed] [Google Scholar]
- 3.Feja KN, Wu F, Roberts K, Loughrey M, Nesin M, Larson E, et al. Risk factors for candidemia in critically ill infants: a matched case-control study. The Journal of pediatrics. 2005;147(2):156–61. doi: 10.1016/j.jpeds.2005.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin DK, Jr, DeLong ER, Steinbach WJ, Cotton CM, Walsh TJ, Clark RH. Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics. 2003;112(3 Pt 1):543–7. doi: 10.1542/peds.112.3.543. [DOI] [PubMed] [Google Scholar]
- 5.Friedman S, Richardson SE, Jacobs SE, O’Brien K. Systemic Candida infection in extremely low birth weight infants: short term morbidity and long term neurodevelopmental outcome. The Pediatric infectious disease journal. 2000;19(6):499–504. doi: 10.1097/00006454-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin DK, DeLong E, Cotten CM, Garges HP, Steinbach WJ, Clark RH. Mortality following blood culture in premature infants: increased with Gram-negative bacteremia and candidemia, but not Gram-positive bacteremia. Journal of perinatology: official journal of the California Perinatal Association. 2004;24(3):175–80. doi: 10.1038/sj.jp.7211068. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin DK, Jr, Stoll BJ, Gantz MG, Walsh MC, Sanchez PJ, Das A, et al. Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics. 2010;126(4):e865–73. doi: 10.1542/peds.2009-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittal M, Dhanireddy R, Higgins RD. Candida sepsis and association with retinopathy of prematurity. Pediatrics. 1998;101(4 Pt 1):654–7. doi: 10.1542/peds.101.4.654. [DOI] [PubMed] [Google Scholar]
- 10.Adams-Chapman I, Bann CM, Das A, Goldberg RN, Stoll BJ, Walsh MC, et al. Neurodevelopmental outcome of extremely low birth weight infants with Candida infection. The Journal of pediatrics. 2013;163(4):961–7. e3. doi: 10.1016/j.jpeds.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA: the journal of the American Medical Association. 2004;292(19):2357–65. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 12.Kossoff EH, Buescher ES, Karlowicz MG. Candidemia in a neonatal intensive care unit: trends during fifteen years and clinical features of 111 cases. The Pediatric infectious disease journal. 1998;17(6):504–8. doi: 10.1097/00006454-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Chitnis AS, Magill SS, Edwards JR, Chiller TM, Fridkin SK, Lessa FC. Trends in Candida central line-associated bloodstream infections among NICUs, 1999–2009. Pediatrics. 2012;130(1):e46–52. doi: 10.1542/peds.2011-3620. [DOI] [PubMed] [Google Scholar]
- 14.Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995–2004. Pediatrics. 2006;117(5):1680–7. doi: 10.1542/peds.2005-1996. [DOI] [PubMed] [Google Scholar]
- 15.Aliaga S, Clark RH, Laughon M, Walsh TJ, Hope WW, Benjamin DK, et al. Changes in the incidence of candidiasis in neonatal intensive care units. Pediatrics. 2014;133(2):236–42. doi: 10.1542/peds.2013-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendel CM. Nosocomial neonatal candidiasis. The Pediatric infectious disease journal. 2005;24(9):831–2. doi: 10.1097/01.inf.0000178291.40568.ef. [DOI] [PubMed] [Google Scholar]
- 17.Saiman L, Ludington E, Dawson JD, Patterson JE, Rangel-Frausto S, Wiblin RT, et al. Risk factors for Candida species colonization of neonatal intensive care unit patients. The Pediatric infectious disease journal. 2001;20(12):1119–24. doi: 10.1097/00006454-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Huang YC, Li CC, Lin TY, Lien RI, Chou YH, Wu JL, et al. Association of fungal colonization and invasive disease in very low birth weight infants. The Pediatric infectious disease journal. 1998;17(9):819–22. doi: 10.1097/00006454-199809000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Manzoni P, Farina D, Galletto P, Leonessa M, Priolo C, Arisio R, et al. Type and number of sites colonized by fungi and risk of progression to invasive fungal infection in preterm neonates in neonatal intensive care unit. Journal of perinatal medicine. 2007;35(3):220–6. doi: 10.1515/JPM.2007.055. [DOI] [PubMed] [Google Scholar]
- 20.Baley JE, Kliegman RM, Boxerbaum B, Fanaroff AA. Fungal colonization in the very low birth weight infant. Pediatrics. 1986;78(2):225–32. [PubMed] [Google Scholar]
- 21.Ali GY, Algohary EH, Rashed KA, Almoghanum M, Khalifa AA. Prevalence of Candida colonization in preterm newborns and VLBW in neonatal intensive care unit: role of maternal colonization as a risk factor in transmission of disease. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(6):789–95. doi: 10.3109/14767058.2011.622005. [DOI] [PubMed] [Google Scholar]
- 22.Waggoner-Fountain LA, Walker MW, Hollis RJ, Pfaller MA, Ferguson JE, 2nd, Wenzel RP, et al. Vertical and horizontal transmission of unique Candida species to premature newborns. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 1996;22(5):803–8. doi: 10.1093/clinids/22.5.803. [DOI] [PubMed] [Google Scholar]
- 23.Bendel CM. Colonization and epithelial adhesion in the pathogenesis of neonatal candidiasis. Seminars in perinatology. 2003;27(5):357–64. doi: 10.1016/s0146-0005(03)00059-4. [DOI] [PubMed] [Google Scholar]
- 24.Kicklighter SD, Springer SC, Cox T, Hulsey TC, Turner RB. Fluconazole for prophylaxis against candidal rectal colonization in the very low birth weight infant. Pediatrics. 2001;107(2):293–8. doi: 10.1542/peds.107.2.293. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. The New England journal of medicine. 2001;345(23):1660–6. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 26.Evans NJ, Rutter N. Development of the epidermis in the newborn. Biology of the neonate. 1986;49(2):74–80. doi: 10.1159/000242513. [DOI] [PubMed] [Google Scholar]
- 27.Neu J. Gastrointestinal development and meeting the nutritional needs of premature infants. The American journal of clinical nutrition. 2007;85(2):629s–34s. doi: 10.1093/ajcn/85.2.629S. [DOI] [PubMed] [Google Scholar]
- 28.Carr R. Neutrophil production and function in newborn infants. British journal of haematology. 2000;110(1):18–28. doi: 10.1046/j.1365-2141.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 29.Correa-Rocha R, Perez A, Lorente R, Ferrando-Martinez S, Leal M, Gurbindo D, et al. Preterm neonates show marked leukopenia and lymphopenia that are associated with increased regulatory T-cell values and diminished IL-7. Pediatric research. 2012;71(5):590–7. doi: 10.1038/pr.2012.6. [DOI] [PubMed] [Google Scholar]
- 30.Bektas S, Goetze B, Speer CP. Decreased adherence, chemotaxis and phagocytic activities of neutrophils from preterm neonates. Acta paediatrica Scandinavica. 1990;79(11):1031–8. doi: 10.1111/j.1651-2227.1990.tb11379.x. [DOI] [PubMed] [Google Scholar]
- 31.Bliss JM, Wong AY, Bhak G, Laforce-Nesbitt SS, Taylor S, Tan S, et al. Candida virulence properties and adverse clinical outcomes in neonatal candidiasis. The Journal of pediatrics. 2012;161(3):441–7. e2. doi: 10.1016/j.jpeds.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman RL, Faix RG. Persistently positive cultures and outcome in invasive neonatal candidiasis. The Pediatric infectious disease journal. 2000;19(9):822–7. doi: 10.1097/00006454-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Benjamin DK, Jr, Poole C, Steinbach WJ, Rowen JL, Walsh TJ. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. 2003;112(3 Pt 1):634–40. doi: 10.1542/peds.112.3.634. [DOI] [PubMed] [Google Scholar]
- 34.Bryant K, Maxfield C, Rabalais G. Renal candidiasis in neonates with candiduria. The Pediatric infectious disease journal. 1999;18(11):959–63. doi: 10.1097/00006454-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Wang LW, Lin CH, Liu CC, Lin YJ. Systemic fungal infection in very low-birth-weight infants. Zhonghua Minguo xiao er ke yi xue hui za zhi [Journal] Zhonghua Minguo xiao er ke yi xue hui. 1996;37(4):272–7. [PubMed] [Google Scholar]
- 36.Lee JH, Hornik CP, Benjamin DK, Jr, Herring AH, Clark RH, Cohen-Wolkowiez M, et al. Risk factors for invasive candidiasis in infants >1500 g birth weight. The Pediatric infectious disease journal. 2013;32(3):222–6. doi: 10.1097/INF.0b013e3182769603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamin DK, Jr, Ross K, McKinney RE, Jr, Benjamin DK, Auten R, Fisher RG. When to suspect fungal infection in neonates: A clinical comparison of Candida albicans and Candida parapsilosis fungemia with coagulase-negative staphylococcal bacteremia. Pediatrics. 2000;106(4):712–8. doi: 10.1542/peds.106.4.712. [DOI] [PubMed] [Google Scholar]
- 38.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117(1):84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 39.Makhoul IR, Kassis I, Smolkin T, Tamir A, Sujov P. Review of 49 neonates with acquired fungal sepsis: further characterization. Pediatrics. 2001;107(1):61–6. doi: 10.1542/peds.107.1.61. [DOI] [PubMed] [Google Scholar]
- 40.Benjamin DK, Jr, Hudak ML, Duara S, Randolph DA, Bidegain M, Mundakel GT, et al. Effect of fluconazole prophylaxis on candidiasis and mortality in premature infants: a randomized clinical trial. JAMA: the journal of the American Medical Association. 2014;311(17):1742–9. doi: 10.1001/jama.2014.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manzoni P, Stolfi I, Pugni L, Decembrino L, Magnani C, Vetrano G, et al. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. The New England journal of medicine. 2007;356(24):2483–95. doi: 10.1056/NEJMoa065733. [DOI] [PubMed] [Google Scholar]
- 42.Parikh TB, Nanavati RN, Patankar CV, Rao S, Bisure K, Udani RH, et al. Fluconazole prophylaxis against fungal colonization and invasive fungal infection in very low birth weight infants. Indian pediatrics. 2007;44(11):830–7. [PubMed] [Google Scholar]
- 43.Yu Y, Du L, Yuan T, Zheng J, Chen A, Chen L, et al. Risk factors and clinical analysis for invasive fungal infection in neonatal intensive care unit patients. American journal of perinatology. 2013;30(7):589–94. doi: 10.1055/s-0032-1329688. [DOI] [PubMed] [Google Scholar]
- 44.Hornik CP, Herring AH, Benjamin DK, Jr, Capparelli EV, Kearns GL, van den Anker J, et al. Adverse events associated with meropenem versus imipenem/cilastatin therapy in a large retrospective cohort of hospitalized infants. The Pediatric infectious disease journal. 2013;32(7):748–53. doi: 10.1097/INF.0b013e31828be70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suojanen JN, Brophy DP, Nasser I. Thrombus on indwelling central venous catheters: the histopathology of “Fibrin sheaths”. Cardiovascular and interventional radiology. 2000;23(3):194–7. doi: 10.1007/s002700010042. [DOI] [PubMed] [Google Scholar]
- 46.Benjamin DK, Jr, Garges H, Steinbach WJ. Candida bloodstream infection in neonates. Seminars in perinatology. 2003;27(5):375–83. doi: 10.1016/s0146-0005(03)00061-2. [DOI] [PubMed] [Google Scholar]
- 47.Cole GT, Halawa AA, Anaissie EJ. The role of the gastrointestinal tract in hematogenous candidiasis: from the laboratory to the bedside. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 1996;22 (Suppl 2):S73–88. doi: 10.1093/clinids/22.supplement_2.s73. [DOI] [PubMed] [Google Scholar]
- 48.Coates EW, Karlowicz MG, Croitoru DP, Buescher ES. Distinctive distribution of pathogens associated with peritonitis in neonates with focal intestinal perforation compared with necrotizing enterocolitis. Pediatrics. 2005;116(2):e241–6. doi: 10.1542/peds.2004-2537. [DOI] [PubMed] [Google Scholar]
- 49.Shetty SS, Harrison LH, Hajjeh RA, Taylor T, Mirza SA, Schmidt AB, et al. Determining risk factors for candidemia among newborn infants from population-based surveillance: Baltimore, Maryland, 1998–2000. The Pediatric infectious disease journal. 2005;24(7):601–4. doi: 10.1097/01.inf.0000168751.11375.d6. [DOI] [PubMed] [Google Scholar]
- 50.Boero M, Pera A, Andriulli A, Ponti V, Canepa G, Palmas F, et al. Candida overgrowth in gastric juice of peptic ulcer subjects on short- and long-term treatment with H2-receptor antagonists. Digestion. 1983;28(3):158–63. doi: 10.1159/000198980. [DOI] [PubMed] [Google Scholar]
- 51.Ciz M, Lojek A. Modulation of neutrophil oxidative burst via histamine receptors. British journal of pharmacology. 2013;170(1):17–22. doi: 10.1111/bph.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Botas CM, Kurlat I, Young SM, Sola A. Disseminated candidal infections and intravenous hydrocortisone in preterm infants. Pediatrics. 1995;95(6):883–7. [PubMed] [Google Scholar]
- 53.Stoll BJ, Temprosa M, Tyson JE, Papile LA, Wright LL, Bauer CR, et al. Dexamethasone therapy increases infection in very low birth weight infants. Pediatrics. 1999;104(5):e63. doi: 10.1542/peds.104.5.e63. [DOI] [PubMed] [Google Scholar]
- 54.Gunn T, Reece ER, Metrakos K, Colle E. Depressed T cells following neonatal steroid treatment. Pediatrics. 1981;67(1):61–7. [PubMed] [Google Scholar]
- 55.Hostetter MK, Lorenz JS, Preus L, Kendrick KE. The iC3b receptor on Candida albicans: subcellular localization and modulation of receptor expression by glucose. The Journal of infectious diseases. 1990;161(4):761–8. doi: 10.1093/infdis/161.4.761. [DOI] [PubMed] [Google Scholar]
- 56.Bishop MJ. Mechanisms of laryngotracheal injury following prolonged tracheal intubation. Chest. 1989;96(1):185–6. doi: 10.1378/chest.96.1.185. [DOI] [PubMed] [Google Scholar]
- 57.Steinbach WJ, Roilides E, Berman D, Hoffman JA, Groll AH, Bin-Hussain I, et al. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. The Pediatric infectious disease journal. 2012;31(12):1252–7. doi: 10.1097/INF.0b013e3182737427. [DOI] [PubMed] [Google Scholar]
- 58.Pammi M, Holland L, Butler G, Gacser A, Bliss JM. Candida parapsilosis is a significant neonatal pathogen: a systematic review and meta-analysis. The Pediatric infectious disease journal. 2013;32(5):e206–16. doi: 10.1097/INF.0b013e3182863a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karlowicz MG, Hashimoto LN, Kelly RE, Jr, Buescher ES. Should central venous catheters be removed as soon as candidemia is detected in neonates? Pediatrics. 2000;106(5):E63. doi: 10.1542/peds.106.5.e63. [DOI] [PubMed] [Google Scholar]
- 60.Faix RG. Invasive neonatal candidiasis: comparison of albicans and parapsilosis infection. The Pediatric infectious disease journal. 1992;11(2):88–93. [PubMed] [Google Scholar]
- 61.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2006;43(1):25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 62.Cahan H, Deville JG. Outcomes of neonatal candidiasis: the impact of delayed initiation of antifungal therapy. International journal of pediatrics. 2011;2011:813871. doi: 10.1155/2011/813871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guida JD, Kunig AM, Leef KH, McKenzie SE, Paul DA. Platelet count and sepsis in very low birth weight neonates: is there an organism-specific response? Pediatrics. 2003;111(6 Pt 1):1411–5. doi: 10.1542/peds.111.6.1411. [DOI] [PubMed] [Google Scholar]
- 64.Manzoni P, Mostert M, Galletto P, Gastaldo L, Gallo E, Agriesti G, et al. Is thrombocytopenia suggestive of organism-specific response in neonatal sepsis? Pediatrics international: official journal of the Japan Pediatric Society. 2009;51(2):206–10. doi: 10.1111/j.1442-200X.2008.02689.x. [DOI] [PubMed] [Google Scholar]
- 65.Montagna MT, Coretti C, Rella A, Barbuti G, Manca F, Montagna O, et al. The role of procalcitonin in neonatal intensive care unit patients with candidemia. Folia microbiologica. 2013;58(1):27–31. doi: 10.1007/s12223-012-0169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oguz SS, Sipahi E, Dilmen U. C-reactive protein and interleukin-6 responses for differentiating fungal and bacterial aetiology in late-onset neonatal sepsis. Mycoses. 2011;54(3):212–6. doi: 10.1111/j.1439-0507.2009.01802.x. [DOI] [PubMed] [Google Scholar]
- 67.Berenguer J, Buck M, Witebsky F, Stock F, Pizzo PA, Walsh TJ. Lysis-centrifugation blood cultures in the detection of tissue-proven invasive candidiasis. Disseminated versus single-organ infection. Diagnostic microbiology and infectious disease. 1993;17(2):103–9. doi: 10.1016/0732-8893(93)90020-8. [DOI] [PubMed] [Google Scholar]
- 68.Thaler M, Pastakia B, Shawker TH, O’Leary T, Pizzo PA. Hepatic candidiasis in cancer patients: the evolving picture of the syndrome. Annals of internal medicine. 1988;108(1):88–100. doi: 10.7326/0003-4819-108-1-88. [DOI] [PubMed] [Google Scholar]
- 69.Alexander BD. Diagnosis of fungal infection: new technologies for the mycology laboratory. Transplant infectious disease: an official journal of the Transplantation Society. 2002;4 (Suppl 3):32–7. doi: 10.1034/j.1399-3062.4.s3.5.x. [DOI] [PubMed] [Google Scholar]
- 70.Marklein G, Josten M, Klanke U, Muller E, Horre R, Maier T, et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for fast and reliable identification of clinical yeast isolates. Journal of clinical microbiology. 2009;47(9):2912–7. doi: 10.1128/JCM.00389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bader O, Weig M, Taverne-Ghadwal L, Lugert R, Gross U, Kuhns M. Improved clinical laboratory identification of human pathogenic yeasts by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011;17(9):1359–65. doi: 10.1111/j.1469-0691.2010.03398.x. [DOI] [PubMed] [Google Scholar]
- 72.Sendid B, Ducoroy P, Francois N, Lucchi G, Spinali S, Vagner O, et al. Evaluation of MALDI-TOF mass spectrometry for the identification of medically-important yeasts in the clinical laboratories of Dijon and Lille hospitals. Medical mycology. 2013;51(1):25–32. doi: 10.3109/13693786.2012.693631. [DOI] [PubMed] [Google Scholar]
- 73.Stone NR, Gorton RL, Barker K, Ramnarain P, Kibbler CC. Evaluation of PNA-FISH yeast traffic light for rapid identification of yeast directly from positive blood cultures and assessment of clinical impact. Journal of clinical microbiology. 2013;51(4):1301–2. doi: 10.1128/JCM.00028-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calderaro A, Martinelli M, Motta F, Larini S, Arcangeletti MC, Medici MC, et al. Comparison of peptide nucleic acid fluorescence in situ hybridization assays with culture-based matrix-assisted laser desorption/ionization-time of flight mass spectrometry for the identification of bacteria and yeasts from blood cultures and cerebrospinal fluid cultures. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013 doi: 10.1111/1469-0691.12490. [DOI] [PubMed] [Google Scholar]
- 75.Aittakorpi A, Kuusela P, Koukila-Kahkola P, Vaara M, Petrou M, Gant V, et al. Accurate and rapid identification of Candida spp. frequently associated with fungemia by using PCR and the microarray-based Prove-it Sepsis assay. Journal of clinical microbiology. 2012;50(11):3635–40. doi: 10.1128/JCM.01461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Balada-Llasat JM, LaRue H, Kamboj K, Rigali L, Smith D, Thomas K, et al. Detection of yeasts in blood cultures by the Luminex xTAG fungal assay. Journal of clinical microbiology. 2012;50(2):492–4. doi: 10.1128/JCM.06375-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paolucci M, Foschi C, Tamburini MV, Ambretti S, Lazzarotto T, Landini MP. Comparison between MALDI-TOF MS and FilmArray Blood Culture Identification panel for rapid identification of yeast from positive blood culture. Journal of microbiological methods. 2014 doi: 10.1016/j.mimet.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 78.Chang SS, Hsieh WH, Liu TS, Lee SH, Wang CH, Chou HC, et al. Multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis - a systemic review and meta-analysis. PloS one. 2013;8(5):e62323. doi: 10.1371/journal.pone.0062323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dunyach C, Bertout S, Phelipeau C, Drakulovski P, Reynes J, Mallie M. Detection and identification of Candida spp. in human serum by LightCycler real-time polymerase chain reaction. Diagnostic microbiology and infectious disease. 2008;60(3):263–71. doi: 10.1016/j.diagmicrobio.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 80.Khlif M, Mary C, Sellami H, Sellami A, Dumon H, Ayadi A, et al. Evaluation of nested and real-time PCR assays in the diagnosis of candidaemia. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2009;15(7):656–61. doi: 10.1111/j.1469-0691.2009.02762.x. [DOI] [PubMed] [Google Scholar]
- 81.Poissy J, Sendid B, Damiens S, Ichi Ishibashi K, Francois N, Kauv M, et al. Presence of Candida cell wall derived polysaccharides in the sera of intensive care unit patients: relation with candidaemia and Candida colonisation. Critical care (London, England) 2014;18(3):R135. doi: 10.1186/cc13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Held J, Kohlberger I, Rappold E, Busse Grawitz A, Hacker G. Comparison of (1->3)-beta-D-glucan, mannan/anti-mannan antibodies, and Cand-Tec Candida antigen as serum biomarkers for candidemia. Journal of clinical microbiology. 2013;51(4):1158–64. doi: 10.1128/JCM.02473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mikulska M, Calandra T, Sanguinetti M, Poulain D, Viscoli C. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: recommendations from the Third European Conference on Infections in Leukemia. Critical care (London, England) 2010;14(6):R222. doi: 10.1186/cc9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alam FF, Mustafa AS, Khan ZU. Comparative evaluation of (1, 3)-beta-D-glucan, mannan and anti-mannan antibodies, and Candida species-specific snPCR in patients with candidemia. BMC infectious diseases. 2007;7:103. doi: 10.1186/1471-2334-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oliveri S, Trovato L, Betta P, Romeo MG, Nicoletti G. Experience with the Platelia Candida ELISA for the diagnosis of invasive candidosis in neonatal patients. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2008;14(4):391–3. doi: 10.1111/j.1469-0691.2007.01938.x. [DOI] [PubMed] [Google Scholar]
- 86.Mularoni A, Furfaro E, Faraci M, Franceschi A, Mezzano P, Bandettini R, et al. High Levels of beta-D-glucan in immunocompromised children with proven invasive fungal disease. Clinical and vaccine immunology: CVI. 2010;17(5):882–3. doi: 10.1128/CVI.00038-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goudjil S, Kongolo G, Dusol L, Imestouren F, Cornu M, Leke A, et al. (1-3)-beta-D-glucan levels in candidiasis infections in the critically ill neonate. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013;26(1):44–8. doi: 10.3109/14767058.2012.722716. [DOI] [PubMed] [Google Scholar]
- 88.Mackay CA, Ballot DE, Perovic O. Serum 1,3-betaD-Glucan assay in the diagnosis of invasive fungal disease in neonates. Pediatric reports. 2011;3(2):e14. doi: 10.4081/pr.2011.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Onishi A, Sugiyama D, Kogata Y, Saegusa J, Sugimoto T, Kawano S, et al. Diagnostic accuracy of serum 1,3-beta-D-glucan for pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. Journal of clinical microbiology. 2012;50(1):7–15. doi: 10.1128/JCM.05267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith PB, Benjamin DK, Jr, Alexander BD, Johnson MD, Finkelman MA, Steinbach WJ. Quantification of 1,3-beta-D-glucan levels in children: preliminary data for diagnostic use of the beta-glucan assay in a pediatric setting. Clinical and vaccine immunology: CVI. 2007;14(7):924–5. doi: 10.1128/CVI.00025-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mokaddas E, Burhamah MH, Khan ZU, Ahmad S. Levels of (1-->3)-beta-D-glucan, Candida mannan and Candida DNA in serum samples of pediatric cancer patients colonized with Candida species. BMC infectious diseases. 2010;10:292. doi: 10.1186/1471-2334-10-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48(5):503–35. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]