Abstract
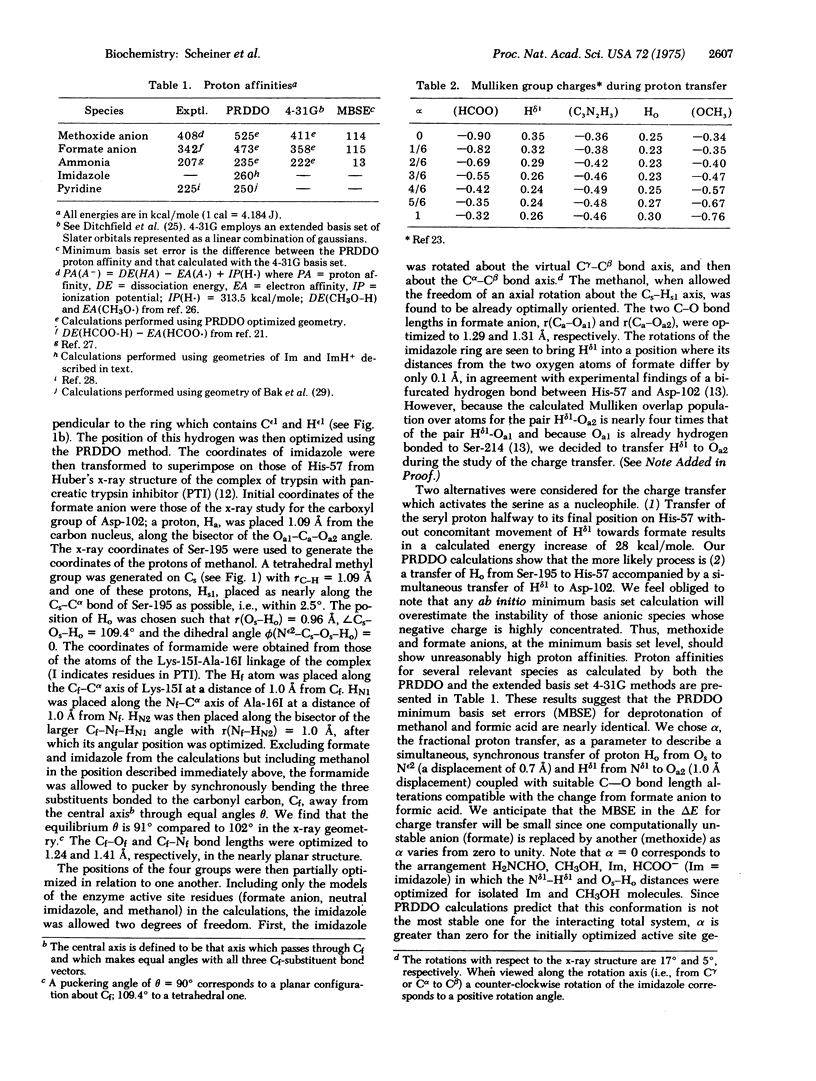
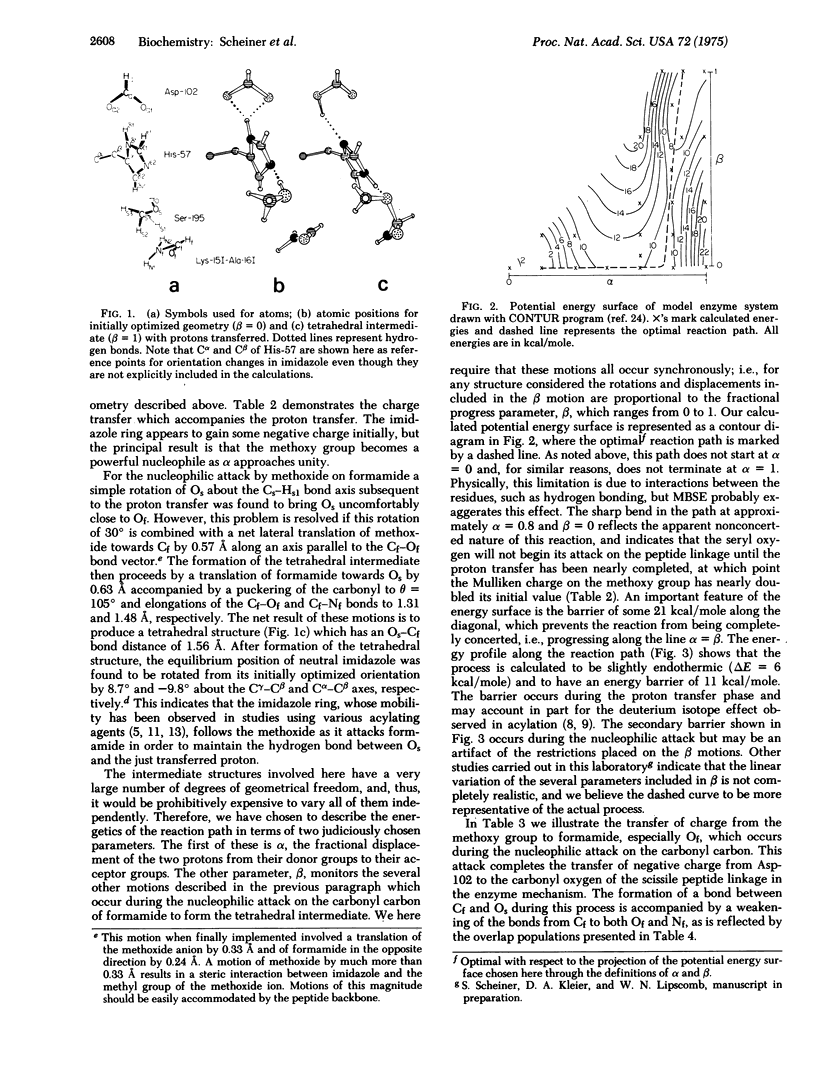
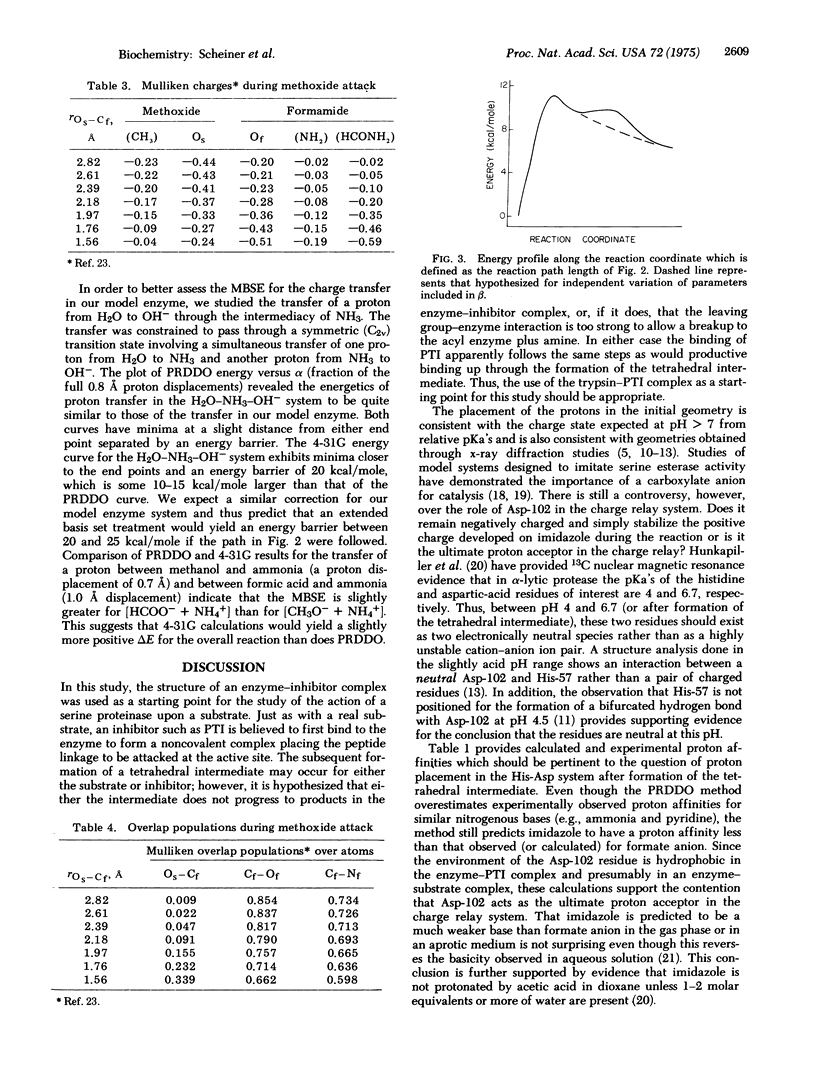
The charge relay ststem and its role in the acylation of serine proteinases is studied using the partial retention of diatomic differential overlap (PRDDO) technique to perform approximate ab initio molecular orbital calculations on a model of the enzyme-substrate complex. The aspartate in the charge relay system is seen to act as the ultimate proton acceptor during the charging of the serine nucleophile. A projection of the potential energy surface is obtained in a subspace corresponding to this charge transfer and to the coupled motions of active site residues and the substrate. These results together with extended basis set results for cruder models suggest that a concerted transfer of protons from Ser-195 to His-57 and from His-57 to Asp-102 occurs with an energy barrier of 20-25 kcal/mole (84-105 kJ/mole). The subsequent nucleophilic attack on the scissile peptide linkage by the charged serine is then seen to proceed energetically downhill to the tetrahedral intermediate. The formation of the tetrahedral intermediate from the Michaelis complex is calculated to be nearly thermoneutral.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender M. L., Killheffer J. V. Chymotrypsins. CRC Crit Rev Biochem. 1973 Apr;1(2):149–199. doi: 10.3109/10409237309102546. [DOI] [PubMed] [Google Scholar]
- Birktoft J. J., Blow D. M. Structure of crystalline -chymotrypsin. V. The atomic structure of tosyl- -chymotrypsin at 2 A resolution. J Mol Biol. 1972 Jul 21;68(2):187–240. doi: 10.1016/0022-2836(72)90210-0. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Bundy H. F., Moore C. L. Chymotrypsin-catalyzed hydrolysis of m-, p-, and o-nitroanilides of N-benzoyl-L-tyrosine. Biochemistry. 1966 Feb;5(2):808–811. doi: 10.1021/bi00866a056. [DOI] [PubMed] [Google Scholar]
- Caplow M. Chymotrypsin catalysis. Evidence for a new intermediate. J Am Chem Soc. 1969 Jun 18;91(13):3639–3645. doi: 10.1021/ja01041a037. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Smallcombe S. H., Whitaker D. R., Richards J. H. Carbon nuclear magnetic resonance studies of the histidine residue in alpha-lytic protease. Implications for the catalytic mechanism of serine proteases. Biochemistry. 1973 Nov 6;12(23):4732–4743. doi: 10.1021/bi00747a028. [DOI] [PubMed] [Google Scholar]
- Inagami T., Patchornik A., York S. S. Participation of an acidic group in the chymotrypsin catalysis. J Biochem. 1969 May;65(5):809–819. doi: 10.1093/oxfordjournals.jbchem.a129081. [DOI] [PubMed] [Google Scholar]
- Krieger M., Kay L. M., Stroud R. M. Structure and specific binding of trypsin: comparison of inhibited derivatives and a model for substrate binding. J Mol Biol. 1974 Feb 25;83(2):209–230. doi: 10.1016/0022-2836(74)90388-x. [DOI] [PubMed] [Google Scholar]
- Lehmann M. S., Koetzle T. F., Hamilton W. C. Precision neutron diffraction structure determination of protein and nucleic acid components. IV. The crystal and molecular structure of the amino acid L-histidine. Int J Pept Protein Res. 1972;4(4):229–239. doi: 10.1111/j.1399-3011.1972.tb03424.x. [DOI] [PubMed] [Google Scholar]
- O'Leary M. H., Kluetz M. D. Identification of the rate-limiting step in the chymotrypsin-catalyzed hydrolysis of N-acetyl-L-tryptophanamide. J Am Chem Soc. 1970 Oct 7;92(20):6089–6090. doi: 10.1021/ja00723a062. [DOI] [PubMed] [Google Scholar]
- Rajender S., Han M., Lumry R. Studies of the chymotrypsinogen family of proteins. IX. Steady-state kinetics of the chymotryptic hydrolysis of N-acetyl-L-tryptophan ethyl ester at pH 8.0. J Am Chem Soc. 1970 Mar 11;92(5):1379–1385. doi: 10.1021/ja00708a045. [DOI] [PubMed] [Google Scholar]
- Rühlmann A., Kukla D., Schwager P., Bartels K., Huber R. Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor. Crystal structure determination and stereochemistry of the contact region. J Mol Biol. 1973 Jul 5;77(3):417–436. doi: 10.1016/0022-2836(73)90448-8. [DOI] [PubMed] [Google Scholar]
- Umeyama H., Imamura A., Nagata C. A molecular orbital study on the enzymic reaction mechanism of alpha-chymotrypsin. J Theor Biol. 1973 Oct;41(3):485–502. doi: 10.1016/0022-5193(73)90057-x. [DOI] [PubMed] [Google Scholar]


