Abstract
Mother-to-child transmission of cytomegalovirus (CMV) and varicella zoster virus (VZV) can lead to severe birth defects and neurologic impairment of infants. Congenital CMV is the most common congenital infection and the leading infectious cause of infant hearing loss and neurologic deficits, complicating up to 1% of all pregnancies globally. While antiviral treatment of congenitally CMV-infected infants can ameliorate the CMV-associated hearing loss and developmental delay, interventions to prevent congenital CMV infection and the associated neurologic impairments are still being evaluated. Moreover, an effective CMV vaccine to protect mothers against CMV acquisition during pregnancy is urgently needed to reduce the sizeable health and economic burden of this disease. In contrast, congenital VZV infection is rare, attributable to the availability of an effective VZV vaccine, high rates of preexisting VZV immunity prior to pregnancy, and poorly efficient in utero VZV transmission. Moreover, passive immunization of exposed pregnant women or infants with VZV hyperimmune globulin can prevent severe disease in those that do not have prior immunity. Active and passive immunization strategies to prevent perinatal CMV infection with similar efficacy to those established to prevent perinatal VZV infections are a critical need in pediatric health.
Keywords: Cytomegalovirus (CMV), varicella zoster virus (VZV)
Introduction
Human CMV is a highly ubiquitous pathogen, with a 45–100% adult seroprevalence rate worldwide [1]. Additionally, CMV is the most common cause of congenital infection, affecting 0.5–2% of all live-born infants [2]. While the majority of affected infants are asymptomatic at birth, 10–15% exhibit signs of CMV-associated sequelae including thrombocytopenia, hepatitis, chorioretinitis, sensorineural hearing loss (SNHL), intrauterine growth restriction, and mental retardation (Figure 1) [3, 4]. An additional 5 to 15% of asymptomatic CMV-infected infants will develop late-onset sequelae, most commonly SNHL, within the first two years of life [3]. Altogether, congenital CMV accounts for 25% of all cases of childhood deafness and results in more long-term pediatric disabilities in U.S. children than any other common causes of birth defects, including Down syndrome and spina bifida (Fig. 2) [4]. Several maternal factors (Table 1) are known to increase the incidence and severity of congenital CMV disease, of which maternal CMV immune status plays an important role. In cases of primary maternal infection, where there is no pre-existing immunity, 40% of women transmit CMV in utero compared to only 1–2% of CMV-seropositive women [5]. Furthermore, neurologic deficits are more common following primary maternal CMV infection, and results in the most severe fetal disease outcomes [5].
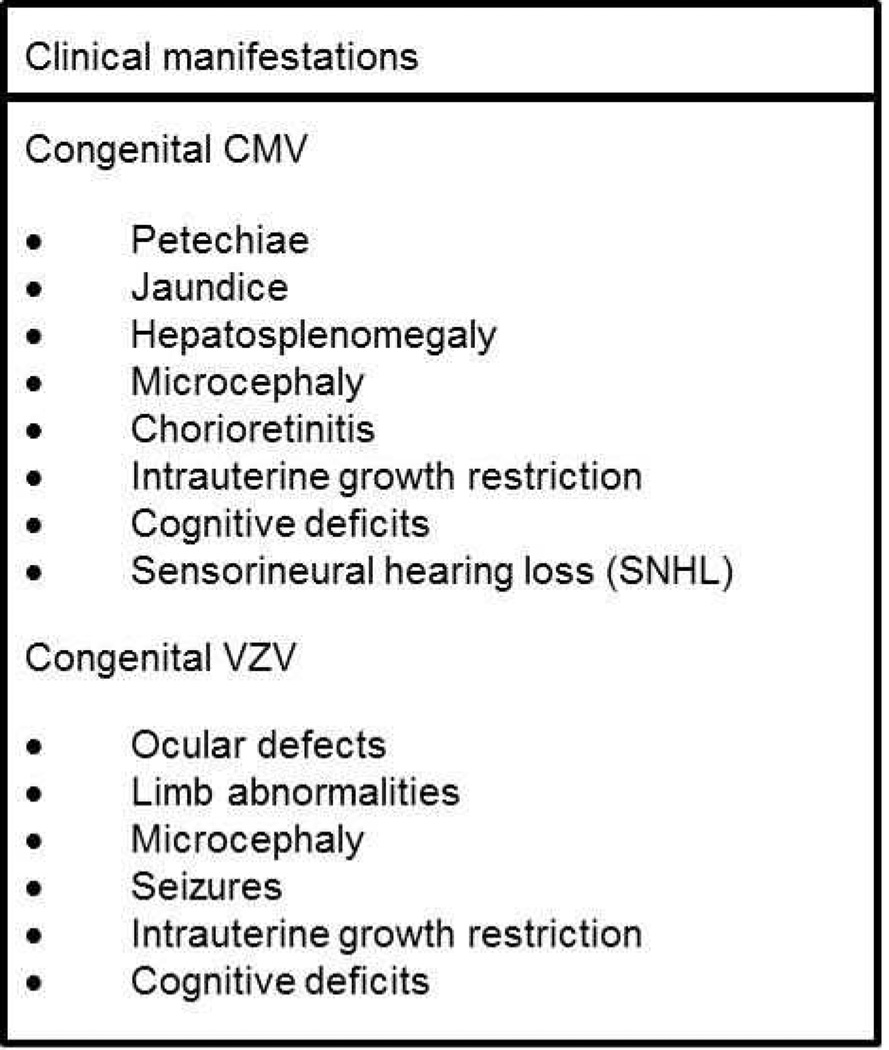
Figure 1. Clinical manifestations of CMV and VZV infection in neonates.
Infants born with congenital CMV or VZV infection can display a wide rane of sequelae, many of which can result in permanent developmental and neurological impairment.
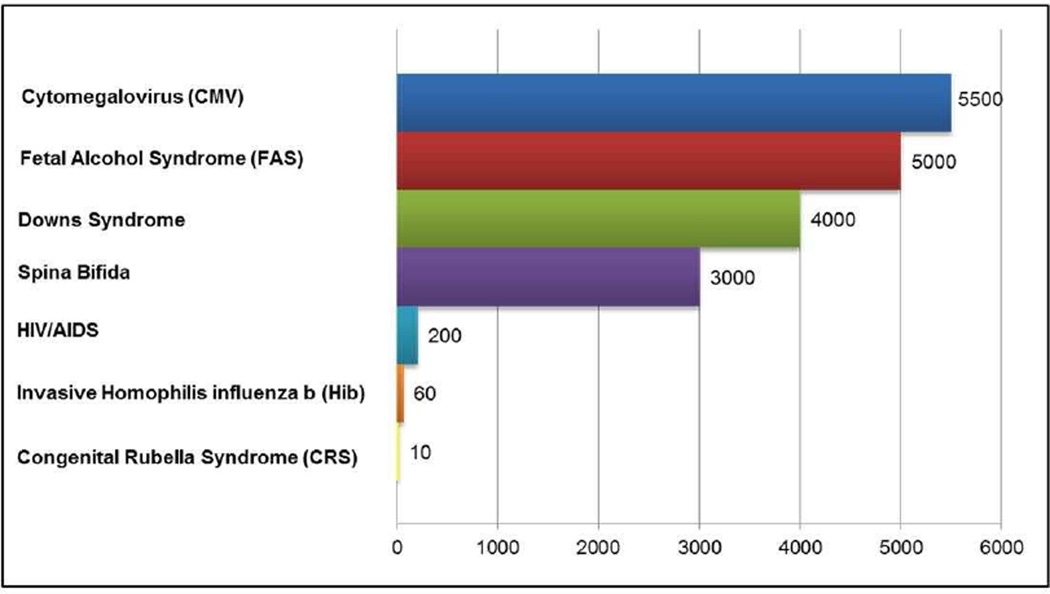
Figure 2. Annual number of U.S. children born with long-term pediatric disabilities.
Congenital CMV infection in the U.S. causes more long-term pediatric disabilities than other common causes of birth defects including FAS, Downs syndrome, spina bifida, HIV, Hib, and CRS
Adapted from Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 2005;5:70–77; with permission.
Table 1.
Maternal risk factors associated with mother-to-child transmission
| CMV | VZV |
|---|---|
| Young age | VZV immune status |
| Non-white race | Acute infection at time of delivery |
| Single marital status | |
| CMV immune status |
Also at risk for developing severe CMV-associated sequelae are premature infants that acquire CMV postnatally either through exposure via breast milk or blood transfusions. While breast milk transmission of CMV is asymptomatic in full-term infants, postnatal acquisition in very low-birth-weight premature infants can be associated with a sepsis-like illness with sequelae including pneumonitis, enteritis, thrombocytopenia, and hepatitis [6, 7]. Although transfusion-associated postnatal CMV infection of premature infants has nearly been eliminated by the use of leukoreduced and CMV-seronegative blood products, encouragement of breast milk feeding to improve health outcomes for premature infants has potentiated the need to address breast milk transmission of CMV in neonatal intensive care settings [8]. However, it remains unclear if postnatal CMV infection of premature infants results in long-term deficits.
Varicella zoster virus (VZV), like CMV a member of the herpesvirus family, can lead to adverse pregnancy and infant outcomes when transmitted following acute maternal infection during pregnancy. Though preexisting VZV immunity has remained high in pregnant women both before and after the advent of VZV vaccination, immunity to VZV is standardly documented during early pregnancy. Infants with the highest risk of acquiring VZV are born to women with acute infection appearing between five days before and two days following delivery, as the infant is exposed to VZV at birth in the setting of limited or no placental VZV-specific IgG transfer. Rarely, VZV can be transmitted in utero during a primary maternal infection, and if this occurs in the first 20 weeks of pregnancy, fetal demise, intrauterine growth restriction, hydrops, limb deformities, microcephaly, and other neurologic defects can result from the congenital infection (Congenital Varicella Syndrome) [9].
Prevalence/Incidence
An estimated 0.7% of all live-born infants are born with congenital CMV infection. Ten to 15% of congenitally infected infants will display symptoms at birth [10] and half of these symptomatic CMV-infected infants will suffer long-term sequelae, often SNHL, mental retardation and microcephaly [2]. Furthermore, 10% of infants who are asymptomatic at birth develop CMV-associated SNHL early in life and approximately 5% are reported to have additional cognitive defects, making it a leading cause of pediatric long-term disability in U.S children (Figure 2) [10].
The incidence of birth defects and pediatric neurologic abnormalities attributable to congenital VZV infection is considerably lower than that of CMV, as the U.S. maternal CMV seroprevalence is approximately 50% compared to greater than 90% for VZV, leaving more women at risk of acute CMV infection during pregnancy. Moreover, the rate of congenital transmission of CMV following acute primary maternal infection is approximately 40%, compared to less than 2% following acute maternal infection with VZV [9, 11].
World Wide/Regional Incidence and Mortality Rates
The incidence of congenital CMV transmission is greatly impacted by CMV seroprevalence in women of childbearing age. In populations of low socioeconomic status (SES), CMV prevalence in pregnant women can reach as high as 80–100% [1]. As approximately two thirds of congenital CMV transmissions occur in CMV-seropositive women, the incidence of congenital CMV infection is high in populations of low socioeconomic status and high CMV-seroprevalence, averaging 1.2% in comparison to the worldwide incidence rate of 0.7% [10]. In the U.S., where CMV seroprevalence is close to 50% in women of childbearing age, there is an increase in the number of primary maternal CMV infections during pregnancy, and as a result more severe infant outcomes [12]. Mortality in infants due to congenital CMV infection ranges from 100–200 in the U.S. each year [13].
Clinical Correlation
Maternal interventions for the prevention/treatment of congenital CMV and perinatal VZV infections
Routine prenatal CMV screening is not currently recommended given the lack of a proven effective intervention to prevent congenital CMV [14, 15]. Furthermore, differentiating primary infection from re-infection or reactivation is difficult due to the poor reliability of IgM antibody assays. With a sensitivity ranging from 50–90% and a high rate of false positivity, IgM is detectable in < 30% of women with an actual primary infection [16–18]. Additionally, IgM can be detectable during reactivation or re-infection and can persist for many months following a primary infection [18, 19]. Maternal immunity demonstrated by IgG positivity alone does not eliminate the potential for congenital CMV since two-thirds of cases result from re-infection with a new CMV strain or reactivation of latent virus [20]. The addition of IgG avidity, a measure of antibody maturity, significantly improves the ability to identify primary infection [21]. The detection of IgM and IgG combined with low IgG avidity suggests a primary CMV infection occurring in the past 2 – 4 months [16, 22].
In addition to vaccine development (see section below), there is ongoing research to determine the potential effectiveness of CMV-specific hyperimmune globulin (HIG) in congenital CMV treatment (Table 2). Initially, Nigro et al. demonstrated some promise for the possible efficacy of CMV-HIG in treating congenital infection, though this was a cohort study [23]. Revello and colleagues recently completed a trial of 124 pregnant women with documented primary CMV infection randomized to receive serial CMV-HIG infusions or placebo. Thirty percent of the CMV-HIG group delivered infants with congenital CMV compared to 44% in the placebo group, which while encouraging, was not statistically significant likely due to inadequate power [24]. Two large-scale ongoing randomized trials of CMV-HIG to prevent congenital CMV will hopefully provide definitive evidence as to its potential efficacy [25, 26].
Table 2.
Prospective studies of CMV Hyperimmune Globulin (HIG) to prevent congenital CMV
| Authors, Year, Study Location |
Phase | Randomized | Blinding | Placebo | Subject # | Study Group | Study Notes | Ref |
|---|---|---|---|---|---|---|---|---|
| Nigro et al. 2005, Italy | I | No | No | No | 45 84 |
Treatment Group Prevention Group |
|
[23] |
| Revello et al. 2014, Italy | II | Yes | Yes | Yes | 61 63 |
Treatment Control |
|
[24] |
| Bio-test AG, Europe | III | Yes | Yes | Yes | Unknown | Treatment Control |
|
[26] |
| NIH-NICHD, United States | III | Yes | Yes | Yes | Planned n=800 | Treatment Control |
|
[25] |
In lieu of vaccination or effective antenatal treatment, prevention or reduction in congenital CMV must focus on educating all women of childbearing potential on preventive strategies. There are several risk factors for infection including lower SES, history of sexually transmitted infections including HPV/abnormal cervical cytology, and very young maternal age (< 15 years). Women at higher risk for primary infection include child care workers and those with young children in the home. In one study, eleven percent of seronegative child care workers seroconverted within the first year of employment [27]. Yeager et al. showed that over half of all family members of young children seroconvert within a year [28], and increasing parity was shown to be an independent risk factor for CMV seroconversion [29]. All women of childbearing potential, regardless of the presence or absence of risk factors, should be counseled that their personal risk of CMV acquisition can be significantly reduced with proper hygiene and behavioral practices.
Regarding VZV, prior history of infection is 97–99% predictive of seropositivity, which confers long-term immunity [30]. Thus, routine prenatal VZV screening based on reported history combined with serology during early pregnancy is recommended [30]. Women who are VZV-seronegative should be vaccinated postpartum. Such a strategy is supported by cost-effectiveness and cost-benefit analyses, given that the associated morbidity and mortality are very high and there is potential for prophylaxis/treatment to prevent congenital infection [31, 32]. If non-immune, a pregnant woman should be advised to avoid contact with infected individuals until they are non-infectious [30]. If a susceptible pregnant woman has exposure to a chickenpox-infected individual, varicella immunoglobulin (VZIG) should be administered within 96 hours of exposure to prevent infection and reduce infant morbidity and mortality [33]. Although unproven through randomized clinical trials, prevention of maternal VZV infection theoretically should prevent congenital VZV. Non-pregnant women of childbearing potential should have documentation of prior history of chickenpox in their medical record with preconception vaccination if no such history exists or if documented IgG negative.
Infant interventions for the prevention/treatment of congenital CMV and perinatal VZV infections
Maternal VZV-specific IgG is highly effective in protecting the infant against neonatal and congenital VZV infection. There are a few settings in which post-exposure prophylaxis with VZIG is recommended for infants who are deficient in maternal VZV-specific IgG. One such scenario is primary maternal infection near the time of delivery, which can lead to severe disease in the newborn, and up to 30% mortality [34]. Similarly, VZIG should be administered to infants born to non-immune mothers and all premature infants born < 28 weeks of gestational age who are postnatally exposed to an individual with acute VZV infection, as both of these settings leaves the infant without maternal antibody protection. If infection occurs in an infant that is deficient in VZV-specific maternal IgG, antiviral treatment with high dose acyclovir should be initiated [35].
There are no currently well-established methods to prevent congenital CMV transmission (see maternal interventions). Yet, as CMV-associated neurologic complications continue to develop throughout the first two years of life in congenitally-infected infants, antiviral suppression of CMV replication may prevent or ameliorate some of these sequelae. The Collaborative Antiviral Study Group (CASG) performed a clinical trial in congenitally CMV-infected infants with CMV-associated disease in the central nervous system with six weeks of intravenous ganciclovir, an antiviral that specifically targets the CMV phosphotransferase UL97, compared to no treatment [36]. This study demonstrated clear improvement in hearing outcome for the ganciclovir-treated infants, though neutropenia, a known side effect of this drug, occurred in two-thirds of the infants treated with ganciclovir. While the clinical improvements with ganciclovir treatment were promising, this long-term intravenous treatment was challenging to implement in infected infants who typically do not require long-term hospitalization. Thus, the same group performed a pharmacokinetic study comparing intravenous ganciclovir with the oral ganciclovir prodrug valganciclovir in neonates. Oral valganciclovir administration was found to achieve similar plasma levels of the active drug and had a similar side effect profile to that of intravenous ganciclovir, thus providing a practical oral option for treatment of congenital CMV disease [37]. In a comparison of infant hearing and developmental outcomes after six weeks versus six months of oral valganciclovir treatment, [38]. oral valganciclovir treatment until six months of age led to improved hearing and developmental outcomes for CMV-infected children. Moreover, the incidence of neutropenia was similar in the placebo and valganciclovir-treated groups between 6 weeks and 6 months of treatment, suggesting that the neutropenia in valganciclovir-treated infants may be at least partly attributable to the viral infection itself. Thus, congenitally CMV-infected infants exhibiting CMV-associated neurologic sequelae should receive valganciclovir treatment and followed closely for suppression of viremia and hematologic abnormalities. However, as 10–15% of congenitally-infected infants who are asymptomatic at birth will develop CMV-associated neurologic sequeale (namely hearing loss) before two years of age, more work is needed to determine whether asymptomatic infants will benefit from this antiviral treatment. Furthermore, it is not known whether premature infants who experience symptomatic postnatal CMV infection via breast milk feeding benefit from antiviral treatment.
Infant CMV diagnostics
The advent of a safe, oral option for effective CMV antiviral treatment for congenitally-infected infants, combined with the high burden of congenital CMV as a major contributor to pediatric disabilities, has made it more critical to institute standard, routine CMV testing so that all congenitally-infected infants may be evaluated for potential antiviral treatment that could impact their future cognitive abilities. As infant serologic assessments are plagued with issues including the presence of maternal IgG and the poor performance and predictive capacity of CMV-specific IgM, detection of the virus is a more practical approach. The current standard of diagnosing congenital CMV infection in infants is a CMV culture from the urine or saliva, including rapid virus detection in culture via immunohistochemistry. Yet, this diagnostic technique is labor-intensive and challenging in laboratories with limited tissue culture facilities. Boppana and colleagues have made considerable progress in developing a high throughput saliva or urine quantitative real time polymerase chain reaction (PCR) assay is highly-sensitive and specific for CMV detection compared to the gold standard of urine CMV culture [39, 40]. While implementation of CMV PCR of dried blood spots would have been a convenient way to integrate this testing into the standard newborn screening program, this assay was found to have low sensitivity [41]. The saliva real time PCR, however, has been validated on dried saliva spots, facilitating batched sample analysis [39]. Therefore, as large scale infant CMV testing is feasible, and treatment of congenitally-infected infants is proven to benefit their long term outcome, the implementation of universal screening for congenital CMV infection is now a top priority for improving pediatric health.
Prospects and Priorities for Maternal CMV Vaccine Research
The large number of infants severely affected by congenital CMV transmission, and the substantial economic burden associated with long-term treatment and care of children with CMV-associated disabilities has prompted the Institute of Medicine to recognize the development of a maternal vaccine to be of the highest priority [42]. However, despite continuous vaccination efforts for the prevention of CMV infection for nearly 40 years, clinical trials still remain at the earliest stages. Summarized below and in table 3 are the important successes and failures of previous vaccination attempts that inform the new directions for current and future CMV vaccine research.
Table 3.
Phase 1 and 2 clinical CMV vaccine trials targeting women of childbearing age
| Platform | Phase | Randomized | Blinding | Placebo | Subject # | Study Group | Study Notes | Ref |
|---|---|---|---|---|---|---|---|---|
| Live-attenuated Towne | I | No | No | No | 10 | Seronegative (pediatric nurses of childbearing age) |
|
[47] |
| Live-attenuated Towne | I | Yes | Partial | Yes | 42 38 |
Seropositive/Seronegative (women of childbearing age with toddlers attending day care) |
|
[46] |
| Live-attenuated Towne | I | Yes | No | No | 63 | Seropositive (women of childbearing age) |
|
[45] |
| Subunit gB/ MF59 or alum adjuvant | I | Yes | Double | Yes | 150 | Seropositive (women of childbearing age) |
|
[54] |
| Subunit gB/ MF59 adjuvant | II | Yes | Double | Yes | 464 | Seronegative (Predominantly African-American) |
|
[55] |
Live-attenuated CMV vaccines
Vaccination against CMV infection began in the early 1970’s with two live-attenuated vaccine candidates, AD169 and the well characterized Towne strain, both of which underwent extensive passaging in human fibroblasts before being tested in humans [43, 44]. Symptoms following vaccination with either strain were mild and restricted to the site of injection and did not result in virus shedding or latency in any of the groups tested, therefore proving to be safe and well tolerated [42–48]. Furthermore, it was discovered that among the different routes evaluated, subcutaneous vaccination was the most effective at inducing the production of CMV-specific neutralizing antibodies and lymphoproliferative cellular responses [43, 44, 49, 50]. Vaccine efficacy of the live-attenuated Towne strain was analyzed in two high risk populations including seronegative renal transplant candidates receiving seropositive donor organs, and seronegative women of childbearing age with young children attending day care. Multiple independent studies in renal transplant recipients reported that vaccination with the Towne strain prior to transplantation did not alter the rate of CMV infection in comparison to non-vaccinated individuals, however vaccination did reduce the severity of disease following primary CMV infection by 84–100% according to a pre-established scoring system [51]. Similarly, vaccination with the Towne strain in seronegative women with children attending day care also failed to protect the women from acquiring CMV, yet child-to-mother transmission was rarely detected in naturally seropositive women [46]. This last observation suggested the Towne vaccine was incapable of eliciting wild-type like immunity, a common problem in the majority of live-attenuated clinical vaccine trials. To address this issue, recent efforts have been made to elicit a stronger and longer-lasting immune response to CMV with the Towne vaccine that include the addition of an IL-12 adjuvant and the creation of several Towne chimeric viruses encoding portions of the genome found in other non-attenuated strains, neither of which have been successful at achieving wild-type immunity [53, 54].
CMV subunit vaccines
The majority of subunit vaccines to date have focused primarily on the surface glycoprotein, gB. The gB protein is directly involved in the attachment and entry of CMV into fibroblasts, and is a major target of neutralizing antibodies present in seropositive plasma. In the first human gB vaccine trial, soluble gB was administered to seronegative adults with either a squalene and water emulsion adjuvant MF59, or aluminum hydroxide [52]. The recipients were given varying doses of the vaccine in three different regimens. Mild discomfort at the site of injection was common, but no serious side effects were reported in any of the treatment groups. Scheduled immunizations at 0, 1, and 6 months elicited the highest neutralizing antibody response between 2 and 6 months after the final dose, and was strongest when combined with MF59. Moreover, the level of neutralizing antibodies induced after the third dose of the gB/MF59 vaccine was above that of naturally infected, seropositive individuals. In a Consistent with this finding, children receiving the same gB/MF59 vaccine regimen at 0, 1 and 6 months were also shown to produce neutralizing antibody titers that exceed those seen in naturally immune sera [53]. In a cohort of CMV-seropositive women, the gB/MF59 vaccine was also capable of boosting antibody and CD4-T cell immune responses [54]. Data from these studies, and its proven safety in transplant recipients, prompted the first and only phase 2, placebo-controlled, randomized, double-blinded trial in seronegative women [55]. Over 400 women were vaccinated with the gB/MF59 vaccine or placebo within 1 year of giving birth, and thus were at high risk for acquiring primary infection. After 42 months, 18/225 vaccinated women and 31/234 placebo-treated women had experienced a CMV infection, yielding a vaccine efficacy of 50%. Among the women in this study, 81 vaccinees and 97 placebo recipients became pregnant. Only 1 of 81 infants born to vaccinated women had confirmed CMV infection and was non-symptomatic. Three infants were born with CMV infection in the placebo-treated group, one of which presented severe sequelae at birth, while the other two remained asymptomatic. Although the numbers in this study are too small to evaluate the efficacy of the gB/MF59 vaccine on preventing mother-to-child transmission of CMV in seronegative women, the results are promising and encourage a future phase 3 trial.
With the present knowledge that CMV contains multiple glycoprotein complexes on the surface of virions involved in cell entry, it is unlikely that production of neutralizing antibodies to a single glycoprotein will provide broad protection against all CMV isolates. Therefore, recent emphasis has been placed on the development of a vaccine that elicits neutralizing antibodies to a second glycoprotein complex consisting of gH, gL, UL128, UL130 and UL131 important for epithelial cell tropism [56]. These studies are still in early preclinical evaluation. Future vaccine platforms that include both the gB protein and pentameric complex as targets will be key to determining the immune responses required for protection against maternal infection and congenital transmission of a breadth of CMV variants. Additional vaccine strategies currently underway include CMV vector and DNA vaccines aimed at eliciting both neutralizing antibody and cell-mediated immune responses [57–60].
Summary/Discussion
While effective interventions to prevent perinatal VZV infection exist, strategies to prevent congenital CMV infection are urgently needed to reduce the high pediatric health and economic burden of this common congenital infection. Long-term antiviral treatment can improve hearing and developmental outcomes for congenitally CMV-infected infants, yet do not prevent the life-long neurologic impairment. The development of active and passive vaccine strategies for impeding maternal CMV acquisition and placental transmission is key to eliminating this common and potentially-preventable cause of pediatric disabilities.
Key Points.
Congenital CMV infection is the leading infectious cause of hearing loss and neurologic deficits, affecting up to 1% of all pregnancies worldwide.
Perinatal VZV infections are rare and primarily preventable through combined active and passive maternal/infant immunization.
Long-term antiviral treatment of infants with congenital CMV infection and associated neurologic sequelae is effective in improving hearing and developmental outcome.
Development of active and passive maternal CMV vaccine strategies is a top priority in pediatric health.
Best Practices Box.
What is the current practice?
Congenital CMV infection:
Screen infants for congenital CMV infection who show signs of congenital infection (including microcephaly, petechial rash, hepatosplenomegaly, hepatitis, thrombocytopenia, fail the newborn hearing screen).
Antiviral treatment of CMV-infected infants with neurologic involvement
What changes in current practice are likely to improve outcomes?
Establishment of the impact of passive CMV hyperimmune globulin on congenital CMV transmission
Implementation of standard congenital CMV screening at birth using infant saliva dried blood spots
Research on the effect of antiviral treatment in congenitally CMV-infected, premature and asymptomatic term infants
Implementation of standard CMV serologic screening and hygiene/behavioral CMV avoidance education for all mothers during pregnancy
Is there a Clinical Algorithm?
Major Recommendations:
Screen infants demonstrating signs consistent with congenital CMV infection with urine or saliva HCMV culture or saliva dried blood spot PCR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cannon M, Shmid D, Hyde T. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 2.Kenneson A, Cannon M. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):254–277. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 3.Britt W. Infectious disease of the fetues and newborn infant. Philadelphia: Elsevier Saunders; 2011. Cytomegalovirus; p. 706. [Google Scholar]
- 4.Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health. 2005;5:70–77. doi: 10.1186/1471-2458-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yow M, Williamson D, Leeds L, et al. Epidemiologic characteristics of cytomegalovirus infection in mothers and their infants. Am J Obstet Gynecol. 1988;158(5):1189–1195. doi: 10.1016/0002-9378(88)90252-9. [DOI] [PubMed] [Google Scholar]
- 6.Lombardi G, Garofoli F, Manzoni P, et al. Breast milk-aquired cytomegalovirus infection in very low birth weight infants. J Matern Fetal Neonatal Med. 2012;25(S3):57–62. doi: 10.3109/14767058.2012.712345. [DOI] [PubMed] [Google Scholar]
- 7.Hamprecht K, Maschmann J, Jahn G, et al. Cytomegalovirus transmission to preterm infants during lactation. J Clin Virol. 2008;41(3):198–205. doi: 10.1016/j.jcv.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia J. Human milk and the premature infant. Ann Nutr Metab. 2013;62(S3):8–14. doi: 10.1159/000351537. [DOI] [PubMed] [Google Scholar]
- 9.Pastuszak AL, Levy M, Schick B, et al. Outcome after maternal varicella infection in the first 20 weeks of pregnancy. New Eng J Med. 1994;330:901–905. doi: 10.1056/NEJM199403313301305. [DOI] [PubMed] [Google Scholar]
- 10.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 11.Enders G, Miller E, Cradock-Watson, et al. Consequences of varicella and herpes zoster in pregancy: prospective study of 1739 cases. Lancet. 1994;343(8912):1548–1551. doi: 10.1016/s0140-6736(94)92943-2. [DOI] [PubMed] [Google Scholar]
- 12.Plosa EJ, Esbenshade JC, Fuller MP, et al. Cytomegalovirus infection. Pediatrics in Review. 2012;33(4):156–163. doi: 10.1542/pir.33-4-156. [DOI] [PubMed] [Google Scholar]
- 13.Ross SA, Boppana SB. Congenital cytomegalovirus infection: outcome and diagnosis. Semin Pediatr Infect Dis. 2005;16(1):44–49. doi: 10.1053/j.spid.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Grangeot-Keros L, Simon B, Audibert F, et al. Should we routinely screen for cytomegalovirus antibody during pregnancy? Intervirology. 1998;41(4–5):158–162. doi: 10.1159/000024930. [DOI] [PubMed] [Google Scholar]
- 15.CDC. Cytomegalovirus (CMV) and Congenital CMV Infection - Clinical Diagnosis & Treatment. [Accessed June 25, 2014]; Available at: http://www.cdc.gov/cmv/clinical/diagnosis-treatment.html.
- 16.Lazzarotto T, Guerra B, Lanari M, et al. New advances in the diagnosis of congenital cytomegalovirus infection. J Clin Virol. 2008;41(3):192–197. doi: 10.1016/j.jcv.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Guerra B, Simonazzi G, Banfi A, et al. Impact of diagnostic and confirmatory tests and prenatal counseling on the rate of pregnancy termination among women with positive cytomegalovirus immunoglobulin M antibody titers. Am J Obstet Gynecol. 2007;196(3):221 e1–221 e6. doi: 10.1016/j.ajog.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 18.Stagno S, Tinker MK, Elrod C, et al. Immunoglobulin M antibodies detected by enzyme-linked immunosorbent assay and radioimmunoassay in the diagnosis of cytomegalovirus infections in pregnant women and newborn infants. J Clin Microbiol. 1985;21(6):930–935. doi: 10.1128/jcm.21.6.930-935.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagay ZJ, Biran G, Ornoy A, et al. Congenital cytomegalovirus infection: a long-standing problem still seeking a solution. Am J Obstet Gynecol. 1996;174(1 Pt 1):241–245. doi: 10.1016/s0002-9378(96)70401-5. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Zhang X, Bialek S, et al. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis. 2011;52(2):e11–e13. doi: 10.1093/cid/ciq085. [DOI] [PubMed] [Google Scholar]
- 21.Lazzarotto T, Spezzacatena P, Pradelli P, et al. Avidity of immunoglobulin G directed against human cytomegalovirus during primary and secondary infections in immunocompetent and immunocompromised subjects. Clin Diagn Lab Immunol. 1997;4(4):469–473. doi: 10.1128/cdli.4.4.469-473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC. Cytomegalovirus (CMV) and Congenital CMV Infection - Interpretation of Laboratory Tests. [Accessed June 25, 2014]; Available at: http://www.cdc.gov/cmv/clinical/lab-tests.html.
- 23.Nigro G, Adler SP, La Torre R, et al. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353(13):1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 24.Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370(14):1316–1326. doi: 10.1056/NEJMoa1310214. [DOI] [PubMed] [Google Scholar]
- 25.NIH. A Randomized Trial to Prevent Congenital Cytomegalovirus (CMV) [Accessed June 25, 2014]; Available at: http://clinicaltrials.gov/show/NCT01376778.
- 26.Biotest AG. Interim analysis of the Cytotect(R) Phase III trial in congenital cytomegalovirus (CMV) infection shows clear indication of efficacy. [Accessed July 30, 2014]; Available at: http://www.biotest.com/ww/en/pub/investor_relations/news/newsdetails.cfm?newsID=1025191. [Google Scholar]
- 27.Pass RF, August AM, Dworsky, et al. Cytomegalovirus infection in day-care center. N Engl J Med. 1982;307(8):477–479. doi: 10.1056/NEJM198208193070804. [DOI] [PubMed] [Google Scholar]
- 28.Yeager AS. Transmission of cytomegalovirus to mothers by infected infants: another reason to prevent transfusion-acquired infections. Pediatr Infect Dis. 1983;2(4):295–297. doi: 10.1097/00006454-198307000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Tookey PA, Ades AE, Peckham CS. Cytomegalovirus prevalence in pregnant women: the influence of parity. Arch Dis Child. 1992;67(7 Spec No):779–783. doi: 10.1136/adc.67.7_spec_no.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marin M, Guris D, Chaves SS, et al. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-4):1–40. [PubMed] [Google Scholar]
- 31.Pinot de Moira A, Edmunds WJ, Breuer J. The cost-effectiveness of antenatal varicella screening with post-partum vaccination of susceptibles. Vaccine. 2006;24(9):1298–1307. doi: 10.1016/j.vaccine.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Rouse DJ, et al. Management of the presumed susceptible varicella (chickenpox)-exposed gravida: a cost-effectiveness/cost-benefit analysis. Obstet Gynecol. 1996;87(6):932–936. doi: 10.1016/0029-7844(96)00025-7. [DOI] [PubMed] [Google Scholar]
- 33.Ogilvie MM. Antiviral prophylaxis and treatment in chickenpox. A review prepared for the UK Advisory Group on Chickenpox on behalf of the British Society for the Study of Infection. J Infect. 1998;36(Suppl 1):31–38. doi: 10.1016/s0163-4453(98)80153-9. [DOI] [PubMed] [Google Scholar]
- 34.Brunell PA. Fetal and neonatal varicella-zoster infections. Semin Perinatol. 1983;7(1):47–56. [PubMed] [Google Scholar]
- 35.Marin M, Bialek SR, Seward JF. Updated Recommendations for use of VariZIG-United States, 2103. MMWR. 2013;62(28):574–576. [PMC free article] [PubMed] [Google Scholar]
- 36.Kimberlin D, Lin CY, Sanches PJ, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143(1):16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- 37.Kimberlin DW, Acosta EP, Sanchez PJ, et al. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J Infect Dis. 2008;197(6):836–845. doi: 10.1086/528376. [DOI] [PubMed] [Google Scholar]
- 38.Kimberlin DW, Jester P, Sanchez PJ, et al. Six months versus six weeks of oral valganciclovir for infants with symptomatic congenital cytomegalovirus (CMV) disease with and without central nervous system (CNS) involvement: Results of a Phase III, randomized, double-blind, placebo-controlled, multinational study. Infectious Diseases Society of America meeting. 2013 Available at: https://idsa.confex.com/idsa/2013/webprogram/Paper43178.html. [Google Scholar]
- 39.Boppana SB, Ross SA, Shimamura M, et al. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. New Eng J Med. 2011;362(22):2111–2118. doi: 10.1056/NEJMoa1006561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross SA, Ahmed A, Palmer AL, et al. Detection of Congenital Cytomegalovirus Infection by Real-Time Polymerase Chain Reaction of Saliva or Urine. J Infect Dis. 2014 doi: 10.1093/infdis/jiu263. Epub May 5, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boppana SB, Ross SA, Novak Z, et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303(14):1375–1382. doi: 10.1001/jama.2010.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arvin AM, Fast P, Myer M, et al. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39(2):233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 43.Elek SD, Stern H. Development of a vaccine against mental retardation caused by cytomegalovirus infection in utero. The Lancet. 1974;303(7845):1–5. doi: 10.1016/s0140-6736(74)92997-3. [DOI] [PubMed] [Google Scholar]
- 44.Plotkin SA, Farquhar J, Hornberger E. Clinical Trials of Immunization with the Towne Strain of Human Cytomegalovirus. J Infect Dis. 1976;134(5):470–475. doi: 10.1093/infdis/134.5.470. [DOI] [PubMed] [Google Scholar]
- 45.Adler SP, Hempfling SH, Starr SE, et al. Safety and immunogenicity of the Towne strain cytomegalovirus vaccine. Pediatr Infect Dis J. 1998;17(3):200–206. doi: 10.1097/00006454-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Adler SP, Starr SE, Plotkin SA, et al. Immunity Induced by Primary Human Cytomegalovirus Infection Protects against Secondary Infection among Women of Childbearing Age. J Infect Dis. 1995;171(1):26–32. doi: 10.1093/infdis/171.1.26. [DOI] [PubMed] [Google Scholar]
- 47.Fleisher GR, Starr SE, Friedman HM, et al. Vaccination of Pediatric Nurses With Live Attenuated Cytomegalovirus. Am J Dis Child. 1982;136(4):294–296. doi: 10.1001/archpedi.1982.03970400012003. [DOI] [PubMed] [Google Scholar]
- 48.Glazer JP, Friedman HM, Grossman RA, et al. Live Cytomegalovirus Vaccination of Renal Tranplant Candidates. Ann Intern Med. 1979;91(5):676–683. doi: 10.7326/0003-4819-91-5-676. [DOI] [PubMed] [Google Scholar]
- 49.Gonczol E, Ianacone J, Furlini G, et al. Humoral Immune Response to Cytomegalovirus Towne Vaccine Strain and to Toledo Low-Passage Strain. J Infect Dis. 1989;159(5):851–859. doi: 10.1093/infdis/159.5.851. [DOI] [PubMed] [Google Scholar]
- 50.Starr SE, Glazer JP, Friedman HM, et al. Specific Cellular and Humoral Immunity after Immunization with Live Towne Strain Cytomegalovirus Vaccine. J Infect Dis. 1981;143(4):585–589. doi: 10.1093/infdis/143.4.585. [DOI] [PubMed] [Google Scholar]
- 51.Plotkin SA. Vaccination against cytomegalovirus, the changeling demon. Pediatr Infect Dis J. 1999;18(4):313–325. doi: 10.1097/00006454-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Pass RF, Duliege AM, Boppana S, et al. A Subunit Cytomegalovirus Vaccine Based on Recombinant Envelope Glycoprotein B and a New Adjuvant. J Infect Dis. 1999;180(4):970–975. doi: 10.1086/315022. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell DK, Holmes SJ, Burke RL, et al. Immunogenicity of a recombinant cytomegalovirus gB vaccine in seronegative toddlers. Pediatr Infect Dis J. 2002;21(2):133–138. doi: 10.1097/00006454-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Sabbaj S, Pass RF, Goepfert PA, et al. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell repsones to cytomegalovirus in chronically infected women. J Infect Dis. 2011;203(11):1534–1541. doi: 10.1093/infdis/jir138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pass RF, Zhang C, Evans A, et al. Vaccine Prevention of Maternal Cytomegalovirus Infection. New Eng J Med. 2009;360(12):1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rychman BJ, Chase MC, Johnson DC. HCMV gH/gL/UL128–131 interferes with virus entry into epithelial cells: Evidence for cell type-specific receptors. PNAS. 2008;105(37):14118–14123. doi: 10.1073/pnas.0804365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adler SP, Plotkin SA, Gonczol E, et al. A Canarypox Vector Expressing Cytomegalovirus (CMV) Glyocprotein B Primes for Antibody Responses to a Live Attenuated CMV Vaccine (Towne) J Infect Dis. 1999;180(3):843–846. doi: 10.1086/314951. [DOI] [PubMed] [Google Scholar]
- 58.Bernstein DI, Reap EA, Katen K, et al. Randomized, double-blind, Phase I trial of an alphavirus replicaon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine. 2010;28(2):484–493. doi: 10.1016/j.vaccine.2009.09.135. [DOI] [PubMed] [Google Scholar]
- 59.Wloch MK, Smith LR, Boutsaboualoy S, et al. Safety and Immunogenicity of a Bivalent Cytomegalovirus DNA Vaccine in Healthy Adult Subjects. J Infect Dis. 2008;197(12):1634–1642. doi: 10.1086/588385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kharfan-Dabaja MA, Boeckh M, Wilck MB, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12(4):290–299. doi: 10.1016/S1473-3099(11)70344-9. [DOI] [PubMed] [Google Scholar]