Abstract
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency among premature infants. Although a large body of research has focused on understanding its pathogenesis, the exact mechanism has not been elucidated. Of particular interest is the potential causative role of infectious culprits in the development of NEC. A variety of reports describe bacterial, viral, and fungal infections occurring in association with NEC; however, no single organism has emerged as being definitively involved in NEC pathogenesis. In this review, the authors summarize the literature on infectious causes of NEC.
Keywords: Necrotizing enterocolitis, Neonate, Bacteria, Virus, Fungi
Key points
-
•
Necrotizing enterocolitis (NEC) is the most common cause of gastrointestinal morbidity and mortality in premature infants.
-
•
The exact role of microbes in the pathogenesis of NEC is still incompletely understood.
-
•
The presence of specific bacteria, viruses, and fungi has been associated with NEC predominantly in relatively rare outbreak situations.
-
•
Aberrant bacterial colonization seems necessary for NEC development but is unlikely to cause disease by itself.
-
•
Future studies are needed to determine how therapeutic interventions on microbial communities may prevent the development of NEC.
Introduction
Necrotizing enterocolitis (NEC) is the most common surgical emergency in premature infants, affecting approximately 7% of infants with less than 1500 g birth weights.1 Universally described risk factors include prematurity, aberrant microbial colonization, and lack of human milk feeding.2 NEC’s clinical presentation is nonspecific and can range from signs limited to the gastrointestinal (GI) tract (eg, feeding intolerance, ileus, abdominal distention, hematochezia) to catastrophic illness with multiorgan failure (eg, lethargy, apnea, metabolic acidosis, shock, disseminated intravascular coagulopathy) and death.3 Since its first mention in the medical literature more than 150 years ago, NEC has stimulated intensive research in its cause; despite seminal discoveries of epidemiologic and molecular risk factors and pathways, the pathogenesis remains unclear.4 One reason for the lack in progress is inclusion of diseases closely resembling classic NEC as a complication of preterm birth, such as spontaneous intestinal perforation (SIP), NEC in term infants, cow-milk intolerance, and viral enteritis.5
The role of bacteria as significant contributors to NEC has been identified since the first systematic descriptions of this disease.6, 7 Pneumatosis intestinalis and portal venous gas are pathognomonic radiographic signs of NEC8 and thought to be caused by anaerobic bacteria, specifically clostridia.9 Gram-negative bacteria have been most frequently associated with NEC, and the epithelial receptor and innate immune sensor Toll-like receptor (TLR) 4 is elevated in the premature intestine and required for the development of experimental NEC.10, 11 NEC can occur in clusters, and seasonal outbreaks of virus-associated NEC cases have been reported.12, 13, 14, 15, 16 Here the authors attempted to summarize the main published data on the role of microbes in NEC.
Bacteria
Bacteria are clearly involved in the pathogenesis of NEC (Table 1 ); despite the paucity of randomized control trials to determine the optimal antimicrobial regimen in premature infants, treatment with intravenous broad-spectrum antibiotics remains a mainstay of the clinical management.17, 18 However, many open questions remain, including the role of specific bacterial overgrowth as the cause or the consequence of NEC, timing of bacterial colonization during fetal/neonatal development, and type of molecular interactions between different microbes and their host.19 Despite the abundance of bacteria in the premature intestine early in life20 and the clinical appearance of gram-negative sepsis, a positive blood culture is uncommon in infants with NEC.21, 22 This finding is surprising given the frequent growth of bacteria in peritoneal fluid.23 In 80 cases of NEC with intestinal perforation, Enterobacteriaceae were present in the peritoneal fluid in 75% of cases, coagulase-negative Staphylococci (CoNS) in 14%, and anaerobes in 6%.23 Despite similar age at the time of intestinal perforation and similar mortality, the distribution of predominant organisms cultured from peritoneal fluid differed significantly between patients with NEC and SIP. Candida species (44%) and CoNS (50%) dominated samples from 36 patients with SIP.23 Specific bacteria have been suggested as important contributing factors in NEC,24, 25 and NEC occurs typically after the first week post partum after the intestine has been colonized. In contrast, one study on human NEC samples using laser capture microdissection and subsequent sequencing combined with fluorescent in situ hybridization and bacterial rRNA-targeting oligonucleotide probes did not detect dominating potential pathogenic bacteria and suggested that NEC is a “non-infectious syndrome.”9
Table 1.
Infectious causes of NEC
| Bacterial | Viral | Fungal |
|---|---|---|
|
Enterococcus (VRE)205 Escherichia coli22, 114, 116, 117, 118, 119 Klebsiella spp22, 112, 113, 114, 115, 116 Pseudomonas aeruginosa123, 124, 125 Salmonella206, 207 Staphylococcus aureus (MRSA)208 Staphylococcus epidermidis48, 212 Ureaplasma urealyticum25, 128, 129 |
Astrovirus15, 184, 185 Cytomegalovirus163, 164, 165 Coronavirus167, 168 Coxsackievirus B2176, 177 Echovirus180 Human immunodeficiency virus (maternal exposure)186, 187, 188 Norovirus16, 147, 149, 150 Rotavirus14, 133, 134, 135 Torovirus170, 171 |
Candida spp194, 197, 198, 199 |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
Bacteria shape normal immune development including the development of T regulatory cells (Treg), which are critical for reducing inflammation-mediated injury.26, 27, 28, 29 Another example is recruitment of intestinal intraepithelial lymphocytes (IEL) after microbial colonization of germ-free mice.30 IEL are reduced in human NEC suggesting that paucity of normal commensals in the newborn gut may alter intestinal immune development.31 Infectious complications of pregnancy, such as chorioamnionitis, increase the risk for NEC either by direct bacterial colonization or through the anatomic and immunologic changes following the inflammatory challenge of the developing intestine.25, 32, 33, 34, 35, 36 Independent epidemiologic association between chorioamnionitis and NEC is difficult to prove, as chorioamnionitis is also the most important risk factor for prematurity and most severe NEC cases occur in extremely premature infants. However, after adjustment for antenatal steroid prophylaxis, gestational age, and surfactant treatment, the presence of intrauterine infection and the fetal inflammatory response syndrome (FIRS) remained independent predictors for NEC in several studies.32, 33 Increased gastric neutrophil counts have been demonstrated in chorioamnionitis-exposed preterm infants, reflecting a proinflammatory state of the gut shortly after birth.37 Moreover, presence of microbes and inflammatory markers in the gut mirror that of the amniotic fluid when chorioamnionitis is present.38 Preterm labor and chorioamnionitis are also linked with abnormal intestinal development and fetal proliferation of activated T cells in the immature intestinal mucosa.35 At the same time, ileum Treg cell proportions are reduced in chorioamnionitis, whereas activated T effector cells are increased.39, 40 Reduced Treg proportions in the small intestinal lamina propria characterize NEC in human disease and in animal models, suggesting the possibility of bacteria-induced fetal immune priming as a risk factor for NEC.41, 42, 43
Gram-Positive Bacteria
The C-type lectin RegIIIγ and its human counterpart, hepatocarcinoma-intestine-pancreas/pancreatic-associated protein (HIP/PAP), are antimicrobial proteins that bind peptidoglycan, a molecule that is exposed on the surface of gram-positive bacteria. RegIIIγ expression is developmentally regulated and dependent on normal microbial ecology.44 Although the exact role and developmental regulation of HIP/PAP is unknown in human infants, lower levels, especially in preterm infants, could lead to aberrant intestinal colonization with gram-positive bacteria.
Staphylococcus epidermidis
Colonization of the maternal genital tract with Staphylococcus sp has been associated with a significantly increased risk for chorioamnionitis (odds ratio 18.4).33 The small intestine is colonized with staphylococci shortly after birth and in patients with or without NEC, specifically in infants delivered via cesarean section.20, 45 CoNS were found to preferentially translocate through the intestinal wall after ischemia-reperfusion injury in mice.46 Importantly, a lack of enteral nutrition and exposure to total parenteral nutrition alone reduce intestinal barrier function.47 CoNS are frequently cultured from postnatal stool samples and seem to increase the risk for NEC development.48
Clostridia species
Clostridia are spore-forming anaerobic motile gram-positive rods. They can be found in soil and the human GI tract and can be considered part of the normal intestinal flora in newborns, especially premature infants exposed to the neonatal intensive care unit (NICU) environment and infants fed formula.49, 50 Therefore, when isolated during disease, it is difficult to establish if they are pathogens or normal flora.51 However, patients with NEC with positive cultures for Clostridia spp have more extensive pneumatosis intestinalis, a higher incidence of portal venous gas, faster progression to more severe necrosis, and intestinal perforation with higher mortality.52, 53 Clostridium spp were significantly more prevalent among samples from a preterm piglet model of NEC.54 Clostridia spp have been implicated in NEC for many years because the clinical presentation of diseases caused by these toxin-producing strains often resemble NEC. For example, pseudomembranous colitis as a result of overgrowth of toxin-producing Clostridium difficile in the colon can present with hematochezia and multiorgan failure.55 Enteritis necroticans, known as pigbel in Papua New Guinea, is a segmental necrotizing infection of the jejunum and ileum caused by Clostridium perfringens, type C.56, 57
Clostridium perfringens
Clostridium perfringens frequently colonizes the intestine of preterm infants within the first 2 weeks post partum.58 Clostridium perfringens types A to E form 12 different toxins: major toxins (eg, α-toxin = phospholipase C), collagenase, protease, hyaluronidase, deoxyribonuclease, enterotoxin, and neuraminidase.59 Clostridium perfringens α-toxin is produced by all 5 types of bacteria (A–E); increases capillary permeability; induces platelet aggregation, hemolysis, and myonecrosis; decreases cardiac contractility; and is lethal.60 Clostridium perfringens was identified as a causative agent of NEC in 22% of cases in one study.61 Compared with the control group (n = 32), the onset of disease was earlier in life, portal venous gas was more common (77%), the clinical course was more severe, and the mortality rate was more than twice as high (44%).61 Another study isolated Clostridium perfringens in patients with fatal outcomes and suggested it has the potential to trigger a fulminant and often lethal course.62 Clostridium perfringens has been declared as a possible risk factor for NEC as it was recognized by molecular techniques in the first 2 weeks post partum in 3 infants who later developed the disease.63 In one study, Clostridium perfringens was isolated from intestinal flora in 40% of infants with NEC compared with 13% of controls (P = .03)64 and has been associated with an NICU outbreak of NEC in another.65 Clostridium perfringens has also been associated with NEC in several animal models.66, 67, 68
Clostridium difficile
Clostridium difficile is part of the commensal intestinal flora in humans but has recently attracted the attention of researchers because of its role as the most common cause of severe and refractory health care–associated diarrhea.69 After intestinal overgrowth following antimicrobial use, toxigenic strains can cause pseudomembranous colitis, ranging from mild diarrhea to fulminant colitis. Clostridium difficile’s 2 major toxins, Clostridium difficile toxin A (TcdA) and Clostridium difficile toxin B (TcdB), disrupt host cell function by inactivating small GTPases that regulate the actin cytoskeleton.70 Both toxins can manifest disease on their own.71 During infancy, asymptomatic colonization with toxin-producing Clostridium difficile is common and has been associated with changes in the intestinal microbiome composition.58, 72, 73, 74, 75 Delivery or exposure to human flora has no effect on colonization, and Clostridium difficile originates from the NICU environment rather than maternal transmission.76, 77 The involvement of Clostridium difficile in NEC is controversial because toxin-producing Clostridium difficile strains are not more frequently recovered in NEC.78 However, Clostridium difficile–associated NEC cases have been described during a Clostridium difficile outbreak.79, 80
Clostridium butyricum
Clostridium butyricum produces butyric acid through fermentation and a specific strain (MIYAIRI 588 strain of Clostridium butyricum) is widely used as a probiotic in Asia.81 It can be isolated from soil, feces of healthy children and adults, as well as soured milk and cheeses. Type E can produce a neurotoxin and has been implicated in cases of botulism.82 Several reports state isolation of toxin-producing Clostridium butyricum from peritoneal fluid, blood, and cerebrospinal fluid of patients with NEC.83 Clostridium butyricum has been suggested as the primary cause of NEC in outbreak situations; but because of a lack of adequate controls, its primary role has been questioned.84, 85 Isolation of Clostridium butyricum in blood samples of infants with NEC may have resulted from mucosal breakdown and transmigration of these bacteria into the bloodstream.86 In a community analysis of bacteria found in tissue specimens from infants with NEC, the presence of Clostridium butyricum and Clostridium parputrificum highly correlated with histologic pneumatosis intestinalis.21 Clostridium butyricum strains isolated from NEC cases can cause cecal lesions in animals with gas cysts, hemorrhagic ulceration, and necrosis.87, 88, 89 Lactose fermentation and production of butyric acid seem to be a prerequisite, and colonization with bifidobacterium was protective.67, 90 Attachment of Clostridium butyricum to the ileal mucosa has been associated with NEC in preterm, cesarean-derived, and formula-fed piglets.54
Gram-Negative Bacteria
Cronobacter sakazakii
With a reported incidence of one infection per 10,660 very low birth weight (VLBW, <1500 g) infants,91 Cronobacter sakazakii (formerly Enterobacter sakazakii)92, 93 infection is rare. Cronobacter sakazakii has been isolated from powdered infant formula worldwide,94, 95 and NICU outbreaks of invasive disease have been reported.96, 97, 98, 99, 100 Meningitis is the most prominent clinical manifestation,101 but outbreaks of NEC occurred in NICUs with isolation of Cronobacter sakazakii from multiple patients’ body fluids and cans with powdered infant formula.102, 103 Cronobacter sakazakii is commonly found in soil, food items, and other environmental sources.104 Therefore, inappropriate hygiene practices including storage, temperature control, and hand, nipple, and bottle cleaning after powdered formula reconstitution may contribute to infection. Powdered formula is not a sterile product, and the World Health Organization recommends formula reconstitution with hot water (>70°C) (http://www.who.int/foodsafety/publications/micro/pif2007/en/).
Cronobacter sakazakii binds to villi in the distal small intestine and can induce NEC from a direct toxic effect to gut epithelium in the rat pup model.105 Cronobacter sakazakii’s best-characterized virulence factor, outer membrane protein A (ompA), binds and invades human epithelial cells103 and brain endothelial cells,106, 107, 108 whereas its enterotoxin functions similarly to lipopolysaccharide (LPS) and modulates the activation of TLR 4.109 OmpA also mediates recruitment of dendritic cells at the expense of neutrophils and macrophages leading to epithelial injury through transforming growth factor-β production and iNOS activation.110, 111
Klebsiella species
Klebsiella sp have been described in NEC outbreaks with nosocomial origin.112, 113 It is also one of the most common organisms responsible for bacteremia in NEC.22, 114, 115 A 1998 outbreak in Johannesburg was significant for isolation of a single clone of an extended-spectrum beta-lactamase–producing Klebsiella in blood cultures of patients with NEC, notable for sudden decompensation leading to shock and severe thrombocytopenia in all cases and for the absence of diarrhea or hematochezia.112
Escherichia coli
E coli is a similarly common organism found in normal gut flora; among infants with NEC, it has been isolated in blood in up to one-third of cases.22, 114 Both E coli and Klebsiella were isolated in feces at markedly higher rates in infants with NEC than those without.116 Several outbreaks of NEC associated with E coli have been described.117, 118 In one report, 15 of 16 infants with suspected or confirmed NEC had either enterotoxigenic E coli or its heat-labile enterotoxin recovered in stool.118 A report of NEC associated with E coli O157:H7 in a term infant resulted in death secondary to widespread intestinal necrosis.119
Pseudomonas
Pseudomonas is well known for its role in nosocomial and immunocompromised infections. It forms biofilms and can colonize hard surfaces and respiratory equipment, with mechanical ventilation as a risk factor for infection. However, Pseudomonas also colonizes the GI tracts of 10% to 42% of newborns120, 121 and 25% to 35% of normal adults.122 Among VLBW infants, it is primarily responsible for late-onset disease (sepsis, pneumonia, NEC). There are several reports of Pseudomonas-associated NEC. A Taiwanese study reports 45 infants with Pseudomonas in the stool, of whom one had NEC, 4 had colonic perforations, and 2 infants died of sepsis.123 Other studies noted an increased rate of NEC in infants with Pseudomonas bacteremia compared with nonbacteremic infants (36% vs 7%, respectively) and with it a much higher mortality rate (up to 50%), especially when signs of septic shock were present.124, 125
Atypical Bacteria
Unique in their lack of a cell wall, Ureaplasma are obligate intracellular mycoplasma that colonize human adult genital tracts. They may be vertically transmitted intrapartum, with nasopharyngeal colonization reported among 22% of NICU patients.126 Colonization is associated with chorioamnionitis,127 a known risk factor for NEC. However, the existence of a direct relationship between colonization with Ureaplasma and development of NEC is controversial. One study found a 2-fold increase in incidence of stage 2 or greater NEC associated with elevated interleukin (IL)-6 and IL-1 beta among infants colonized with Ureaplasma (12.3% vs 5.5%).25 Two other groups disagreed and found no increased incidence of NEC associated with Ureaplasma colonization.128, 129
Viruses
Rotavirus
A double-stranded DNA member of the Reovirus family, rotavirus causes GI disease by invading enterocytes and disrupting their absorptive and digestive activities.130 Fecal excretion of rotavirus can be found in up to half of infants in the newborn nursery.131 Although most infants shed the virus asymptomatically, 8% to 30% of infants present with vomiting and diarrhea.131, 132 Rotavirus infection tends to peak in the late winter/early spring, though introduction of a vaccine in young children has interrupted this seasonality.130 Several outbreaks of NEC have been associated with rotavirus infection with virus isolated from stool or serologic diagnosis and concomitant evidence of infection among a significant portion of NICU staff members.133, 134 Risk factors for the development of serious GI disease included low birth weight and younger age.133 Notably, rotavirus-associated NEC has been found to cause less severe disease compared with NEC without rotavirus.14 Anatomic distinctions were also noted: left-sided, more distal colonic pneumatosis intestinalis in rotavirus NEC compared with right-sided, ileal pneumatosis in nonrotavirus NEC.14, 135
Norovirus
Norovirus (Norwalk virus), a nonenveloped positive-sense single-stranded RNA virus,136 is the most important cause of foodborne outbreaks of gastroenteritis137, 138 and the second leading cause (after rotavirus) of gastroenteritis in young children.139 Both individual infections and outbreaks most commonly occur during winter.98 Seventeen percent of 75 premature infants less than 32 weeks’ gestation shed the virus in their stool over the first 4 weeks after admission in a NICU in Sydney.140 Norovirus prevalence was 1.9%, representing roughly half of all infants in that cohort who shed the virus.140 Controversy exists regarding the best methods for viral identification, with one report noting several norovirus-positive cases by enzyme-linked immunosorbent assay that were not corroborated by reverse transcription polymerase chain reaction or electron microscopy.141 The specificity of each aforementioned method is reportedly greater than 90%, and positivity in 2 of the 3 tests confirms norovirus infection.142 Norovirus has been thought to primarily affect the small intestine based on pathologic findings of villus blunting, crypt hypertrophy, and edema among adults infected with norovirus143, 144, 145 and mononuclear infiltrate and apoptosis in the jejunum and ileum of pediatric small bowel transplant recipients.146 However, a recent report described 3 premature infants with norovirus infections with radiographic evidence of extensive colonic pneumatosis and pathologic insult (fibrosis and hyperplastic vessels) limited to the colon.147 Apnea was noted as the primary presentation of norovirus infection in a preterm infant who subsequently developed watery diarrhea and positive stool cultures.148 Several small outbreaks associated with NEC have been described. The largest involved 8 cases of NEC with a 25% mortality rate and noted that, in comparison with nonoutbreak NEC cases, those associated with norovirus had significantly lower levels of neutrophil band forms.149 An outbreak of 8 cases of norovirus among premature infants was marked by abdominal distention, apnea, and increased gastric residuals. Vomiting and acute diarrhea were not predominant clinical features (27% and 0%, respectively), but one infant with proven norovirus developed NEC.150 A case-control study noted an increased prevalence of norovirus in stools of infants with NEC compared with non-NEC controls (40% vs 9%, respectively) and suggested an etiologic role of norovirus in the pathogenesis of NEC.16
Cytomegalovirus
Cytomegalovirus (CMV), a double-stranded DNA herpesvirus, is well known to cause serious neonatal disease in its congenital form but has also been implicated in NEC. CMV transmission may occur via transplacental, intrapartum, or postpartum routes. Rates of perinatal CMV infection in premature infants have been reported to be as high as 15% to 20%.151, 152 Given their immunocompromised status, premature infants are at particular risk for postnatal infection from breast milk (transmission rates 5%–37%)153, 154, 155 or via transfused blood products.156, 157, 158, 159 Among immunocompromised patients, CMV enteritis is common and marked by diarrhea, hematochezia, and toxic megacolon.160 In infants, however, CMV enteritis is unusual; the virus is a disputed player in the development of NEC. Patients may present with diarrhea or with disease resembling NEC but without distinguishing features, such as intestinal pneumatosis.161 However, several case reports linking confirmed cases of NEC to CMV infections have been reported, with clinical manifestations including abdominal compartment syndrome,162 viremia and sepsis,163 and colonic strictures.164 In one particularly severe case, fulminant NEC leading to death was associated with stool culture positive for CMV but with a notable reduction in diversity of bacterial flora, prompting speculation that intestinal CMV infection may predispose infants to NEC by altering intestinal immune responses and promoting secondary bacterial infection.165
Coronavirus (Torovirus)
Coronaviruses are enveloped viruses with positive-sense RNA genomes that are known to cause respiratory166 and serious GI disease among infants.167, 168 A coronavirus outbreak was associated with hemorrhagic NEC, and viral particles were visualized both in intestinal and fecal specimens.168 In another outbreak of NEC, coronavirus was detected in stool in 23 of 32 (72%) infants. Sixty percent of bedside nurses also shed the virus in stool, prompting speculation of nosocomial transmission.167 As members of the coronavirus family, toroviruses are a known agent of diarrhea in cattle and horses and have been associated with GI disease in children. Torovirus infections are known to occur year-round, with a substantial portion thought to be acquired nosocomially.169 Its association in neonatal disease was first described in 1982, when “virus-like particles” similar to coronavirus were detected in the stool of 80% of infants in an outbreak of bloody diarrhea, bilious gastric aspirates, and abdominal distention.170 Transmission was thought to be vertical because all but one mother had flulike or GI symptoms within 2 weeks of delivery, and viral particles were detected in meconium of several infants.170 One study reported the detection of torovirus in the stools of 48% of its patients with NEC and in 60% of those with stage III disease, although the presence or absence of torovirus did not affect mortality.171
Enteroviruses
Enteroviruses are positive-sense, single-stranded RNA viruses encompassing multiple serotypes, including 2 specific viruses that have been associated with NEC: coxsackievirus and echovirus. These infections are seasonal, with most cases occurring during summer and fall.172, 173, 174, 175 Among 27 infants with enterovirus, 3 had NEC marked by fevers, abdominal distention, and bloody diarrhea and one had coxsackievirus B and died following exploratory surgery revealing dusky jejunum.176 Another fatal case of NEC associated with widespread coxsackievirus B infection demonstrated ischemic ileum with subserosal hemorrhage; the child’s parents were both febrile at the time of birth.177 The clinical presentation of echovirus infection among premature infants ranges from asymptomatic to diarrheal illness178 to upper and lower respiratory tract infections.179 An outbreak of echovirus type 22 in a NICU resulted in a diarrheal illness among 12 premature infants, 6 (50%) of whom developed stage I NEC and one had pneumatosis intestinalis, but all survived. Identification of echovirus was via stool culture in most infants, though a few only had increased serum antibody titers.180
Astrovirus
As single-stranded RNA viruses, astroviruses were first described in infants during an outbreak of gastroenteritis in a newborn nursery.181 Few reports on the pathology of human astrovirus infection are available, but one study in a child following bone marrow transplant revealed villous blunting and inflammatory cell infiltrate in the duodenum and jejunum (not consistent with graft-versus-host disease).182 Alternatively, intestinal astrovirus infection in a turkey model leads to rearrangement of the actin cytoskeleton on ultrastructural examination and evidence of sodium malabsorption secondary to redistribution of sodium-hydrogen exchangers.183 There are multiple reports associating astrovirus with NEC.15, 184, 185 One report detected astrovirus in the stools of 6% of infants with either gastroenteritis or NEC. Infants with astrovirus more frequently acquired NEC (9 of 14) than those with norovirus (1 of 8) or rotavirus (2 of 12).184 Compared with uninfected infants, those with astrovirus had increased hematochezia (54% vs 15%) and Bell stage II and III NEC (21% vs 4%).185
Human Immunodeficiency Virus
One case-control study suggests that maternal human immunodeficiency virus (HIV) infection places premature infants at higher risk for the development of NEC (at a rate of 8.8% vs 1.2% in children of HIV-positive and HIV-negative mothers, respectively).186 Additional case reports describe development of NEC in 2 infants born to HIV-positive mothers; one infant also had trisomy 21.187, 188 All HIV-positive mothers received antiretroviral drugs during pregnancy and/or labor, and all infants received antiretrovirals after birth; no infant was HIV positive. The investigators speculate that the reduced production of IL-12 and/or use of zidovudine may have predisposed infants of HIV-positive mothers to NEC.186
Fungi
Candida
Candida is a classically dimorphic organism that produces both yeast and hyphal forms, though certain species differ slightly (Candida glabrata does not form hyphae, and Candida parapsilosis forms pseudohyphae). In VLBW infants, fungal colonization occurs in the first week of life at an estimated rate of 27%, with Candida spp making up most of the organisms.189 Candida albicans is isolated in more than 60% of cases of candidemia.190 Among the NICU population, the risk factors for both colonization and invasive disease include the use of central venous lines, intravenous lipids, and histamine H2 receptor antagonists.191, 192, 193 It is unclear whether intestinal colonization with Candida spp is protective or a risk factor for NEC. In one study, none of 7 infants with NEC had viable fungal organisms detected in stool.194 Further complicating the picture is the frequent association of Candida with SIP.195, 196 When Candida is linked to NEC, however, the results can be severe197; 27% of fatal cases of surgically treated NEC were associated with Candida sepsis, an outcome complicated by late diagnosis occurring either within 48 hours of death or at autopsy.198 On pathologic examination of 84 patients with NEC, yeast and pseudohyphae were detected in both the intestinal lumen and wall.199 Antifungal prophylaxis has been shown in a randomized controlled trial200 to have benefit in reducing both colonization and invasive disease201, 202 but not NEC201 or overall mortality.203
Summary
NEC is a common and devastating problem for premature infants. Bacterial colonization seems to be a necessary but not sufficient contributor to NEC. Although intestinal pathogens may cause NEC-like illness in animal models or occasional clinical outbreaks, they are not detected in most cases of classic NEC.204 NEC outbreaks have been associated with clusters of viral GI infections, but the clinical presentation may vary and often affect the large intestine. Future studies are needed to determine the impact of host-specific GI tract microbial communities on the development of NEC.
Best practices.
What is the current practice?
NEC
The guidelines of the Surgical Infection Society and the Infectious Diseases Society of America recommend fluid resuscitation, bowel decompression, antimicrobial therapy, and surgical intervention (laparotomy or drainage) if needed.18 Recommended antibiotics for complicated intra-abdominal infections in infants include combinations of ampicillin, gentamicin, and cefotaxime with or without anaerobic coverage with metronidazole, piperacillin-tazobactam, or meropenem.17, 18 In spite of their frequent use, the safety and efficacy of various antimicrobial combination treatment strategies for NEC has not been established in randomized controlled trials. The American Pediatric Surgical Association Outcomes and Clinical Trials Committee advises probiotics to decrease the incidence of NEC, and human milk should be used when possible.209 They conclude that there is a lack of evidence-based data to support definitive recommendations for the type of surgical treatment or length of antimicrobial therapy.
What changes in current practice are likely to improve outcomes?
Prevention of NEC is the most effective strategy because once the disease becomes clinically evident, a mucosal and systemic inflammatory cascade has already been activated and multiorgan injury is likely. For the same reasons, earlier diagnosis of NEC before clinical onset is an important goal.210
Major recommendations
Medical management consists of bowel decompression, discontinuation of enteral feedings and medications, maintaining intravascular volume and electrolyte balance, and initiating broad-spectrum antibiotics based on known sensitivities of prevalent pathogens in the individual NICU. Typical regimens include ampicillin plus gentamicin to cover for common intestinal bacteria. Often the addition of a third antibiotic that provides more targeted anaerobic coverage (eg, clindamycin or metronidazole) is indicated when there is evidence of pneumatosis or bowel perforation. As an alternative, piperacillin-tazobactam may offer the advantage of broad-spectrum antimicrobial coverage including typical anaerobes of the intestinal flora. However, downsides are variable penetration into the cerebrospinal fluid and concerns for the emergence of drug resistance. Second-line therapy for severely ill infants can include meropenem and vancomycin in cases of positive cultures with resistant organisms, possible central nervous system infection, perforated bowel, and/or failed first-line or alternative therapy. Almost all of the drugs mentioned do not have a Food and Drug Administration label for use in this population because safety and efficacy data are lacking. Supportive management may require respiratory and blood pressure support and correcting anemia and thrombocytopenia and/or other coagulation defects. Serial abdominal radiographs are often recommended to monitor for intestinal pneumatosis, portal venous gas, and pneumoperitoneum. Early consultation with a pediatric surgeon is advised. Intestinal perforation or evidence of bowel necrosis is a common indication for operative management. As a preventive measure, a more consistent practice style including the implementation of early breast milk feedings, standardized feeding regimens, and reduction of unnecessary antibiotics is recommended.
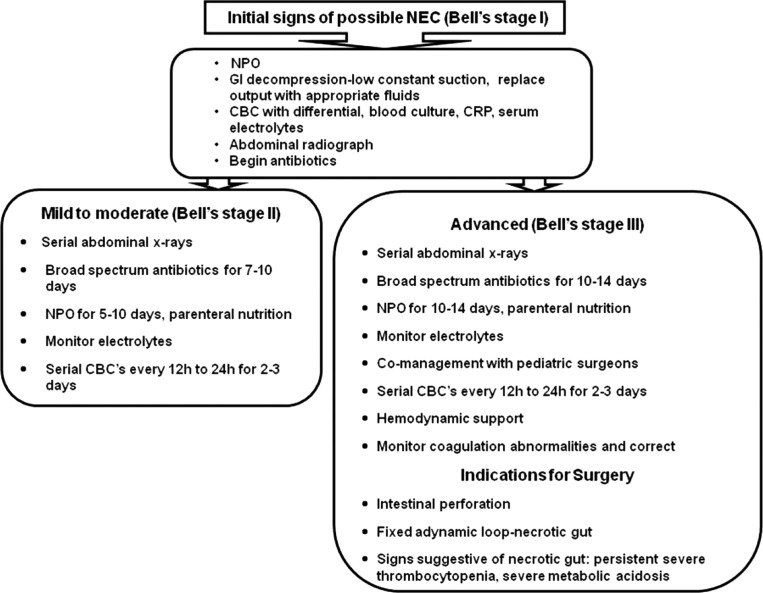
Clinical algorithms
- The management of patients with NEC (medical and/or surgical) can be guided by Bell staging criteria as reported recently by Sharma and Hudak211 (Fig. 1 ).
Fig. 1.
Clinical decision algorithms. CBC, complete blood cell count; CRP, C-reactive protein; NPO, nil per os (nothing by mouth).(Adapted from Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol 2013;40(1):27–51.) Rating for the strength of the evidence
C (Recommendation based on consensus, usual practice, expert opinion, disease-oriented evidence, and case series for studies of diagnosis, treatment, prevention, or screening)
Summary statement
NEC is a multifactorial disease, but bacteria and other microorganisms have been uniformly implicated somewhere along the pathogenic process. Because no specific microorganism can be considered causative in most cases of NEC, broad-spectrum antimicrobial therapy remains a mainstay in NEC treatment. More research is needed to determine the optimum therapy and to develop effective strategies for NEC prevention.
Footnotes
Disclosures: J.L. Wynn is supported by funding from the National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS)GM106143. J.H. Weitkamp has been supported by award number K08HD061607 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NIH/NICHD), the Vanderbilt University Medical Center's Digestive Disease Research Center sponsored by NIH grant P30DK058404 and CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences (NCATS).
References
- 1.Lemons J.A., Bauer C.R., Oh W. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107(1):E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 2.Neu J., Walker W.A. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanto W.P., Jr., Hunter J.E., Stoll B.J. Recognition and medical management of necrotizing enterocolitis. Clin Perinatol. 1994;21(2):335–346. [PubMed] [Google Scholar]
- 4.Obladen M. Necrotizing enterocolitis–150 years of fruitless search for the cause. Neonatology. 2009;96(4):203–210. doi: 10.1159/000215590. [DOI] [PubMed] [Google Scholar]
- 5.Gordon P.V., Swanson J.R., Attridge J.T. Emerging trends in acquired neonatal intestinal disease: is it time to abandon Bell's criteria? J Perinatol. 2007;27(11):661–671. doi: 10.1038/sj.jp.7211782. [DOI] [PubMed] [Google Scholar]
- 6.Berdon W.E., Grossman H., Baker D.H. Necrotizing enterocolitis in the premature infant. Radiology. 1964;83:879–887. doi: 10.1148/83.5.879. [DOI] [PubMed] [Google Scholar]
- 7.Willi H. Über eine bösartige Enteritis bei Säuglingen des ersten Trimenons. Ann Pediatr. 1944;162:87–112. [Google Scholar]
- 8.Buonomo C. The radiology of necrotizing enterocolitis. Radiol Clin North Am. 1999;37(6):1187–1198. doi: 10.1016/s0033-8389(05)70256-6. vii. [DOI] [PubMed] [Google Scholar]
- 9.Smith B., Bode S., Petersen B.L. Community analysis of bacteria colonizing intestinal tissue of neonates with necrotizing enterocolitis. BMC Microbiol. 2011;11:73. doi: 10.1186/1471-2180-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leaphart C.L., Cavallo J., Gribar S.C. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179(7):4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 11.Neal M.D., Sodhi C.P., Dyer M. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J Immunol. 2013;190(7):3541–3551. doi: 10.4049/jimmunol.1202264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snyder C.L., Hall M., Sharma V. Seasonal variation in the incidence of necrotizing enterocolitis. Pediatr Surg Int. 2010;26(9):895–898. doi: 10.1007/s00383-010-2675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meinzen-Derr J., Morrow A.L., Hornung R.W. Epidemiology of necrotizing enterocolitis temporal clustering in two neonatology practices. J Pediatr. 2009;154(5):656–661. doi: 10.1016/j.jpeds.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma R., Garrison R.D., Tepas J.J., 3rd Rotavirus-associated necrotizing enterocolitis: an insight into a potentially preventable disease? J Pediatr Surg. 2004;39(3):453–457. doi: 10.1016/j.jpedsurg.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Bagci S., Eis-Hubinger A.M., Franz A.R. Detection of astrovirus in premature infants with necrotizing enterocolitis. Pediatr Infect Dis J. 2008;27(4):347–350. doi: 10.1097/INF.0b013e318162a17a. [DOI] [PubMed] [Google Scholar]
- 16.Stuart R.L., Tan K., Mahar J.E. An outbreak of necrotizing enterocolitis associated with norovirus genotype GII.3. Pediatr Infect Dis J. 2010;29(7):644–647. doi: 10.1097/inf.0b013e3181d824e1. [DOI] [PubMed] [Google Scholar]
- 17.Bell M.J., Ternberg J.L., Bower R.J. The microbial flora and antimicrobial therapy of neonatal peritonitis. J Pediatr Surg. 1980;15(4):569–573. doi: 10.1016/s0022-3468(80)80775-5. [DOI] [PubMed] [Google Scholar]
- 18.Solomkin J.S., Mazuski J.E., Bradley J.S. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 19.Cilieborg M.S., Boye M., Sangild P.T. Bacterial colonization and gut development in preterm neonates. Early Hum Dev. 2012;88(Suppl 1):S41–S49. doi: 10.1016/j.earlhumdev.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 20.Romano-Keeler J., Moore D.J., Wang C. Early life establishment of site-specific microbial communities in the gut. Gut Microbes. 2014;5(2):192–201. doi: 10.4161/gmic.28442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark R.H., Gordon P., Walker W.M. Characteristics of patients who die of necrotizing enterocolitis. J Perinatol. 2012;32(3):199–204. doi: 10.1038/jp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bizzarro M.J., Ehrenkranz R.A., Gallagher P.G. Concurrent bloodstream infections in infants with necrotizing enterocolitis. J Pediatr. 2014;164(1):61–66. doi: 10.1016/j.jpeds.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Mollitt D.L., Tepas J.J., 3rd, Talbert J.L. The microbiology of neonatal peritonitis. Arch Surg. 1988;123(2):176–179. doi: 10.1001/archsurg.1988.01400260056006. [DOI] [PubMed] [Google Scholar]
- 24.Mollitt D.L., Tepas J.J., Talbert J.L. The role of coagulase-negative Staphylococcus in neonatal necrotizing enterocolitis. J Pediatr Surg. 1988;23(1 Pt 2):60–63. doi: 10.1016/s0022-3468(88)80542-6. [DOI] [PubMed] [Google Scholar]
- 25.Okogbule-Wonodi A.C., Gross G.W., Sun C.C. Necrotizing enterocolitis is associated with ureaplasma colonization in preterm infants. Pediatr Res. 2011;69(5 Pt 1):442–447. doi: 10.1203/PDR.0b013e3182111827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto S., Setoyama H., Umesaki Y. Differential induction of major histocompatibility complex molecules on mouse intestine by bacterial colonization. Gastroenterology. 1992;103(6):1777–1782. doi: 10.1016/0016-5085(92)91434-6. [DOI] [PubMed] [Google Scholar]
- 27.Mazmanian S.K., Liu C.H., Tzianabos A.O. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Mahony C., Scully P., O'Mahony D. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4(8):e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imaoka A., Matsumoto S., Setoyama H. Proliferative recruitment of intestinal intraepithelial lymphocytes after microbial colonization of germ-free mice. Eur J Immunol. 1996;26(4):945–948. doi: 10.1002/eji.1830260434. [DOI] [PubMed] [Google Scholar]
- 31.Weitkamp J.H., Rosen M.J., Zhao Z. Small intestinal intraepithelial TCRgammadelta+ T lymphocytes are present in the premature intestine but selectively reduced in surgical necrotizing enterocolitis. PLoS One. 2014;9(6):e99042. doi: 10.1371/journal.pone.0099042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau J., Magee F., Qiu Z. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am J Obstet Gynecol. 2005;193(3 Pt 1):708–713. doi: 10.1016/j.ajog.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Seliga-Siwecka J.P., Kornacka M.K. Neonatal outcome of preterm infants born to mothers with abnormal genital tract colonisation and chorioamnionitis: a cohort study. Early Hum Dev. 2013;89(5):271–275. doi: 10.1016/j.earlhumdev.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Been J.V., Lievense S., Zimmermann L.J. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. J Pediatr. 2013;162(2):236–242.e2. doi: 10.1016/j.jpeds.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Wolfs T.G., Buurman W.A., Zoer B. Endotoxin induced chorioamnionitis prevents intestinal development during gestation in fetal sheep. PLoS One. 2009;4(6):e5837. doi: 10.1371/journal.pone.0005837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolfs T.G., Kallapur S.G., Knox C.L. Antenatal ureaplasma infection impairs development of the fetal ovine gut in an IL-1-dependent manner. Mucosal Immunol. 2013;6(3):547–556. doi: 10.1038/mi.2012.97. [DOI] [PubMed] [Google Scholar]
- 37.Arnon S., Grigg J., Silverman M. Association between pulmonary and gastric inflammatory cells on the first day of life in preterm infants. Pediatr Pulmonol. 1993;16(1):59–61. doi: 10.1002/ppul.1950160112. [DOI] [PubMed] [Google Scholar]
- 38.Miralles R., Hodge R., McParland P.C. Relationship between antenatal inflammation and antenatal infection identified by detection of microbial genes by polymerase chain reaction. Pediatr Res. 2005;57(4):570–577. doi: 10.1203/01.PDR.0000155944.48195.97. [DOI] [PubMed] [Google Scholar]
- 39.Luciano A.A., Yu H., Jackson L.W. Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PLoS One. 2011;6(2):e16698. doi: 10.1371/journal.pone.0016698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfs T.G., Kallapur S.G., Polglase G.R. IL-1alpha mediated chorioamnionitis induces depletion of FoxP3+ cells and ileal inflammation in the ovine fetal gut. PLoS One. 2011;6(3):e18355. doi: 10.1371/journal.pone.0018355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weitkamp J.H., Rudzinski E., Koyama T. Ontogeny of FOXP3(+) regulatory T cells in the postnatal human small intestinal and large intestinal lamina propria. Pediatr Dev Pathol. 2009;12(6):443–449. doi: 10.2350/08-09-0533.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weitkamp J.H., Koyama T., Rock M.T. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut. 2013;62(1):73–82. doi: 10.1136/gutjnl-2011-301551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dingle B.M., Liu Y., Fatheree N.Y. FoxP3(+) regulatory T cells attenuate experimental necrotizing enterocolitis. PLoS One. 2013;8(12):e82963. doi: 10.1371/journal.pone.0082963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cash H.L., Whitham C.V., Behrendt C.L. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dominguez-Bello M.G., Costello E.K., Contreras M. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo C.C., Shih H.H., Chiu C.H. Translocation of coagulase-negative bacterial staphylococci in rats following intestinal ischemia-reperfusion injury. Biol Neonate. 2004;85(3):151–154. doi: 10.1159/000075065. [DOI] [PubMed] [Google Scholar]
- 47.Kansagra K., Stoll B., Rognerud C. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol. 2003;285(6):G1162–G1170. doi: 10.1152/ajpgi.00243.2003. [DOI] [PubMed] [Google Scholar]
- 48.Stewart C.J., Marrs E.C., Magorrian S. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatr. 2012;101(11):1121–1127. doi: 10.1111/j.1651-2227.2012.02801.x. [DOI] [PubMed] [Google Scholar]
- 49.Ferraris L., Butel M.J., Campeotto F. Clostridia in premature neonates' gut: incidence, antibiotic susceptibility, and perinatal determinants influencing colonization. PLoS One. 2012;7(1):e30594. doi: 10.1371/journal.pone.0030594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stark P.L., Lee A. Clostridia isolated from the feces of infants during the first year of life. J Pediatr. 1982;100(3):362–365. doi: 10.1016/s0022-3476(82)80430-7. [DOI] [PubMed] [Google Scholar]
- 51.Alfa M.J., Robson D., Davi M. An outbreak of necrotizing enterocolitis associated with a novel clostridium species in a neonatal intensive care unit. Clin Infect Dis. 2002;35(Suppl 1):S101–S105. doi: 10.1086/341929. [DOI] [PubMed] [Google Scholar]
- 52.Kosloske A.M., Ulrich J.A. A bacteriologic basis for the clinical presentations of necrotizing enterocolitis. J Pediatr Surg. 1980;15(4):558–564. doi: 10.1016/s0022-3468(80)80773-1. [DOI] [PubMed] [Google Scholar]
- 53.Kosloske A.M., Ulrich J.A., Hoffman H. Fulminant necrotising enterocolitis associated with Clostridia. Lancet. 1978;2(8098):1014–1016. doi: 10.1016/s0140-6736(78)92337-1. [DOI] [PubMed] [Google Scholar]
- 54.Azcarate-Peril M.A., Foster D.M., Cadenas M.B. Acute necrotizing enterocolitis of preterm piglets is characterized by dysbiosis of ileal mucosa-associated bacteria. Gut Microbes. 2011;2(4):234–243. doi: 10.4161/gmic.2.4.16332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singer D.B., Cashore W.J., Widness J.A. Pseudomembranous colitis in a preterm neonate. J Pediatr Gastroenterol Nutr. 1986;5(2):318–320. [PubMed] [Google Scholar]
- 56.Lallouette P., Bizzini B., Maro B. Studies on the immunostimulating and anti-tumour activity of a fraction isolated from Corynebacterium granulosum. Dev Biol Stand. 1977;38:111–113. [PubMed] [Google Scholar]
- 57.Petrillo T.M., Beck-Sague C.M., Songer J.G. Enteritis necroticans (pigbel) in a diabetic child. N Engl J Med. 2000;342(17):1250–1253. doi: 10.1056/NEJM200004273421704. [DOI] [PubMed] [Google Scholar]
- 58.Blakey J.L., Lubitz L., Barnes G.L. Development of gut colonisation in pre-term neonates. J Med Microbiol. 1982;15(4):519–529. doi: 10.1099/00222615-15-4-519. [DOI] [PubMed] [Google Scholar]
- 59.McDonel J.L. Clostridium perfringens toxins (type A, B, C, D, E) Pharmacol Ther. 1980;10(3):617–655. doi: 10.1016/0163-7258(80)90031-5. [DOI] [PubMed] [Google Scholar]
- 60.Flores-Diaz M., Alape-Giron A. Role of Clostridium perfringens phospholipase C in the pathogenesis of gas gangrene. Toxicon. 2003;42(8):979–986. doi: 10.1016/j.toxicon.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 61.Dittmar E., Beyer P., Fischer D. Necrotizing enterocolitis of the neonate with Clostridium perfringens: diagnosis, clinical course, and role of alpha toxin. Eur J Pediatr. 2008;167(8):891–895. doi: 10.1007/s00431-007-0614-9. [DOI] [PubMed] [Google Scholar]
- 62.Schlapbach L.J., Ahrens O., Klimek P. Clostridium perfringens and necrotizing enterocolitis. J Pediatr. 2010;157(1):175. doi: 10.1016/j.jpeds.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 63.de la Cochetiere M.F., Piloquet H., des Robert C. Early intestinal bacterial colonization and necrotizing enterocolitis in premature infants: the putative role of Clostridium. Pediatr Res. 2004;56(3):366–370. doi: 10.1203/01.PDR.0000134251.45878.D5. [DOI] [PubMed] [Google Scholar]
- 64.Blakey J.L., Lubitz L., Campbell N.T. Enteric colonization in sporadic neonatal necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 1985;4(4):591–595. doi: 10.1097/00005176-198508000-00017. [DOI] [PubMed] [Google Scholar]
- 65.Kotsanas D., Carson J.A., Awad M.M. Novel use of tryptose sulfite cycloserine egg yolk agar for isolation of Clostridium perfringens during an outbreak of necrotizing enterocolitis in a neonatal unit. J Clin Microbiol. 2010;48(11):4263–4265. doi: 10.1128/JCM.01724-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyakawa M.E., Saputo J., Leger J.S. Necrotizing enterocolitis and death in a goat kid associated with enterotoxin (CPE)-producing Clostridium perfringens type A. Can Vet J. 2007;48(12):1266–1269. [PMC free article] [PubMed] [Google Scholar]
- 67.Waligora-Dupriet A.J., Dugay A., Auzeil N. Evidence for clostridial implication in necrotizing enterocolitis through bacterial fermentation in a gnotobiotic quail model. Pediatr Res. 2005;58(4):629–635. doi: 10.1203/01.PDR.0000180538.13142.84. [DOI] [PubMed] [Google Scholar]
- 68.Cilieborg M.S., Boye M., Molbak L. Preterm birth and necrotizing enterocolitis alter gut colonization in pigs. Pediatr Res. 2011;69(1):10–16. doi: 10.1203/PDR.0b013e3181ff2a89. [DOI] [PubMed] [Google Scholar]
- 69.Dubberke E.R., Butler A.M., Yokoe D.S. Multicenter study of Clostridium difficile infection rates from 2000 to 2006. Infect Control Hosp Epidemiol. 2010;31(10):1030–1037. doi: 10.1086/656245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pruitt R.N., Lacy D.B. Toward a structural understanding of Clostridium difficile toxins A and B. Front Cell Infect Microbiol. 2012;2:28. doi: 10.3389/fcimb.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuehne S.A., Cartman S.T., Heap J.T. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467(7316):711–713. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 72.Jacquot A., Neveu D., Aujoulat F. Dynamics and clinical evolution of bacterial gut microflora in extremely premature patients. J Pediatr. 2011;158(3):390–396. doi: 10.1016/j.jpeds.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 73.Chang J.Y., Shin S.M., Chun J. Pyrosequencing-based molecular monitoring of the intestinal bacterial colonization in preterm infants. J Pediatr Gastroenterol Nutr. 2011;53(5):512–519. doi: 10.1097/MPG.0b013e318227e518. [DOI] [PubMed] [Google Scholar]
- 74.Rousseau C., Levenez F., Fouqueray C. Clostridium difficile colonization in early infancy is accompanied by changes in intestinal microbiota composition. J Clin Microbiol. 2011;49(3):858–865. doi: 10.1128/JCM.01507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Donta S.T., Myers M.G. Clostridium difficile toxin in asymptomatic neonates. J Pediatr. 1982;100(3):431–434. doi: 10.1016/s0022-3476(82)80454-x. [DOI] [PubMed] [Google Scholar]
- 76.Al-Jumaili I.J., Shibley M., Lishman A.H. Incidence and origin of Clostridium difficile in neonates. J Clin Microbiol. 1984;19(1):77–78. doi: 10.1128/jcm.19.1.77-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.el-Mohandes A.E., Keiser J.F., Refat M. Prevalence and toxigenicity of Clostridium difficile isolates in fecal microflora of preterm infants in the intensive care nursery. Biol Neonate. 1993;63(4):225–229. doi: 10.1159/000243935. [DOI] [PubMed] [Google Scholar]
- 78.Lishman A.H., Al Jumaili I.J., Elshibly E. Clostridium difficile isolation in neonates in a special care unit. Lack of correlation with necrotizing enterocolitis. Scand J Gastroenterol. 1984;19(3):441–444. [PubMed] [Google Scholar]
- 79.Han V.K., Sayed H., Chance G.W. An outbreak of Clostridium difficile necrotizing enterocolitis: a case for oral vancomycin therapy? Pediatrics. 1983;71(6):935–941. [PubMed] [Google Scholar]
- 80.Mathew O.P., Bhatia J.S., Richardson C.J. An outbreak of Clostridium difficile necrotizing enterocolitis. Pediatrics. 1984;73(2):265–266. [PubMed] [Google Scholar]
- 81.Seki H., Shiohara M., Matsumura T. Prevention of antibiotic-associated diarrhea in children by Clostridium butyricum MIYAIRI. Pediatr Int. 2003;45(1):86–90. doi: 10.1046/j.1442-200x.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 82.McCroskey L.M., Hatheway C.L., Fenicia L. Characterization of an organism that produces type E botulinal toxin but which resembles Clostridium butyricum from the feces of an infant with type E botulism. J Clin Microbiol. 1986;23(1):201–202. doi: 10.1128/jcm.23.1.201-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sturm R., Staneck J.L., Stauffer L.R. Neonatal necrotizing enterocolitis associated with penicillin-resistant, toxigenic Clostridium butyricum. Pediatrics. 1980;66(6):928–931. [PubMed] [Google Scholar]
- 84.Howard F.M., Flynn D.M., Bradley J.M. Outbreak of necrotising enterocolitis caused by Clostridium butyricum. Lancet. 1977;2(8048):1099–1102. doi: 10.1016/s0140-6736(77)90546-3. [DOI] [PubMed] [Google Scholar]
- 85.Gothefors L., Blenkharn I. Clostridium butyricum and necrotising enterocolitis. Lancet. 1978;1(8054):52–53. doi: 10.1016/s0140-6736(78)90406-3. [DOI] [PubMed] [Google Scholar]
- 86.Mitchell R.G., Etches P.C., Day D.G. Non-toxigenic clostridia in babies. J Clin Pathol. 1981;34(2):217–220. doi: 10.1136/jcp.34.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Popoff M.R., Ravisse P. Lesions produced by Clostridium butyricum strain CB 1002 in ligated intestinal loops in guinea pigs. J Med Microbiol. 1985;19(3):351–357. doi: 10.1099/00222615-19-3-351. [DOI] [PubMed] [Google Scholar]
- 88.Popoff M.R., Szylit O., Ravisse P. Experimental cecitis in gnotoxenic chickens monoassociated with Clostridium butyricum strains isolated from patients with neonatal necrotizing enterocolitis. Infect Immun. 1985;47(3):697–703. doi: 10.1128/iai.47.3.697-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bousseboua H., Le Coz Y., Dabard J. Experimental cecitis in gnotobiotic quails monoassociated with Clostridium butyricum strains isolated from patients with neonatal necrotizing enterocolitis and from healthy newborns. Infect Immun. 1989;57(3):932–936. doi: 10.1128/iai.57.3.932-936.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Butel M.J., Roland N., Hibert A. Clostridial pathogenicity in experimental necrotising enterocolitis in gnotobiotic quails and protective role of bifidobacteria. J Med Microbiol. 1998;47(5):391–399. doi: 10.1099/00222615-47-5-391. [DOI] [PubMed] [Google Scholar]
- 91.Stoll B.J., Hansen N., Fanaroff A.A. Enterobacter sakazakii is a rare cause of neonatal septicemia or meningitis in VLBW infants. J Pediatr. 2004;144(6):821–823. doi: 10.1016/j.jpeds.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 92.Machens H.G., Ringe B., Ziemer G. A new procedure for abdominal wound closure after pediatric liver transplantation: the “sandwich” technique. Surgery. 1994;115(2):255–256. [PubMed] [Google Scholar]
- 93.Kucerova E., Clifton S.W., Xia X.Q. Genome sequence of Cronobacter sakazakii BAA-894 and comparative genomic hybridization analysis with other Cronobacter species. PLoS One. 2010;5(3):e9556. doi: 10.1371/journal.pone.0009556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muytjens H.L., Roelofs-Willemse H., Jaspar G.H. Quality of powdered substitutes for breast milk with regard to members of the family Enterobacteriaceae. J Clin Microbiol. 1988;26(4):743–746. doi: 10.1128/jcm.26.4.743-746.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chap J., Jackson P., Siqueira R. International survey of Cronobacter sakazakii and other Cronobacter spp. in follow up formulas and infant foods. Int J Food Microbiol. 2009;136(2):185–188. doi: 10.1016/j.ijfoodmicro.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 96.Hoque A., Ahmed T., Shahidullah M. Isolation and molecular identification of Cronobacter spp. from powdered infant formula (PIF) in Bangladesh. Int J Food Microbiol. 2010;142(3):375–378. doi: 10.1016/j.ijfoodmicro.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 97.Weir E. Powdered infant formula and fatal infection with Enterobacter sakazakii. CMAJ. 2002;166(12):1570. [PMC free article] [PubMed] [Google Scholar]
- 98.Ahmed S.M., Lopman B.A., Levy K. A systematic review and meta-analysis of the global seasonality of norovirus. PLoS One. 2013;8(10):e75922. doi: 10.1371/journal.pone.0075922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bar-Oz B., Preminger A., Peleg O. Enterobacter sakazakii infection in the newborn. Acta Paediatr. 2001;90(3):356–358. [PubMed] [Google Scholar]
- 100.Clark N.C., Hill B.C., O'Hara C.M. Epidemiologic typing of Enterobacter sakazakii in two neonatal nosocomial outbreaks. Diagn Microbiol Infect Dis. 1990;13(6):467–472. doi: 10.1016/0732-8893(90)90078-a. [DOI] [PubMed] [Google Scholar]
- 101.Urmenyi A.M., Franklin A.W. Neonatal death from pigmented coliform infection. Lancet. 1961;1(7172):313–315. doi: 10.1016/s0140-6736(61)91481-7. [DOI] [PubMed] [Google Scholar]
- 102.van Acker J., de Smet F., Muyldermans G. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J Clin Microbiol. 2001;39(1):293–297. doi: 10.1128/JCM.39.1.293-297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Townsend S., Hurrell E., Forsythe S. Virulence studies of Enterobacter sakazakii isolates associated with a neonatal intensive care unit outbreak. BMC Microbiol. 2008;8:64. doi: 10.1186/1471-2180-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hunter C.J., Bean J.F. Cronobacter: an emerging opportunistic pathogen associated with neonatal meningitis, sepsis and necrotizing enterocolitis. J Perinatol. 2013;33(8):581–585. doi: 10.1038/jp.2013.26. [DOI] [PubMed] [Google Scholar]
- 105.Hunter C.J., Singamsetty V.K., Chokshi N.K. Enterobacter sakazakii enhances epithelial cell injury by inducing apoptosis in a rat model of necrotizing enterocolitis. J Infect Dis. 2008;198(4):586–593. doi: 10.1086/590186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singamsetty V.K., Wang Y., Shimada H. Outer membrane protein A expression in Enterobacter sakazakii is required to induce microtubule condensation in human brain microvascular endothelial cells for invasion. Microb Pathog. 2008;45(3):181–191. doi: 10.1016/j.micpath.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nair M.K., Venkitanarayanan K., Silbart L.K. Outer membrane protein A (OmpA) of Cronobacter sakazakii binds fibronectin and contributes to invasion of human brain microvascular endothelial cells. Foodborne Pathog Dis. 2009;6(4):495–501. doi: 10.1089/fpd.2008.0228. [DOI] [PubMed] [Google Scholar]
- 108.Bensasson M., Perez-Busquier M., Dorfmann H. Special radiographic features of the hand in patients with articular chondrocalcinosis. A controlled study. Ann Radiol (Paris) 1975;18(7):701–710. [PubMed] [Google Scholar]
- 109.Pagotto F.J., Nazarowec-White M., Bidawid S. Enterobacter sakazakii: infectivity and enterotoxin production in vitro and in vivo. J Food Prot. 2003;66(3):370–375. doi: 10.4315/0362-028x-66.3.370. [DOI] [PubMed] [Google Scholar]
- 110.Emami C.N., Mittal R., Wang L. Recruitment of dendritic cells is responsible for intestinal epithelial damage in the pathogenesis of necrotizing enterocolitis by Cronobacter sakazakii. J Immunol. 2011;186(12):7067–7079. doi: 10.4049/jimmunol.1100108. [DOI] [PubMed] [Google Scholar]
- 111.Emami C.N., Mittal R., Wang L. Role of neutrophils and macrophages in the pathogenesis of necrotizing enterocolitis caused by Cronobacter sakazakii. J Surg Res. 2012;172(1):18–28. doi: 10.1016/j.jss.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gregersen N., Van Nierop W., Von Gottberg A. Klebsiella pneumoniae with extended spectrum beta-lactamase activity associated with a necrotizing enterocolitis outbreak. Pediatr Infect Dis J. 1999;18(11):963–967. doi: 10.1097/00006454-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 113.Hill H.R., Hunt C.E., Matsen J.M. Nosocomial colonization with Klebsiella, type 26, in a neonatal intensive-care unit associated with an outbreak of sepsis, meningitis, and necrotizing enterocolitis. J Pediatr. 1974;85(3):415–419. doi: 10.1016/s0022-3476(74)80133-2. [DOI] [PubMed] [Google Scholar]
- 114.Boccia D., Stolfi I., Lana S. Nosocomial necrotising enterocolitis outbreaks: epidemiology and control measures. Eur J Pediatr. 2001;160(6):385–391. doi: 10.1007/s004310100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stone H.H., Kolb L.D., Geheber C.E. Bacteriologic considerations in perforated necrotizing enterocolitis. South Med J. 1979;72(12):1540–1544. doi: 10.1097/00007611-197912000-00015. [DOI] [PubMed] [Google Scholar]
- 116.Bell M.J., Feigin R.D., Ternberg J.L. Evaluation of gastrointestinal microflora in necrotizing enterocolitis. J Pediatr. 1978;92(4):589–592. doi: 10.1016/s0022-3476(78)80296-0. [DOI] [PubMed] [Google Scholar]
- 117.Speer M.E., Taber L.H., Yow M.D. Fulminant neonatal sepsis and necrotizing enterocolitis associated with a “nonenteropathogenic” strain of Escherichia coli. J Pediatr. 1976;89(1):91–95. doi: 10.1016/s0022-3476(76)80939-0. [DOI] [PubMed] [Google Scholar]
- 118.Cushing A.H. Necrotizing enterocolitis with Escherichia coli heat-labile enterotoxin. Pediatrics. 1983;71(4):626–630. [PubMed] [Google Scholar]
- 119.Guner Y.S., Malhotra A., Ford H.R. Association of Escherichia coli O157:H7 with necrotizing enterocolitis in a full-term infant. Pediatr Surg Int. 2009;25(5):459–463. doi: 10.1007/s00383-009-2365-3. [DOI] [PubMed] [Google Scholar]
- 120.Borderon E., Thieffry J.C., Jamet O. Observations on the intestinal colonization by Pseudomonas aeruginosa in newborn infants. Biol Neonate. 1990;57(2):88–97. doi: 10.1159/000243168. [DOI] [PubMed] [Google Scholar]
- 121.Jefferies J.M., Cooper T., Yam T. Pseudomonas aeruginosa outbreaks in the neonatal intensive care unit–a systematic review of risk factors and environmental sources. J Med Microbiol. 2012;61(Pt 8):1052–1061. doi: 10.1099/jmm.0.044818-0. [DOI] [PubMed] [Google Scholar]
- 122.Olson B., Weinstein R.A., Nathan C. Epidemiology of endemic Pseudomonas aeruginosa: why infection control efforts have failed. J Infect Dis. 1984;150(6):808–816. doi: 10.1093/infdis/150.6.808. [DOI] [PubMed] [Google Scholar]
- 123.Cheng Y.L., Lee H.C., Yeung C.Y. Clinical significance in previously healthy children of Pseudomonas aeruginosa in the stool. Pediatr Neonatol. 2009;50(1):13–17. doi: 10.1016/S1875-9572(09)60024-3. [DOI] [PubMed] [Google Scholar]
- 124.Henderson A., Maclaurin J., Scott J.M. Pseudomonas in a Glasgow baby unit. Lancet. 1969;2(7615):316–317. doi: 10.1016/s0140-6736(69)90071-3. [DOI] [PubMed] [Google Scholar]
- 125.Leigh L., Stoll B.J., Rahman M. Pseudomonas aeruginosa infection in very low birth weight infants: a case-control study. Pediatr Infect Dis J. 1995;14(5):367–371. doi: 10.1097/00006454-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 126.Rudd P.T., Carrington D. A prospective study of chlamydial, mycoplasmal, and viral infections in a neonatal intensive care unit. Arch Dis Child. 1984;59(2):120–125. doi: 10.1136/adc.59.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shurin P.A., Alpert S., Bernard Rosner B.A. Chorioamnionitis and colonization of the newborn infant with genital mycoplasmas. N Engl J Med. 1975;293(1):5–8. doi: 10.1056/NEJM197507032930102. [DOI] [PubMed] [Google Scholar]
- 128.Ozdemir R., Erdeve O., Yurttutan S. Letter to the editor Re: Okogbule-Wonodi et al. Pediatr Res 69:442–447. Pediatr Res. 2011;70(4):423–424. doi: 10.1203/PDR.0b013e31822f58ed. [author reply: 424] [DOI] [PubMed] [Google Scholar]
- 129.Perzigian R.W., Adams J.T., Weiner G.M. Ureaplasma urealyticum and chronic lung disease in very low birth weight infants during the exogenous surfactant era. Pediatr Infect Dis J. 1998;17(7):620–625. doi: 10.1097/00006454-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 130.Cox E., Christenson J.C. Rotavirus. Pediatr Rev. 2012;33(10):439–445. doi: 10.1542/pir.33-10-439. [quiz: 446–7] [DOI] [PubMed] [Google Scholar]
- 131.Murphy A.M., Albrey M.B., Crewe E.B. Rotavirus infections of neonates. Lancet. 1977;2(8049):1149–1150. doi: 10.1016/S0140-6736(77)91538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chrystie I.L., Totterdell B.M., Banatvala J.E. Asymptomatic endemic rotavirus infections in the newborn. Lancet. 1978;1(8075):1176–1178. doi: 10.1016/S0140-6736(78)90967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rotbart H.A., Nelson W.L., Glode M.P. Neonatal rotavirus-associated necrotizing enterocolitis: case control study and prospective surveillance during an outbreak. J Pediatr. 1988;112(1):87–93. doi: 10.1016/S0022-3476(88)80128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rotbart H.A., Levin M.J., Yolken R.H. An outbreak of rotavirus-associated neonatal necrotizing enterocolitis. J Pediatr. 1983;103(3):454–459. doi: 10.1016/S0022-3476(83)80427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Keller K.M., Schmidt H., Wirth S. Differences in the clinical and radiologic patterns of rotavirus and non-rotavirus necrotizing enterocolitis. Pediatr Infect Dis J. 1991;10(10):734–738. doi: 10.1097/00006454-199110000-00003. [DOI] [PubMed] [Google Scholar]
- 136.Jiang X., Wang M., Wang K. Sequence and genomic organization of Norwalk virus. Virology. 1993;195(1):51–61. doi: 10.1006/viro.1993.1345. [DOI] [PubMed] [Google Scholar]
- 137.Scallan E., Hoekstra R.M., Angulo F.J. Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis. 2011;17(1):7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Widdowson M.A., Sulka A., Bulens S.N. Norovirus and foodborne disease, United States, 1991–2000. Emerg Infect Dis. 2005;11(1):95–102. doi: 10.3201/eid1101.040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patel M.M., Widdowson M.A., Glass R.I. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14(8):1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Naing Z., Rayner B., Killikulangara A. Prevalence of viruses in stool of premature neonates at a neonatal intensive care unit. J Paediatr Child Health. 2013;49(3):E221–E226. doi: 10.1111/jpc.12113. [DOI] [PubMed] [Google Scholar]
- 141.Wiechers C., Bissinger A.L., Hamprecht K. Apparently non-specific results found using a norovirus antigen immunoassay for fecal specimens from neonates. J Perinatol. 2008;28(1):79–81. doi: 10.1038/sj.jp.7211849. [DOI] [PubMed] [Google Scholar]
- 142.Rabenau H.F., Sturmer M., Buxbaum S. Laboratory diagnosis of norovirus: which method is the best? Intervirology. 2003;46(4):232–238. doi: 10.1159/000072433. [DOI] [PubMed] [Google Scholar]
- 143.Agus S.G., Dolin R., Wyatt R.G. Acute infectious nonbacterial gastroenteritis: intestinal histopathology. Histologic and enzymatic alterations during illness produced by the Norwalk agent in man. Ann Intern Med. 1973;79(1):18–25. doi: 10.7326/0003-4819-79-1-18. [DOI] [PubMed] [Google Scholar]
- 144.Schreiber D.S., Blacklow N.R., Trier J.S. The mucosal lesion of the proximal small intestine in acute infectious nonbacterial gastroenteritis. N Engl J Med. 1973;288(25):1318–1323. doi: 10.1056/NEJM197306212882503. [DOI] [PubMed] [Google Scholar]
- 145.Dolin R., Levy A.G., Wyatt R.G. Viral gastroenteritis induced by the Hawaii agent. Jejunal histopathology and serologic response. Am J Med. 1975;59(6):761–768. doi: 10.1016/0002-9343(75)90461-1. [DOI] [PubMed] [Google Scholar]
- 146.Morotti R.A., Kaufman S.S., Fishbein T.M. Calicivirus infection in pediatric small intestine transplant recipients: pathological considerations. Hum Pathol. 2004;35(10):1236–1240. doi: 10.1016/j.humpath.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 147.Pelizzo G., Nakib G., Goruppi I. Isolated colon ischemia with norovirus infection in preterm babies: a case series. J Med Case Rep. 2013;7(1):108. doi: 10.1186/1752-1947-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kamaluddeen M., Lodha A., Akierman A. Non-Rotavirus infection causing apnea in a neonate. Indian J Pediatr. 2009;76(10):1051–1052. doi: 10.1007/s12098-009-0199-6. [DOI] [PubMed] [Google Scholar]
- 149.Turcios-Ruiz R.M., Axelrod P., St John K. Outbreak of necrotizing enterocolitis caused by norovirus in a neonatal intensive care unit. J Pediatr. 2008;153(3):339–344. doi: 10.1016/j.jpeds.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Armbrust S., Kramer A., Olbertz D. Norovirus infections in preterm infants: wide variety of clinical courses. BMC Res Notes. 2009;2:96. doi: 10.1186/1756-0500-2-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mussi-Pinhata M.M., Yamamoto A.Y., do Carmo Rego M.A. Perinatal or early-postnatal cytomegalovirus infection in preterm infants under 34 weeks gestation born to CMV-seropositive mothers within a high-seroprevalence population. J Pediatr. 2004;145(5):685–688. doi: 10.1016/j.jpeds.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 152.de Cates C.R., Gray J., Roberton N.R. Acquisition of cytomegalovirus infection by premature neonates. J Infect. 1994;28(1):25–30. doi: 10.1016/s0163-4453(94)94037-1. [DOI] [PubMed] [Google Scholar]
- 153.Miron D., Brosilow S., Felszer K. Incidence and clinical manifestations of breast milk-acquired Cytomegalovirus infection in low birth weight infants. J Perinatol. 2005;25(5):299–303. doi: 10.1038/sj.jp.7211255. [DOI] [PubMed] [Google Scholar]
- 154.Lanzieri T.M., Dollard S.C., Josephson C.D. Breast milk-acquired cytomegalovirus infection and disease in VLBW and premature infants. Pediatrics. 2013;131(6):e1937–e1945. doi: 10.1542/peds.2013-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hamprecht K., Maschmann J., Vochem M. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357(9255):513–518. doi: 10.1016/S0140-6736(00)04043-5. [DOI] [PubMed] [Google Scholar]
- 156.Adler S.P., Chandrika T., Lawrence L. Cytomegalovirus infections in neonates acquired by blood transfusions. Pediatr Infect Dis. 1983;2(2):114–118. doi: 10.1097/00006454-198303000-00009. [DOI] [PubMed] [Google Scholar]
- 157.Yeager A.S., Grumet F.C., Hafleigh E.B. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981;98(2):281–287. doi: 10.1016/s0022-3476(81)80662-2. [DOI] [PubMed] [Google Scholar]
- 158.Preiksaitis J.K., Brown L., McKenzie M. Transfusion-acquired cytomegalovirus infection in neonates. A prospective study. Transfusion. 1988;28(3):205–209. doi: 10.1046/j.1537-2995.1988.28388219143.x. [DOI] [PubMed] [Google Scholar]
- 159.Kim A.R., Lee Y.K., Kim K.A. Transfusion-related cytomegalovirus infection among very low birth weight infants in an endemic area. J Korean Med Sci. 2006;21(1):5–10. doi: 10.3346/jkms.2006.21.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Reyes C., Pereira S., Warden M.J. Cytomegalovirus enteritis in a premature infant. J Pediatr Surg. 1997;32(11):1545–1547. doi: 10.1016/S0022-3468(97)90448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cheong J.L., Cowan F.M., Modi N. Gastrointestinal manifestations of postnatal cytomegalovirus infection in infants admitted to a neonatal intensive care unit over a five year period. Arch Dis Child Fetal Neonatal Ed. 2004;89(4):F367–F369. doi: 10.1136/adc.2003.032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee S.L., Johnsen H., Applebaum H. Cytomegalovirus enterocolitis presenting as abdominal compartment syndrome in a premature neonate. World J Pediatr. 2012;8(1):80–82. doi: 10.1007/s12519-011-0307-3. [DOI] [PubMed] [Google Scholar]
- 163.Tengsupakul S., Birge N.D., Bendel C.M. Asymptomatic DNAemia heralds CMV-associated NEC: case report, review, and rationale for preemption. Pediatrics. 2013;132(5):e1428–e1434. doi: 10.1542/peds.2013-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gessler P., Bischoff G.A., Wiegand D. Cytomegalovirus-associated necrotizing enterocolitis in a preterm twin after breastfeeding. J Perinatol. 2004;24(2):124–126. doi: 10.1038/sj.jp.7211042. [DOI] [PubMed] [Google Scholar]
- 165.Tran L., Ferris M., Norori J. Necrotizing enterocolitis and cytomegalovirus infection in a premature infant. Pediatrics. 2013;131(1):e318–e322. doi: 10.1542/peds.2011-1971. [DOI] [PubMed] [Google Scholar]
- 166.Gagneur A., Sizun J., Vallet S. Coronavirus-related nosocomial viral respiratory infections in a neonatal and paediatric intensive care unit: a prospective study. J Hosp Infect. 2002;51(1):59–64. doi: 10.1053/jhin.2002.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chany C., Moscovici O., Lebon P. Association of coronavirus infection with neonatal necrotizing enterocolitis. Pediatrics. 1982;69(2):209–214. [PubMed] [Google Scholar]
- 168.Moscovici O., Chany C., Lebon P. Association of coronavirus infection with hemorrhagic entercolitis in newborn infants. C R Seances Acad Sci D. 1980;290(13):869–872. [PubMed] [Google Scholar]
- 169.Jamieson F.B., Wang E.E., Bain C. Human torovirus: a new nosocomial gastrointestinal pathogen. J Infect Dis. 1998;178(5):1263–1269. doi: 10.1086/314434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vaucher Y.E., Ray C.G., Minnich L.L. Pleomorphic, enveloped, virus-like particles associated with gastrointestinal illness in neonates. J Infect Dis. 1982;145(1):27–36. doi: 10.1093/infdis/145.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lodha A., de Silva N., Petric M. Human torovirus: a new virus associated with neonatal necrotizing enterocolitis. Acta Paediatr. 2005;94(8):1085–1088. doi: 10.1111/j.1651-2227.2005.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 172.Pruekprasert P., Stout C., Patamasucon P. Neonatal enterovirus infection. J Assoc Acad Minor Phys. 1995;6(4):134–138. [PubMed] [Google Scholar]
- 173.Ehrnst A., Eriksson M. Epidemiological features of type 22 echovirus infection. Scand J Infect Dis. 1993;25(3):275–281. doi: 10.3109/00365549309008499. [DOI] [PubMed] [Google Scholar]
- 174.Moore M., Kaplan M.H., McPhee J. Epidemiologic, clinical, and laboratory features of Coxsackie B1-B5 infections in the United States, 1970–79. Public Health Rep. 1984;99(5):515–522. [PMC free article] [PubMed] [Google Scholar]
- 175.Jenista J.A., Powell K.R., Menegus M.A. Epidemiology of neonatal enterovirus infection. J Pediatr. 1984;104(5):685–690. doi: 10.1016/s0022-3476(84)80944-0. [DOI] [PubMed] [Google Scholar]
- 176.Lake A.M., Lauer B.A., Clark J.C. Enterovirus infections in neonates. J Pediatr. 1976;89(5):787–791. doi: 10.1016/s0022-3476(76)80808-6. [DOI] [PubMed] [Google Scholar]
- 177.Johnson F.E., Crnic D.M., Simmons M.A. Association of fatal Coxsackie B2 viral infection and necrotizing enterocolitis. Arch Dis Child. 1977;52(10):802–804. doi: 10.1136/adc.52.10.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nakao T., Miura R., Sato M. ECHO virus type 22 infection in a premature infant. Tohoku J Exp Med. 1970;102(1):61–68. doi: 10.1620/tjem.102.61. [DOI] [PubMed] [Google Scholar]
- 179.Berkovich S., Pangan J. Recoveries of virus from premature infants during outbreaks of respiratory disease: the relation of ECHO virus type 22 to disease of the upper and lower respiratory tract in the premature infant. Bull N Y Acad Med. 1968;44(4):377–387. [PMC free article] [PubMed] [Google Scholar]
- 180.Birenbaum E., Handsher R., Kuint J. Echovirus type 22 outbreak associated with gastro-intestinal disease in a neonatal intensive care unit. Am J Perinatol. 1997;14(8):469–473. doi: 10.1055/s-2007-994182. [DOI] [PubMed] [Google Scholar]
- 181.Madeley C.R., Cosgrove B.P. Letter: viruses in infantile gastroenteritis. Lancet. 1975;2(7925):124. doi: 10.1016/s0140-6736(75)90020-3. [DOI] [PubMed] [Google Scholar]
- 182.Sebire N.J., Malone M., Shah N. Pathology of astrovirus associated diarrhoea in a paediatric bone marrow transplant recipient. J Clin Pathol. 2004;57(9):1001–1003. doi: 10.1136/jcp.2004.017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nighot P.K., Moeser A., Ali R.A. Astrovirus infection induces sodium malabsorption and redistributes sodium hydrogen exchanger expression. Virology. 2010;401(2):146–154. doi: 10.1016/j.virol.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bagci S., Eis-Hubinger A.M., Yassin A.F. Clinical characteristics of viral intestinal infection in preterm and term neonates. Eur J Clin Microbiol Infect Dis. 2010;29(9):1079–1084. doi: 10.1007/s10096-010-0965-4. [DOI] [PubMed] [Google Scholar]
- 185.Chappe C., Minjolle S., Dabadie A. Astrovirus and digestive disorders in neonatal units. Acta Paediatr. 2012;101(5):e208–e212. doi: 10.1111/j.1651-2227.2011.02569.x. [DOI] [PubMed] [Google Scholar]
- 186.Desfrere L., de Oliveira I., Goffinet F. Increased incidence of necrotizing enterocolitis in premature infants born to HIV-positive mothers. AIDS. 2005;19(14):1487–1493. doi: 10.1097/01.aids.0000183123.09206.07. [DOI] [PubMed] [Google Scholar]
- 187.Schmitz T., Weizsaecker K., Feiterna-Sperling C. Exposure to HIV and antiretroviral medication as a potential cause of necrotizing enterocolitis in term neonates. AIDS. 2006;20(7):1082–1083. doi: 10.1097/01.aids.0000222089.74905.0f. [DOI] [PubMed] [Google Scholar]
- 188.van der Meulen E.F., Bergman K.A., Kamps A.W. Necrotising enterocolitis in a term neonate with trisomy 21 exposed to maternal HIV and antiretroviral medication. Eur J Pediatr. 2009;168(1):113–114. doi: 10.1007/s00431-008-0715-0. [DOI] [PubMed] [Google Scholar]
- 189.Baley J.E., Kliegman R.M., Boxerbaum B. Fungal colonization in the very low birth weight infant. Pediatrics. 1986;78(2):225–232. [PubMed] [Google Scholar]
- 190.Fridkin S.K., Kaufman D., Edwards J.R. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995–2004. Pediatrics. 2006;117(5):1680–1687. doi: 10.1542/peds.2005-1996. [DOI] [PubMed] [Google Scholar]
- 191.Saiman L., Ludington E., Dawson J.D. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr Infect Dis J. 2001;20(12):1119–1124. doi: 10.1097/00006454-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 192.Saiman L., Ludington E., Pfaller M. Risk factors for candidemia in neonatal intensive care unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J. 2000;19(4):319–324. doi: 10.1097/00006454-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 193.Benjamin D.K., Jr., Stoll B.J., Gantz M.G. Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics. 2010;126(4):e865–e873. doi: 10.1542/peds.2009-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stewart C.J., Nelson A., Scribbins D. Bacterial and fungal viability in the preterm gut: NEC and sepsis. Arch Dis Child Fetal Neonatal Ed. 2013;98(4):F298–F303. doi: 10.1136/archdischild-2012-302119. [DOI] [PubMed] [Google Scholar]
- 195.Coates E.W., Karlowicz M.G., Croitoru D.P. Distinctive distribution of pathogens associated with peritonitis in neonates with focal intestinal perforation compared with necrotizing enterocolitis. Pediatrics. 2005;116(2):e241–e246. doi: 10.1542/peds.2004-2537. [DOI] [PubMed] [Google Scholar]
- 196.Mintz A.C., Applebaum H. Focal gastrointestinal perforations not associated with necrotizing enterocolitis in very low birth weight neonates. J Pediatr Surg. 1993;28(6):857–860. doi: 10.1016/0022-3468(93)90345-l. [DOI] [PubMed] [Google Scholar]
- 197.Parra-Herran C.E., Pelaez L., Sola J.E. Intestinal candidiasis: an uncommon cause of necrotizing enterocolitis (NEC) in neonates. Fetal Pediatr Pathol. 2010;29(3):172–180. doi: 10.3109/15513811003777342. [DOI] [PubMed] [Google Scholar]
- 198.Smith S.D., Tagge E.P., Miller J. The hidden mortality in surgically treated necrotizing enterocolitis: fungal sepsis. J Pediatr Surg. 1990;25(10):1030–1033. doi: 10.1016/0022-3468(90)90212-r. [DOI] [PubMed] [Google Scholar]
- 199.Ballance W.A., Dahms B.B., Shenker N. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr. 1990;117(1 Pt 2):S6–S13. doi: 10.1016/s0022-3476(05)81124-2. [DOI] [PubMed] [Google Scholar]
- 200.Kaufman D., Boyle R., Hazen K.C. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345(23):1660–1666. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 201.Kaufman D.A., Morris A., Gurka M.J. Fluconazole prophylaxis in preterm infants: a multicenter case-controlled analysis of efficacy and safety. Early Hum Dev. 2014;90(Suppl 1):S87–S90. doi: 10.1016/S0378-3782(14)70026-X. [DOI] [PubMed] [Google Scholar]
- 202.Healy C.M., Campbell J.R., Zaccaria E. Fluconazole prophylaxis in extremely low birth weight neonates reduces invasive candidiasis mortality rates without emergence of fluconazole-resistant Candida species. Pediatrics. 2008;121(4):703–710. doi: 10.1542/peds.2007-1130. [DOI] [PubMed] [Google Scholar]
- 203.Benjamin D.K., Jr., Hudak M.L., Duara S. Effect of fluconazole prophylaxis on candidiasis and mortality in premature infants: a randomized clinical trial. JAMA. 2014;311(17):1742–1749. doi: 10.1001/jama.2014.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ullrich T., Tang Y.W., Correa H. Absence of gastrointestinal pathogens in ileum tissue resected for necrotizing enterocolitis. Pediatr Infect Dis J. 2012;31(4):413–414. doi: 10.1097/INF.0b013e318242534a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Raskind C.H., Dembry L.M., Gallagher P.G. Vancomycin-resistant enterococcal bacteremia and necrotizing enterocolitis in a preterm neonate. Pediatr Infect Dis J. 2005;24(10):943–944. doi: 10.1097/01.inf.0000180987.00122.5d. [DOI] [PubMed] [Google Scholar]
- 206.Pumberger W., Novak W. Fatal neonatal Salmonella enteritidis sepsis. J Perinatol. 2000;20(1):54–56. doi: 10.1038/sj.jp.7200298. [DOI] [PubMed] [Google Scholar]
- 207.Stein H., Beck J., Solomon A. Gastroenteritis with necrotizing enterocolitis in premature babies. Br Med J. 1972;2(5814):616–619. doi: 10.1136/bmj.2.5814.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Overturf G.D., Sherman M.P., Scheifele D.W. Neonatal necrotizing enterocolitis associated with delta toxin-producing methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 1990;9(2):88–91. doi: 10.1097/00006454-199002000-00005. [DOI] [PubMed] [Google Scholar]
- 209.Downard C.D., Renaud E., St Peter S.D. American Pediatric Surgical Association Outcomes Clinical Trials Committee. Treatment of necrotizing enterocolitis: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg. 2012;47(11):2111–2122. doi: 10.1016/j.jpedsurg.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 210.Weitkamp J.H. More than a gut feeling: predicting surgical necrotising enterocolitis. Gut. 2014;63(8):1205–1206. doi: 10.1136/gutjnl-2013-305928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sharma R., Hudak M.L. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol. 2013;40(1):27–51. doi: 10.1016/j.clp.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ng P.C., Lewindon P.J., Siu Y.K. Bacterial contaminated breast milk and necrotizing enterocolitis in preterm twins. J Hosp Infect. 1995;31(2):105–110. doi: 10.1016/0195-6701(95)90165-5. [DOI] [PubMed] [Google Scholar]