INTRODUCTION: NATURE OF THE PROBLEM
Pathogenesis and Natural History of Clostridium difficile Infection
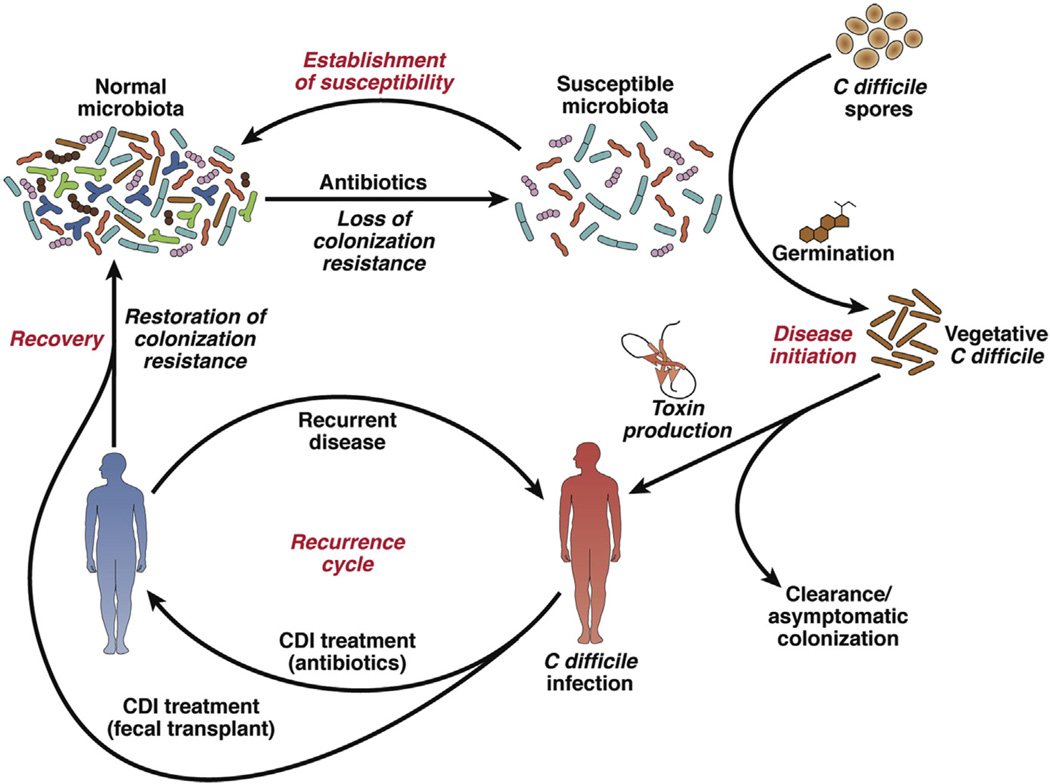
The human gut microbiota is a diverse ecosystem consisting of thousands of bacterial species.1 It is thought that one role of this ecosystem is to protect against invasion by pathogens.2,3 The predominant understanding of the pathogenesis of CDI is that it requires disruption of the gut microbiota as a prerequisite for the onset of symptomatic disease (Fig. 1).4 This disruption usually occurs through exposure to antibiotics, which alter the composition and function of the microbiome to a state susceptible to CDI.5 After exposure to C difficile spores, patients can either become asymptomatically colonized or develop symptomatic infection.6,7 Colonization follows germination of the C difficile spores and vegetative outgrowth. Subsequent expression of the toxins TcdA and TcdB, the main virulence factors of C difficile, results in epithelial damage and symptomatic infection. CDI can be self-limited8,9 but usually requires treatment with antibiotics that have activity against C difficile,10 although the treatments are nonspecific and have activity against other gut bacteria. Features of infection include diarrhea, leukocytosis, fever, or pseudomembranous colitis.10 Some patients can experience severe disease, including signs and symptoms such as abdominal pain, ileus, or septic shock that results in admission to an intensive care unit (ICU), abdominal surgery such as colectomy, or even death.11
Fig. 1.
Pathogenesis of Clostridium difficile infection (CDI). This figure shows how a healthy gut microbiota (upper left corner) is altered by antibiotics to a susceptible state in which either asymptomatic colonization or symptomatic CDI can occur. Some patients do not have recovery of the microbiome back to a healthy state and experience recurrent CDI. Fecal microbiota transplantation can help restore the microbiome to a state resistant to CDI. (Adapted from Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology 2014;146(6):1547–53; with permission.)
RECURRENT CLOSTRIDIUM DIFFICILE INFECTION
After recovery from infection, some patients retain a microbiome susceptible to CDI and can have recurrent disease (see Fig. 1),12 due to either recrudescence of the original infection or reinfection with a new strain.13 After an initial episode of CDI, 15% to 20% of successfully treated patients suffer a recurrence and up to 45% of those patients can have a second recurrence; however, less than 5% of all patients who have an initial episode of CDI can enter a cycle of recurrent disease with multiple recurrences.14–16 Conventional therapies for recurrent CDI include extended pulses and/or tapering doses of either metronidazole or (usually) vancomycin.10,17 Multiple regimens exist and the duration varies from 4 to 10 weeks, followed in some instances by either rifaximin or fidaxomicin as a cap or chaser after initial therapy.10,18,19 The possible mechanism by which this acts involves agents with a narrower spectrum of antimicrobial activity, allowing the microbiome to recover while still suppressing C difficile activity.
Some patients have recurrent CDI recalcitrant to these treatments and relapse soon after antibiotics are stopped. A trial of chronic, low-dose antibiotics to suppress CDI is an option, although there are downsides to this strategy—increased antimicrobial resistance, continued microbiome disruption, and breakthroughs requiring retreatment at higher dosages. Faced with this prospect, other therapeutic options are often discussed in these patients. It is unclear why patients experience recurrent infection, but host factors such as antibody response to toxins,20 microbial factors such as C difficile strain,21,22 and community factors such as persistent disruption of the gut microbiome23 all may play a role. Augmentation of the immune response through intravenous infusion of immunoglobulin has variable efficacy.24 A strain-dependent differential recurrence rate for treatment, either of primary CDI or of a first recurrence, with the antibiotic fidaxomicin has been demonstrated.12,25 However, it is not clear how this translates into clinical benefit, especially for those with multiple recurrences. Restoration of the gut microbiome is the treatment strategy that has garnered the most attention and has gained acceptance among practitioners for treatment of recurrent CDI.26
Role of the Gut Microbiota in Recurrent Clostridium difficile Infection
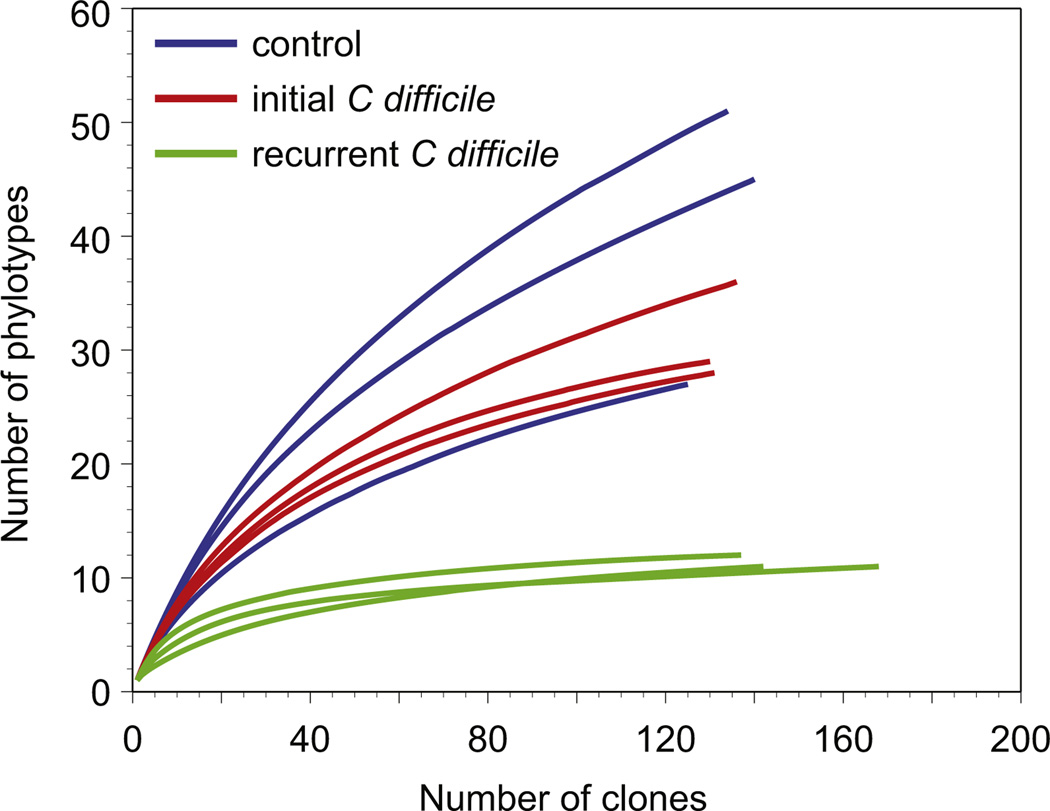
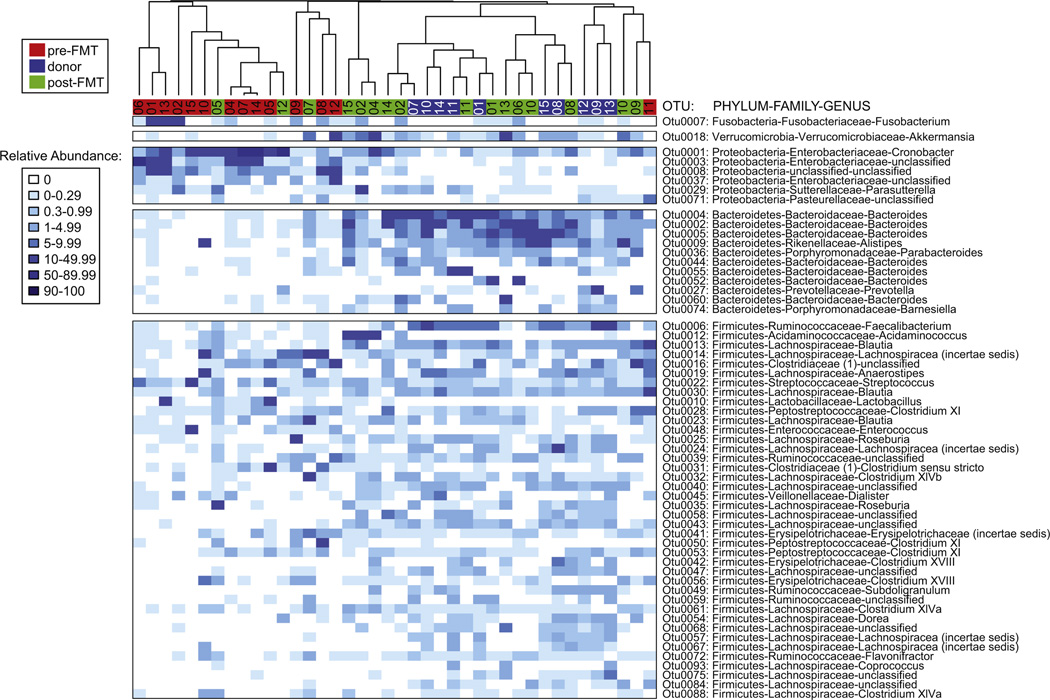
As noted earlier, disruption of the normal indigenous gut microbiota is a prerequisite for the development of CDI. The potential mechanisms by which the indigenous microbiota normally prevents colonization by pathogens such as C difficile are not fully understood. It is likely that multiple mechanisms play a role, including competition for nutritional niches, production of metabolites that are deleterious to C difficile, stimulating host immune responses, and modulating the physiology of the pathogen, as summarized in recent reviews.4,27 Disruption of the indigenous microbiota with antibiotics alters the community structure and function, resulting in the loss of colonization resistance. In patients who are successfully treated for CDI with standard antibiotic therapy, it is assumed that the indigenous microbiota recovers to the point that normal function is restored and colonization resistance returns. In patients who develop recurrent CDI, this functional recovery of the microbiota does not occur. Patients with recurrent CDI have a microbiota characterized by lower-than-normal community diversity (Fig. 2).23 In itself, lowered diversity is not a mechanism that permits continued persistence of C difficile; rather, this low diversity is a marker of continued disruption of microbiota community structure and function. This persistence of a structurally and functionally deficient microbiota provides the rationale for use of fecal microbiota transfer to treat recurrent CDI. By providing access to members of the microbiota that can carry on specific functions that mediate colonization resistance, FMT can reverse the damage. It has been demonstrated that, after successful FMT for CDI, the patient’s stool more closely resembles the composition (and thus the function) of the donated stool (Fig. 3).28
Fig. 2.
Diversity of the gut microbiome and recurrent Clostridium difficile infection. Rarefaction curve analysis indicates that patients with recurrent infection have lower diversity of their gut microbiome compared with healthy controls or patients with a successfully treated initial infection. (Adapted from Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile–associated diarrhea. J Infect Dis 2008;197(3):435–8; with permission.)
Fig. 3.
Composition of the gut microbiome after stool transplant. The microbial composition of the patient’s stool after transplant is similar to the donor’s stool and is more diverse than before transplant. (Adapted from Seekatz AM, Aas J, Gessert CE, et al. Recovery of the gut microbiome following fecal microbiota transplantation. mBio 2014;5(3):e00893–914; with permission.)
In addition to the microbial composition of the stool, fecal contents include a wide range of metabolites. The role of these metabolites in symptomatic recovery from recurrent CDI after FMT has not been determined, although data from human studies and animal models describe changes in bile salt composition.5,29 The nature of these changes in bile salts is uncertain, but they may play a role in immediate symptom relief reported by some patients30 or be involved in the mechanism by which the microbiome influences subsequent recovery and colonization resistance.
OVERVIEW OF FECAL MICROBIOTA TRANSPLANTATION
Historical Perspective
FMT, which uses healthy stool to restore the microbiome to a state resistant to CDI, has recently reemerged as a safe and effective option for treatment of recurrent CDI. FMT is not new to modern times, as there are reports of its use in ancient China for various purposes.31 It was first described as a treatment of pseudomembranous colitis in the 1950s32 and then is not well-described in the literature again until 1983, when Schwann and colleagues30 published the use of fecal enema to treat a 65-year-old woman with CDI, who had symptomatic resolution within 24 hours. The number of protocols and possible routes of administration increased: Aas and colleagues33 reported using FMT via nasogastric infusion of stool in 1994; Persky and Brandt34 performed FMT via colonoscopy in 2000; and in 2010, Silverman and colleagues35 reported a case series of self-administered FMTs via fecal retention enemas by patients in their own homes. In the past several years, the use of FMT for CDI has become widespread.
Stool Preparation Methods
Preparation and infusion of donor stool for FMT takes myriad forms, as reported in the published protocols.36–38 Diluents typically include tap water or normal saline, but yogurt, milk, and mixtures with psyllium husk have also been used. Some protocols call for gentle agitation of stool with the diluent, while others blend the whole preparation. Often, stool is collected and prepared within hours of administration, but frozen stool preparations collected weeks or months before FMT have also been successfully used.39 The amount of prepared stool infusate also varies but is generally at least 50 g.
Routes of Instillation
The sites of stool instillation include the stomach, duodenum, and proximal/distal large intestine.37,38 Infusion into the upper GI tract takes place through a nasogastric or nasojejunal tube or via gastroscopy. Infusion into the lower GI tract takes place using retention enemas, which the patients self-administer in some protocols,35 or via colonoscopy, which usually infuses the donor stool into the terminal ileum and other sites more distal as the colonoscope is withdrawn.
Recipient Preparation
It is generally recommended that recipients withhold any antibiotic therapy for 24 to 48 hours before FMT, if possible, as presence of antibiotics in the GI tract adversely affects the health of the donated stool and decreases the efficacy of the transplant. A bowel preparation or lavage, often with a solution of polyethylene glycol, can be performed and is especially common in protocols using a colonoscope.36 Recipients are typically screened for blood-borne pathogens (Table 1) to establish whether there is evidence of prior infection, which can be helpful post-FMT if a transmission is suspected. Some protocols call for use of an antimotility agent such as loperamide before FMT, to aid in retention of the transplant.36
Table 1.
Screening tests for potentially transmissible infectious pathogens in donors and recipients undergoing fecal microbiota transplantation
| Pathogen/Infection | Usual Tests | Recipient, Donor, or Both |
Part of Routine or Extended Screening |
|---|---|---|---|
| Hepatitis A/B/C | Serum antibodies; serum PCR | Both | Routine |
| HIV | Third- or fourth-generation serum ELISA; serum RNA PCR if recent seroconversion possible | Both | Routine |
| Syphilis | Nontreponemal serum test followed by treponemal confirmatory test if positive (eg, serum RPR followed by TP-PA) | Both | Routine |
| Enteric bacterial pathogens (Salmonella species, E coli, Shigella species, and others) | Routine stool culture | Donor | Routine |
| Enteric helminths and protozoa | Stool microscopy for ova and parasites; antigen ELISAs for Giardia and Cryptosporidium species | Donor | Routine |
| Clostridium difficile | Stool EIA for bacterial products and/or PCR | Donor | Routine |
| Epstein-Barr virus | Serum antibodies; PCR | Both | Extended (HSCT and SOT patients) |
| Cytomegalovirus | Serum antibodies; PCR | Both | Extended (HSCT and SOT patients) |
| Others (Helicobacter pylori, HTLV, and many others) | Various tests | Usually donor only | Extended (research protocols) |
Donor Type and Screening
Donor eligibility also varies between protocols. In general, it is preferred to use donors who are in generally good health and have normal bowel movements. Although many protocols use donors known to the patients, some use universal standard donors. Screening tests typically used are listed in Table 1. In addition, some absolute contraindications to donation for FMT have been proposed (Table 2), including high-risk behaviors such as intravenous drug use and conditions such as inflammatory bowel disease (IBD).
Table 2.
Proposed contraindications to donation for fecal microbiota transplantation
| Risk Factor/Condition | Absolute or Relative Contraindication |
|---|---|
| Known HIV or viral hepatitis infection or recent exposure (12 mo) | Absolute |
| High-risk sexual behaviors (sexual contact with someone infected with HIV/viral hepatitis, men who have sex with men, sex for money) | Absolute |
| Use of illicit drugs | Relative (can consider if in remission and in distant past) |
| Recent tattoo or body piercing (12 mo) | Relative |
| Incarceration or history of incarceration | Relative |
| Risk factors for Creutzfeldt-Jakob disease | Absolute |
| Recent travel (6 mo) to regions where endemic diarrheal illness is prevalent | Relative |
| Past or present irritable bowel syndrome, inflammatory bowel disease, or gastrointestinal malignancy/known polyposis | Absolute |
| Recent antibiotic use (3–6 mo or more) | Absolute (consider delaying FMT if able and another donor unavailable) |
| Ingestion of allergen with known recipient allergy (eg, tree nuts) | Relative (delay FMT if recent ingestion and donor abstains) |
| Others (eg, metabolic syndrome, major gastrointestinal surgery [such as gastric bypass], systemic autoimmune disease, atopic disorders [asthma, eczema, eosinophilic disorders of the gastrointestinal tract]) | Relative/unknown |
Synthetic Stool/Frozen Stool
Use of a single universal donor is attractive, as it can expedite the FMT process and obviates frequent repeated screenings. In lieu of having this donor provide stool on demand, frozen stool preparations have been used for FMT, even in capsule form.39,40 A clinical trial is also underway that uses a synthetic microbiota suspension, derived from intestinal sources, for FMT.41 A nonprofit organization, OpenBiome, takes care of donor selection/screening and stool preparation (www.OpenBiome.org). They ship prepared stool for nasogastric or colonoscopic administration that can be used immediately after thawing or stored at −20°C for up to 6 months. The proposed 2014 revision to the US Food and Drug Administration (FDA) guidance statement on FMT requires that the donor be known to the treating physician or recipient.42 If the draft is accepted without modification, then services such as those provided by OpenBiome may no longer be available in the United States.
Repeated Fecal Microbiota Transplantations
Although success rates for FMT via a single infusion are high, repeated infusions take place in many protocols and can increase the overall efficacy.37 Most clinicians do not repeat FMT routinely without evaluating the first FMT for clinical success.
CLINICAL OUTCOMES FROM FECAL MICROBIOTA TRANSPLANTATION FOR RECURRENT CLOSTRIDIUM DIFFICILE INFECTION
Case Series and Case Reports
Two large systematic reviews of FMT for CDI have been published.37,38 The first, by Gough and colleagues,37 included published articles, abstracts from conference proceedings, and unpublished data solicited from investigators. This comprehensive search included case data on 317 patients treated via FMT for recurrent CDI, but no controlled trials were found. The routes used for FMT included distal infusion into the GI tract via retention enema (35%) or colonoscopy (42%) and proximal infusion via nasogastric/nasojejunal tube or gastroscope (23%). The investigators did look at some of the variability in protocols and found differences in resolution rates: infusion into the upper GI tract (76%) versus lower GI tract (89%–96%), related donors (93%) versus unrelated donors (84%), male donors (86%) versus female donors (100%), tap water as a diluent (99%) versus saline (86%), and volume of FMT infusate greater than 500 mL (97%) versus less than 500 mL (80%). Although these findings are interesting, it is not possible to draw any definite conclusions, as that would require controlled trials examining each of the variables.
Regardless of this variability, 92% of patients overall had resolution of their recurrent CDI after one or, from these data, more treatments. After only 1 treatment, 89% had resolution of symptoms. Of the 4% of patients who had relapsed CDI after the first FMT, 87.5% had resolution after one or more repeat FMTs.
A second review by Kassam and colleagues38 in 2013 was more limited in scope as the investigators included only completed, published studies that were peer reviewed and had a sample size of 10 or more patients. Similar to the review by Gough and colleagues,37 of the 273 patients included in this review, 89.7% had resolution of CDI with FMT. A subgroup analysis showed that FMT into the lower GI tract had a higher resolution rate (91.4%) than FMT into the upper GI tract (82.3%).
Another systematic review of FMT in general, not just for CDI, reinforced the overall efficacy and benign safety profile of FMT for CDI.43 Other individual case series and reports not included in these reviews have been published, but they are all similar in finding an excellent resolution rate for CDI treated by FMT.39,44–48
Clinical Trials
In 2013, van Nood and colleagues published the first randomized controlled trial on FMT for recurrent CDI via duodenal infusion. Patients were randomized to receive vancomycin for 5 days followed by FMT (n = 16), vancomycin alone for 14 days (n = 13), or vancomycin for 14 days with bowel lavage (rapid administration of a large volume of polyethylene glycol solution) (n = 13). The primary outcome was cure defined as absence of diarrhea or persistent diarrhea from another cause, with 3 consecutive stool tests negative for C difficile toxin. The study was stopped early after an interim analysis, as 94% of patients in the FMT group achieved cure (81% were cured after 1 infusion) versus 31% or 23% in the vancomycin alone or vancomycin with bowel lavage groups, respectively. Based on these findings, off-protocol FMT was offered to 18 patients in the other treatment arms, and this achieved an 83% cure rate.
In a pilot trial published by Youngster and colleagues,49 patients were randomized to receive FMT via either colonoscopy or nasogastric tube from a frozen fecal suspension. A total of only 10 patients in each arm were enrolled, but they did not show a statistically significant difference in efficacy between administration routes. Following this, Youngster and colleagues40 conducted an open-label feasibility study using frozen fecal capsules for FMT in 20 patients with 3 or more episodes of CDI and failure of vancomycin taper(s) or 2 or more episodes of severe CDI requiring hospitalization. Resolution occurred in 14 (70%) patients after a single treatment, and 4 of the 6 non-responders had resolution on retreatment for an overall efficacy of 90%. There are several other clinical trials studying FMT for CDI underway that are not yet completed.41,50–54
Other Clostridium difficile Infection-Related Indications
The success of FMT in treating recurrent CDI has spurred interest in its role in treating primary CDI or severe CDI. Few data exist for the use of FMT in primary CDI. Lofgren and colleagues55 constructed a mathematical model of CDI in an ICU and assessed the role of various treatments, including FMT, on primary CDI. The investigators showed that, compared with conventional treatments the model predicted a decreased median incidence of recurrent CDI in patients with primary CDI treated by FMT. In addition to being a mathematical model and not a real-world study, there were several limitations to the model itself that make it difficult to draw general conclusions about FMT for primary CDI.56 FMT for severe disease also has been little described in the literature. Although several published case reports suggest that it is effective,57–60 one recent documented death after FMT for severe CDI underscores the need for more research into the safety and efficacy of FMT for this indication.61
COMPLICATIONS AND CONCERNS WITH FECAL MICROBIOTA TRANSPLANTATION FOR CLOSTRIDIUM DIFFICILE INFECTION
Short-term Complications
In all the published literature noted earlier, there were no serious adverse effects directly attributable to FMT, but symptoms of an irritable colon (constipation, diarrhea, cramping, bloating) were reported shortly after FMT and were usually transient (<48 hours).37 In the special population of immunocompromised patients, CDI has demonstrated safety overall; however, patients with IBD may be at increased risk of adverse events. A recent case series focusing on immunocompromised patients reported that 14% of patients with IBD experienced a disease flare after FMT for CDI, some requiring hospitalization.45 No cases of infectious complications such as septicemia were reported. Other studies have also found an increased risk of IBD flare or other symptoms such as fever and elevated inflammatory markers after FMT, both for CDI and for other indications.62–64 Deaths involving FMT have been reported: one death occurred from aspiration pneumonia during sedation for colonoscopy for FMT,45 although this was not directly related to the procedure. In a more concerning case, one patient with severe CDI failed FMT and died afterward from toxic megacolon and shock, although it is uncertain whether and to what degree FMT or withdrawal of antibiotics with activity against CDI after FMT contributed to the outcome.61 Although some evidence for safety exists, FMT is largely untested in patients with severe CDI57–60 and the fatal case of toxic megacolon noted earlier is of concern. One study of a single-agent probiotic in critically ill patients with acute pancreatitis found an increase in mortality,65 raising the concern for use of probiotics or FMT in patients in the ICU. Further research is needed to determine if FMT for severe CDI has an acceptable safety profile.
Long-term Complications
Although safe in most patients in the short run, the long-term safety profile of FMT has yet to be established. Some of the concern over the safety of FMT stems from an incomplete understanding of the complex interplay between the specific composition of the gut microbiome and the host. The intestinal microbiota has been associated with colon cancer, diabetes, obesity, and atopic disorders such as asthma.66 Whether FMT can place the recipient at increased risk of developing these conditions and if proper screening and selection of donor stool can mitigate such risk are unknown.
One study evaluated 77 patients in terms of efficacy and safety 3 to 68 months (mean 17 months) after FMT.67 Although the scope of the primary survey was limited to symptoms and recurrence of CDI, some patients did report the development of new conditions, including autoimmune disease, ovarian cancer, myocardial infarction, and stroke. These concerns underscore the need for more longitudinal studies on patients who have undergone FMT.
Regulatory Environment
FMT has now become accepted and is in widespread use, drawing the attention of the United States Department of Health and Human Services’ FDA in 2013, which published guidance suggesting that stool should be regulated as a biologic agent.68 The FDA feels that an Investigational New Drug application is required for FMT but intends to exercise enforcement discretion in certain use situations, such as treatment of recurrent CDI. The FDA is planning to revise this guidance statement in 2014 but has not yet finalized the policy.42
SUMMARY
Both among physicians and patients, there has been a growing acceptance of FMT as a viable treatment option for recurrent CDI.26,69 Performing FMT does involve choosing among numerous specific parameters and navigating some logistic hurdles. These include informed consent; donor type, selection, and screening; preparation of the stool for infusion; and route of administration. Despite the numerous options for these parameters, the overall efficacy of FMT remains high. Although in the short term FMT seems to be safe, the long-term safety has not yet been established. Fewer data on the use of FMT for primary CDI or severe CDI exist. The regulatory status of FMT in the United States, especially concerning stool banks, is not solidly defined and remains in flux, but this has not discouraged its widespread use.
KEY POINTS.
Disruption of the gut microbiome is a prerequisite for Clostridium difficile infection (CDI) and can persist after treatment.
C difficile can cause recurrent infection that can be difficult to manage with conventional treatments, which do not restore the microbiome to a healthy state.
Fecal microbiota transplantation (FMT), which takes stool from a healthy donor and infuses it into the gastrointestinal (GI) tract of the recipient, is highly effective in treating recurrent CDI and is safe in the short term.
Despite numerous protocols with significant variation in the stool sources, methods for preparation, and routes of instillation, the effectiveness of FMT generally remains high.
The long-term safety of FMT has not been established, and changes in the microbiome have been associated with several medical conditions.
Acknowledgments
Funding: This work was supported by grants from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant number U19-AI090871), the Claude D. Pepper Older Americans Independence Center (grant number AG-024824), and the Michigan Institute for Clinical and Health Research (grant number 2UL1TR000433). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Footnotes
Conflicts of interest: Authors have no reported conflicts.
REFERENCES
- 1.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van der Waaij D, Berghuis-de Vries JM, Lekkerkerk-van der Wees JE. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. Epidemiol Infect. 1971;69(03):405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vollaard E, Clasener H. Colonization resistance. Antimicrob Agents Chemother. 1994;38(3):409. doi: 10.1128/aac.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology. 2014;146(6):1547–1553. doi: 10.1053/j.gastro.2014.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theriot CM, Koenigsknecht MJ, Carlson PE, Jr, et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samore MH, DeGirolami PC, Tlucko A, et al. Clostridium difficile colonization and diarrhea at a tertiary care hospital. Clin Infect Dis. 1994;18(2):181–187. doi: 10.1093/clinids/18.2.181. [DOI] [PubMed] [Google Scholar]
- 7.Riggs MM, Sethi AK, Zabarsky TF, et al. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis. 2007;45(8):992–998. doi: 10.1086/521854. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett JG. Treatment of antibiotic-associated pseudomembranous colitis. Rev Infect Dis. 1984;6(Suppl 1):S235–S241. doi: 10.1093/clinids/6.supplement_1.s235. [DOI] [PubMed] [Google Scholar]
- 9.Olson MM, Shanholtzer CJ, Lee JT, Jr, et al. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982–1991. Infect Control Hosp Epidemiol. 1994;15(6):371–381. doi: 10.1086/646934. [DOI] [PubMed] [Google Scholar]
- 10.Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 11.McDonald LC, Coignard B, Dubberke E, et al. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007;28(2):140–145. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 12.Figueroa I, Johnson S, Sambol SP, et al. Relapse versus reinfection: recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin. Clin Infect Dis. 2012;55(Suppl 2):S104–S109. doi: 10.1093/cid/cis357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eyre DW, Babakhani F, Griffiths D, et al. Whole-genome sequencing demonstrates that fidaxomicin is superior to vancomycin for preventing reinfection and relapse of infection with Clostridium difficile. J Infect Dis. 2014;209(9):1446–1451. doi: 10.1093/infdis/jit598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe. 2009;15(6):285–289. doi: 10.1016/j.anaerobe.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Huebner ES, Surawicz CM. Treatment of recurrent Clostridium difficile diarrhea. Gastroenterol Hepatol. 2006;2(3):203–208. [PMC free article] [PubMed] [Google Scholar]
- 16.Borody TJ, Warren EF, Leis SM, et al. Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol. 2004;38(6):475–483. doi: 10.1097/01.mcg.0000128988.13808.dc. [DOI] [PubMed] [Google Scholar]
- 17.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 18.Garey KW, Ghantoji SS, Shah DN, et al. A randomized, double-blind, placebo-controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhoea in patients with Clostridium difficile infection. J Antimicrob Chemother. 2011;66(12):2850–2855. doi: 10.1093/jac/dkr377. [DOI] [PubMed] [Google Scholar]
- 19.Johnson S, Gerding DN. Fidaxomicin ‘chaser’ regimen following vancomycin for patients with multiple C difficile recurrences. Clin Infect Dis. 2012;56(2):309–310. doi: 10.1093/cid/cis833. [DOI] [PubMed] [Google Scholar]
- 20.Kyne L, Warny M, Qamar A, et al. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251):189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 21.Crook DW, Walker AS, Kean Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis. 2012;55(Suppl 2):S93–S103. doi: 10.1093/cid/cis499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abou Chakra CN, Pepin J, Sirard S, et al. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS One. 2014;9(6):e98400. doi: 10.1371/journal.pone.0098400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile–associated diarrhea. J Infect Dis. 2008;197(3):435–438. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 24.O’Horo J, Safdar N. The role of immunoglobulin for the treatment of Clostridium difficile infection: a systematic review. Int J Infect Dis. 2009;13(6):663–667. doi: 10.1016/j.ijid.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55(Suppl 2):S154–S161. doi: 10.1093/cid/cis462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakken JS, Polgreen PM, Beekmann SE, et al. Treatment approaches including fecal microbiota transplantation for recurrent Clostridium difficile infection (RCDI) among infectious disease physicians. Anaerobe. 2013;24:20–24. doi: 10.1016/j.anaerobe.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Britton RA, Young VB. Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends Microbiol. 2012;20(7):313–319. doi: 10.1016/j.tim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seekatz AM, Aas J, Gessert CE, et al. Recovery of the gut microbiome following fecal microbiota transplantation. MBio. 2014;5(3):e00893–e00914. doi: 10.1128/mBio.00893-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weingarden AR, Chen C, Bobr A, et al. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol. 2014;306(4):G310–G319. doi: 10.1152/ajpgi.00282.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwan A, Sjolin S, Trottestam U, et al. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet. 1983;322(8354):845. doi: 10.1016/s0140-6736(83)90753-5. [DOI] [PubMed] [Google Scholar]
- 31.Zhang F, Luo W, Shi Y, et al. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107(11):1755. doi: 10.1038/ajg.2012.251. [DOI] [PubMed] [Google Scholar]
- 32.Eiseman B, Silen W, Bascom GS, et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–859. [PubMed] [Google Scholar]
- 33.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36(5):580–585. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- 34.Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile–associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol. 2000;95(11):3283–3285. doi: 10.1111/j.1572-0241.2000.03302.x. [DOI] [PubMed] [Google Scholar]
- 35.Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010;8(5):471–473. doi: 10.1016/j.cgh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53(10):994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 38.Kassam Z, Lee CH, Yuan Y, et al. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108(4):500–508. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton MJ, Weingarden AR, Sadowsky MJ, et al. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(5):761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 40.Youngster I, Russell GH, Pindar C, et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312(17):1772–1778. doi: 10.1001/jama.2014.13875. http://dx.doi.org/10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 41.Rebiotix Inc. ClinicalTrials.gov. [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [Accessed July 1, 2014]. Microbiota restoration therapy for recurrent Clostridium difficile-associated diarrhea (PUNCH CD) Available at: http://clinicaltrials.gov/ct2/show/NCT01925417. NLM Identifier: NCT01925417. [Google Scholar]
- 42.Draft guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. [Accessed July 1, 2014];U.S. Department of Health and Human Services, Food and Drug Administration. Available at: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm387023.htm.
- 43.Sha S, Liang J, Chen M, et al. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther. 2014;39(10):1003–1032. doi: 10.1111/apt.12699. [DOI] [PubMed] [Google Scholar]
- 44.Kassam Z, Hundal R, Marshall JK, et al. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med. 2012;172(2):191–193. doi: 10.1001/archinte.172.2.191. [DOI] [PubMed] [Google Scholar]
- 45.Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dutta SK, Girotra M, Garg S, et al. Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2014;12(9):1572–1576. doi: 10.1016/j.cgh.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 47.Friedman-Moraco RJ, Mehta AK, Lyon GM, et al. Fecal microbiota transplantation for refractory Clostridium difficile colitis in solid organ transplant recipients. Am J Transplant. 2014;14(2):477–480. doi: 10.1111/ajt.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emanuelsson F, Claesson BE, Ljungström L, et al. Faecal microbiota transplantation and bacteriotherapy for recurrent Clostridium difficile infection: a retrospective evaluation of 31 patients. Scand J Infect Dis. 2014;46(2):89–97. doi: 10.3109/00365548.2013.858181. [DOI] [PubMed] [Google Scholar]
- 49.Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58(11):1515–1522. doi: 10.1093/cid/ciu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.University Health Network Toronto. ClinicalTrials.gov. [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [Accessed July 1, 2014]. Oral vancomycin followed by fecal transplant versus tapering oral vancomycin. Available at: http://clinicaltrials.gov/ct2/show/NCT01226992. NLM Identifier: NCT01226992. [Google Scholar]
- 51.Tel-Aviv Sourasky Medical Center. ClinicalTrials.gov. [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [Accessed July 1, 2014]. Transplantation of fecal microbiota for Clostridium difficile infection. Available at: http://clinicaltrials.gov/ct2/show/NCT01958463. NLM Identifier: NCT01958463. [Google Scholar]
- 52.Hadassah Medical Organization. ClinicalTrials.gov. [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [Accessed July 1, 2014]. Efficacy and safety of fecal microbiota transplantation for severe Clostridium difficile associated colitis. Available at: http://clinicaltrials.gov/ct2/show/NCT01959048. NLM Identifier: NCT01959048. [Google Scholar]
- 53.University Hospital Tuebingen. ClinicalTrials.gov. [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [Accessed July 1, 2014]. Fecal microbiota transplantation in recurrent or refractory Clostridium difficile colitis (TOCSIN) Available at: http://clinicaltrials.gov/ct2/show/NCT01942447. NLM Identifier: NCT01942447. [Google Scholar]
- 54.Duke University. ClinicalTrials.gov. [Internet] Bethesda (MD): National Library of Medicine (US); 2000. [Accessed July 1, 2014]. Stool transplants to treat refractory Clostridium difficile colitis. Available at: http://clinicaltrials.gov/ct2/show/NCT02127398. NLM Identifier: NCT02127398. [Google Scholar]
- 55.Lofgren ET, Moehring RW, Anderson DJ, et al. A mathematical model to evaluate the routine use of fecal microbiota transplantation to prevent incident and recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;35(1):18–27. doi: 10.1086/674394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rao K, Young VB, Aronoff DM. Commentary: fecal microbiota therapy: ready for prime time? Infect Control Hosp Epidemiol. 2014;35(1):28–30. doi: 10.1086/674395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neemann K, Eichele DD, Smith PW, et al. Fecal microbiota transplantation for fulminant Clostridium difficile infection in an allogeneic stem cell transplant patient. Transpl Infect Dis. 2012;14(6):E161–E165. doi: 10.1111/tid.12017. [DOI] [PubMed] [Google Scholar]
- 58.Trubiano JA, Gardiner B, Kwong JC, et al. Faecal microbiota transplantation for severe Clostridium difficile infection in the intensive care unit. Eur J Gastroenterol Hepatol. 2013;25(2):255–257. doi: 10.1097/MEG.0b013e32835b2da9. [DOI] [PubMed] [Google Scholar]
- 59.Gallegos-Orozco J, Paskvan-Gawryletz C, Gurudu S, et al. Successful colonoscopic fecal transplant for severe acute Clostridium difficile pseudomembranous colitis. Rev Gastroenterol Mex. 2011;77(1):40–42. [PubMed] [Google Scholar]
- 60.You DM, Franzos MA, Holman RP. Successful treatment of fulminant Clostridium difficile infection with fecal bacteriotherapy. Ann Intern Med. 2008;148(8):632–633. doi: 10.7326/0003-4819-148-8-200804150-00024. [DOI] [PubMed] [Google Scholar]
- 61.Solari PR, Fairchild PG, Noa LJ, et al. Tempered enthusiasm for fecal transplant. Clin Infect Dis. 2014;59(2):319. doi: 10.1093/cid/ciu278. [DOI] [PubMed] [Google Scholar]
- 62.De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11(8):1036–1038. doi: 10.1016/j.cgh.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 63.Angelberger S, Reinisch W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108(10):1620–1630. doi: 10.1038/ajg.2013.257. [DOI] [PubMed] [Google Scholar]
- 64.Kump PK, Gröchenig HP, Lackner S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19(10):2155–2165. doi: 10.1097/MIB.0b013e31829ea325. [DOI] [PubMed] [Google Scholar]
- 65.Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9613):651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 66.Sekirov I, Russell SL, Antunes LC, et al. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 67.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(7):1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 68.Guidance for industry: enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. [Accessed July 1, 2014];U.S. Department of Health and Human Services, Food and Drug Administration. Available at: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm361379.htm.
- 69.Zipursky JS, Sidorsky TI, Freedman CA, et al. Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis. 2012;55(12):1652–1658. doi: 10.1093/cid/cis809. [DOI] [PubMed] [Google Scholar]