Abstract
Aim
The neural cardiac therapy for heart failure (NECTAR-HF) was a randomized sham-controlled trial designed to evaluate whether a single dose of vagal nerve stimulation (VNS) would attenuate cardiac remodelling, improve cardiac function and increase exercise capacity in symptomatic heart failure patients with severe left ventricular (LV) systolic dysfunction despite guideline recommended medical therapy.
Methods
Patients were randomized in a 2 : 1 ratio to receive therapy (VNS ON) or control (VNS OFF) for a 6-month period. The primary endpoint was the change in LV end systolic diameter (LVESD) at 6 months for control vs. therapy, with secondary endpoints of other echocardiography measurements, exercise capacity, quality-of-life assessments, 24-h Holter, and circulating biomarkers.
Results
Of the 96 implanted patients, 87 had paired datasets for the primary endpoint. Change in LVESD from baseline to 6 months was −0.04 ± 0.25 cm in the therapy group compared with −0.08 ± 0.32 cm in the control group (P = 0.60). Additional echocardiographic parameters of LV end diastolic dimension, LV end systolic volume, left ventricular end diastolic volume, LV ejection fraction, peak V02, and N-terminal pro-hormone brain natriuretic peptide failed to show superiority compared to the control group. However, there were statistically significant improvements in quality of life for the Minnesota Living with Heart Failure Questionnaire (P = 0.049), New York Heart Association class (P = 0.032), and the SF-36 Physical Component (P = 0.016) in the therapy group.
Conclusion
Vagal nerve stimulation as delivered in the NECTAR-HF trial failed to demonstrate a significant effect on primary and secondary endpoint measures of cardiac remodelling and functional capacity in symptomatic heart failure patients, but quality-of-life measures showed significant improvement.
Keywords: Vagal stimulation, Heart failure, Autonomic nervous system
See page 404 for the editorial comment on this article (doi:10.1093/eurheartj/ehu363)
Introduction
Increased sympathetic activation and reduced parasympathetic tone, as reflected by reduced baroreflex sensitivity and/or decreased heart rate variability, are potentially important pathophysiological contributors to the progression of heart failure (HF) irrespective of aetiology, and are associated with poor outcome.1,2 Experimental augmentation of parasympathetic tone has recently emerged as a potential therapeutic approach to normalizing autonomic imbalance and inhibiting the progression of HF.3,4 The effect of enhancing parasympathetic tone via direct vagal nerve stimulation (VNS) was recently assessed in a non-randomized observational study of 32 HF patients with left ventricular (LV) systolic dysfunction, with results suggesting that vagal stimulation favourably influenced quality of life, exercise capacity, and LV remodelling.5,6
The neural cardiac therapy for heart failure (NECTAR-HF) trial (www.clinicaltrials.gov, NCT01385176) is the first randomized sham-controlled trial designed to evaluate whether right cervical VNS is safe and might attenuate cardiac remodelling, improve cardiac function, increase exercise capacity, enhance quality of life, and favourably impact circulating biomarkers in symptomatic HF patients with severe LV systolic dysfunction receiving guideline recommended medical therapy.7
Methods
The NECTAR-HF study design has been published previously.8 The complete protocol can be viewed in the Supplementary materials online that accompany this manuscript. Twenty-seven centres across Western Europe were approved for participation in the study by the appropriate ethics committees and regulatory agencies. The study was conducted in accordance with the Declaration of Helsinki, ISO 14155: 2011 and all other applicable regulations as determined by the country of submission. Participants provided written informed consent prior to enrolment.
Patient eligibility criteria
Patients were required to have a documented LV ejection fraction (LVEF) of ≤ 35%, an LV end diastolic dimension (LVEDD) of ≥55 mm, and a New York Heart Association (NYHA) classification of II or III. Patients also had to be treated with medical therapy per European heart failure guidelines7 for at least 30 days prior to enrolment. Key exclusion criteria included persistent or permanent atrial fibrillation, cardiac resynchronization (CRT) for <1 year or a QRS of >130 ms without CRT, type I diabetes, type II diabetes for >5 years, sleep disordered breathing that had been treated for <6 months, a surgically correctable cause of HF, recent HF hospitalization, or myocardial infarction (30 or 90 days, respectively), or an indication for dialysis.
Therapy
In all patients, implantation of the VNS system was performed within 45 days of the pre-implant screening period. Figure 1 shows the implanted system. A self-sizing bipolar helical lead was implanted around the right vagus nerve in the cervical region and the lead body was tunnelled over the clavicle. The terminal end was connected to the pulse generator that was implanted in a subcutaneous pectoral pocket. During the implant procedure, the NECTAR-HF system was tested for system integrity and to verify effective stimulation. Prior to discharge from the hospital, patients were instructed to charge their implanted device once a week for an hour by placing the wireless external charger over the generator. Patients returned to the clinic 14 ± 5 days after the implant for baseline assessment. At the completion of baseline testing, patients were randomized in a 2 : 1 block permuted manner to either therapy or control.
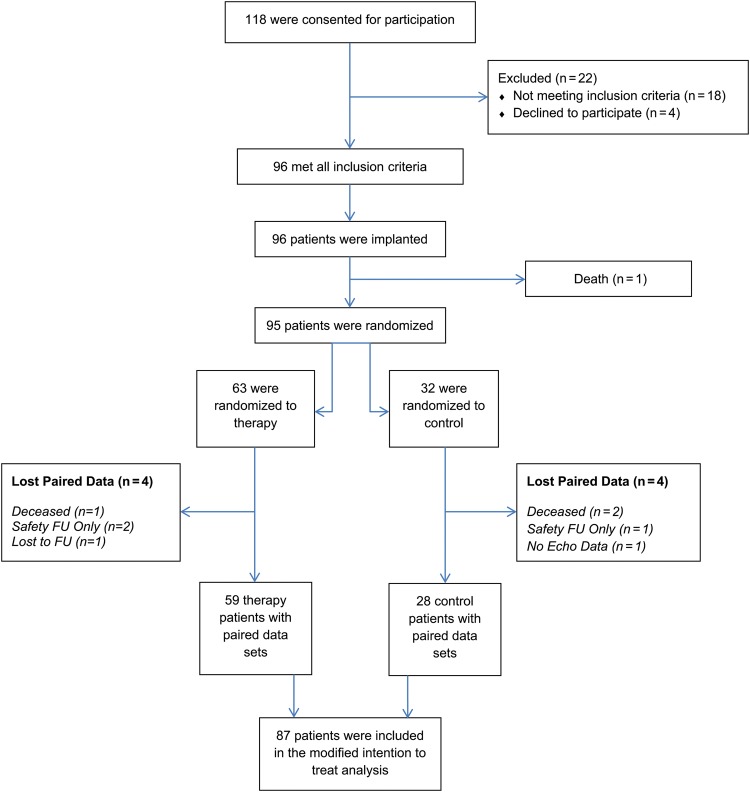
Figure 1.
Consort diagram for neural cardiac therapy for heart failure.
During therapy titration, the stimulation amplitude was increased until patients either experienced side effects that were unpleasant (e.g. neck pain or coughing), or the maximum allowed current for chronic stimulation was reached (4 mA). The recommended settings were 20 Hz, with 10 s on, 50 s off, and a pulse width of 300 µs. So as to maintain blinding, regardless of group randomization, all patients underwent therapy titration until first detectable level of stimulation, such as a tickling sensation in the throat, coughing, or voice alteration, but in the control group stimulation was turned off at the end of the visit.
Following the baseline visit, all patients returned for three stimulation titrations within the next 30 days. After the final titration, the 6-month time window began. Therapy patients received active stimulation; control patients' devices remained off. Additional follow-up visits were performed at 3 and 6 months for device checks, adverse event reporting, and/or endpoint assessments. Patients who had a defibrillator underwent routine device checks to assess for possible system interference.
Blinding
Programming of the generator was performed by a physician un-blinded to treatment assignment, while all other investigators and site study staff involved in endpoint data collection were blinded to randomization. Before and after titrations, a standard statement was read to patients informing them that they may feel sensations during titrations, or on a chronic basis, but that these sensations were not indicative that they were receiving therapeutic stimulation. At scheduled visits, patients were queried on whether they believed they were assigned to the therapy or control group. To assess the degree to which patients were blinded to treatment assignment, a blinding index analysis was performed. A detailed description of the analysis has been described elsewhere.9
At the end of 6 months, all patients in the control group had their devices turned ON to receive active therapy. Additional follow-up visits occur every 3 months through 18 month. Because NECTAR-HF is an on-going trial, the present report is limited to the results of the 6 month randomized and controlled phase.
Endpoints and sample size
The change in left ventricular end systolic diameter (LVESD) from baseline (randomization visit) to 6 months was selected as primary efficacy endpoint. Echocardiography exams were performed by treatment-blinded sonographers who were certified by the echocardiography core lab (Brigham and Women's Hospital, Boston, MA, USA). Details of the echocardiography views and analysis can be found in the Supplementary materials online.
Secondary endpoints included LV end systolic volume (LVESV), LVEF, peak VO2, N-terminal pro-hormone brain natriuretic peptide (NT-proBNP), Holter derived indices of autonomic nerve modulation (standard deviation of normal to normal beats, SDNN; standard deviation of the average normal to normal beats, SDANN; root mean square of successive differences, RMSSD), Minnesota Living with Heart Failure Questionnaire © (MLHFQ),9 the Short Form 36 Health Survey (SF-36)10 and NYHA functional class. All procedures for echocardiography, cardiopulmonary exercise testing, quality-of-life and plasma collection are described in the main design paper.8 NT-proBNP (Roche Elecsys ECLIA) was quantified at the Clinical Reference Laboratory (Lenexa, KS, USA) and the 45-biomarker Human InflammationMAP® v. 1.0 (multiplexed immunoassay) was performed at Myriad RBM (Austin, TX, USA).
Statistical analysis
The primary endpoint of LVESD was analysed using a modified intention to treat analysis on the final dataset, where only patients with paired datasets from baseline and 6 months were included in the analysis. Sample size calculation and the statistical analysis plan have been described elsewhere (Supplementary materials online),8 but was estimated that the 96 patients study was powered to detect a 5 ± 6 mm difference in LVESD between groups using a 2 : 1 randomization, assuming 40% data attrition.
An as-treated sub-analysis was performed including only patients who received per-protocol therapy for at least 85% of the randomization period. The primary endpoint of LVESD was tested using a general linear model, with LVESD as the outcome and randomization group, and baseline LVESD as a covariate in the model. Statistical testing was performed at a significance level of 5%.
Testing of all secondary outcomes, except NYHA, was performed using the same methodology that was used for the primary endpoint. NYHA was assessed using a Cochran-Armitage test for trend, in which the number of NYHA classes changed from baseline to 6 months was compared between the randomization groups. Exploratory analyses were performed on pre-specified endpoints and subgroups as defined in the Statistical Analysis Plan (see Supplementary materials online). All-cause mortality will be further formally evaluated as the primary safety endpoint at 18 months as specified in the protocol.
Results
Of the 118 patients enrolled, 96 were found to be eligible and were implanted across 24 centres (7 sites with 5–10 implants, 1 site with 13 implants). As shown in Figure 2, 87 patients completed the 6-month study having paired echocardiography exams. In addition, 86 patients had paired blood samples, and 83 had paired exercise tests available.
Figure 2.
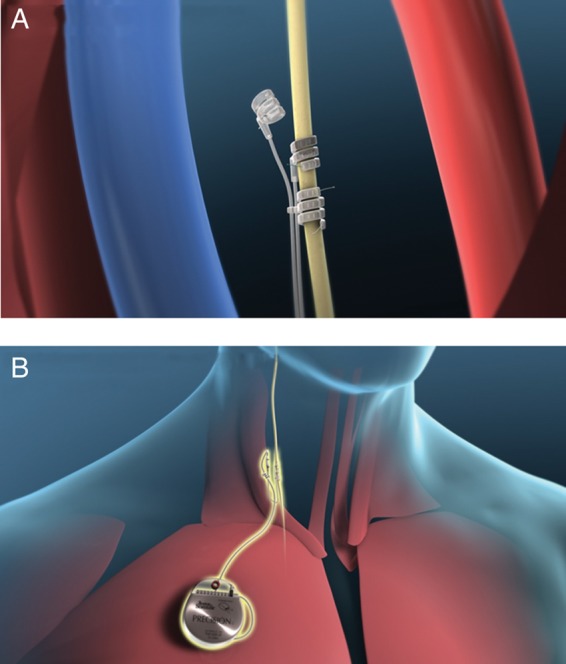
(A) Investigational bipolar helical vagal cuff. (B) Precision(TM) Pulse Generator and implanted lead.
Patient characteristics
Table 1 summarizes the patient characteristics. Body mass index was significantly greater and diuretic usage higher in the control group, while statin usage was statistically higher in the therapy group. No patients were treated with ivabradine. All other variables, including efficacy endpoints, were balanced between the therapy and control groups. The median pre-implant NT-proBNP was 882 pg/ml (inter-quartile range, IQR: 488–1926) and 879 (IQR: 370–1843) for control and therapy patients, respectively. The median pre-implant CRP levels were 2.6 μg/mL (IQR: 1.9–12.0) and 2.1 μg/mL (IQR: 1.0–4.7) for control and therapy patients, respectively.
Table 1.
Baseline patient characteristics at enrolment for the therapy and control groups
| Therapy (N = 63) | Control (N = 32) | P-value | |
|---|---|---|---|
| Demographics | |||
| Gender: male, n (%) | 56 (89) | 26 (81) | 0.31 |
| Age | 59.8 ± 12.2 | 59.3 ± 10.1 | 0.87 |
| Body mass index | 28.6 ± 5.9 | 31.2 ± 5.1 | 0.02 |
| NYHA II/III | 7/51 | 7/22 | 0.22 |
| ICD/CRT-D/No device | 51/5/7 | 22/4/6 | 0.68 |
| Resting heart rate (bpm) | 68.2 ± 13.2 | 71.3 ± 12.9 | 0.24 |
| Blood pressure (mmHg) | |||
| Systolic | 118 ± 17 | 115 ± 16 | 0.39 |
| Diastolic | 73 ± 10 | 73 ± 13 | 0.98 |
| Clinical history | |||
| Ischaemic heart failure, n (%) | 44 (70) | 20 (63) | 0.47 |
| Hypertension, n (%) | 29 (46) | 21 (66) | 0.07 |
| Renal disease n (%) | 12 (19) | 9 (28) | 0.31 |
| Heart failure hospitalization past 6 months, n (%) | 8 (13) | 4 (13) | 0.98 |
| Previous myocardial infarction, n (%) | 42 (67) | 19 (59) | 0.48 |
| Non-insulin dependent diabetes, n (%) | 14 (22) | 9 (28) | 0.53 |
| Sleep apnoea, n (%) | 9 (14) | 3 (9) | 0.50 |
| Cardiovascular medications | |||
| B-blockers, n (%) | 59 (94) | 30 (94) | 0.99 |
| Angiotensin converting enzyme inhibitor, n (%) | 51 (81) | 24 (75) | 0.50 |
| Angiotensin receptor blocker, n (%) | 17 (27) | 7 (22) | 0.59 |
| Mineralocorticoid receptor antagonist, n (%) | 43 (68) | 23 (72) | 0.72 |
| Loop diuretics, n (%) | 54 (86) | 32 (100) | 0.02 |
| Statin, n (%) | 50 (79) | 19 (59) | 0.04 |
Therapy
The mean stimulation amplitude for the therapy patients at the end of the 3rd titration was 1.24 ± 0.74 mA, and 1.42 ± 0.80 mA after the 3-month follow-up visit. The self-reported threshold for activating the laryngeal fibres at the 3rd titration visit was 0.99 ± 0.67 mA, and 1.17 ± 0.74 mA at the 3-month follow-up visit. During the full 6-month randomization period, there were 10 patients that were defined as having lost therapy due to magnet reset of the device (six patients), failure to adequately re-charge the stimulator (two patients), system explant due to infection (one patient), and elective deactivation of the device (one patient).
Blinding
Table 2 shows the results of a blinding assessment that was performed at the 6-month follow-up visit. The blinding index ranged from 0.31 in the inactive therapy group to 0.70 in the active therapy arm.
Table 2.
At the 6-month follow-up visit, patients were asked what to which group they believed they were randomized
| 6 months | ||||
|---|---|---|---|---|
| Randomization group | Patients' response to blinding |
Blinding Index (95% CI) | ||
| On | Off | Did not know | ||
| Off | 7 (24.1%) | 16 (55.2%) | 6 (20.7%) | 0.31 (0.08, 0.54) |
| On | 44 (77.2%) | 4 (7.0%) | 9 (15.8%) | 0.70 (0.48, 0.92) |
Data are presented as N (%). A blinding index of 0 means blinding was perfect, and score of 1 would be completely un-blinded.
Efficacy endpoints
Table 3 shows a summary of the primary and secondary endpoint outcomes. Analysis of the primary endpoint of LVESD revealed comparable changes from baseline to 6 months in the therapy and control groups. Other echocardiographic endpoints (LVEDD, LVESV, left ventricular end diastolic volume, and LVEF) and the additional endpoints of exercise capacity (peak VO2) and NT-proBNP likewise showed comparable changes in the two groups. Analysis of MLHFQ and SF-36 demonstrated statistically significant improvement with VNS treatment when compared with control. Figure 3 displays the NYHA results, showing that 62% of patients in the therapy group improved their functional class at least one point compared with only 45% of control patients (P = 0.032). Additional analyses, performed using pure intention to treat (imputed missing data), failed to show significance in echocardiography related measures. An as-treated analysis, excluding patients defined as having lost therapy (described above) did not alter the results of the primary or secondary endpoint outcomes. Finally, indexing the echocardiography analysis to body surface area did not impact the results.
Table 3.
Changes in primary and secondary efficacy endpoints from baseline to 6 months for therapy and control patients
| Endpoint | N | Therapy |
Control |
P-valuea | ||
|---|---|---|---|---|---|---|
| Baseline | 6-month | Baseline | 6-month | |||
| LVESD (cm) | 86 | 4.9 ± 0.9 | 4.9 ± 0.8 | 5.2 ± 0.7 | 5.1 ± 0.8 | 0.60 |
| LVEDD (cm) | 86 | 5.9 ± 0.7 | 5.8 ± 0.7 | 6.0 ± 0.6 | 6.0 ± 0.7 | 0.84 |
| LVEDV (ml) | 86 | 218.3 ± 67.1 | 207.4 ± 68.5 | 235.4 ± 46.7 | 221.3 ± 49.3 | 0.36 |
| LVESV (ml) | 86 | 154.7 ± 58.5 | 142.5 ± 57.1 | 164.0 ± 39.2 | 152.1 ± 43.8 | 0.86 |
| LVEF (%) | 86 | 30.5 ± 6.0 | 32.7 ± 6.4 | 30.8 ± 4.2 | 32.1 ± 5.6 | 0.27 |
| Peak VO2 (ml/kg/min) | 83 | 15.6 ± 3.9 | 15.8 ± 4.4 | 15.2 ± 3.3 | 14.7 ± 3.6 | 0.26 |
| MLHFQ score | 87 | 44.4 ± 22.2 | 35.8 ± 20.8 | 42.8 ± 25.1 | 41.8 ± 24.3 | 0.049 |
| SF-36 physical | 85 | 36.3 ± 7.6 | 41.2 ± 7.9 | 37.7 ± 7.9 | 38.4 ± 8.4 | 0.02 |
| SF-36 mental | 85 | 41.2 ± 7.9 | 43.8 ± 10.8 | 40.7 ± 10.9 | 41.1 ± 10.7 | 0.24 |
| NT-proBNP | 84 | 879 (370–1843) | 930 (409–1938) | 882 (488–1926) | 839 (302–1847) | 0.41 |
LVESD, left ventricular end systolic diameter; LVEDD, left ventricular end diastolic diameter; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire.
Data presented as mean ± SD for all values except NT-proBNP which is median (inter-quartile range). MLWHFQ intergroup difference, 95% confidence interval = −7.7 (−14.3, −0.03); SF-36 intergroup difference (95% confidence interval) = 3.7 (0.7, −6.7).
aComparing the therapy and control deltas from baseline to 6 months.
Figure 3.
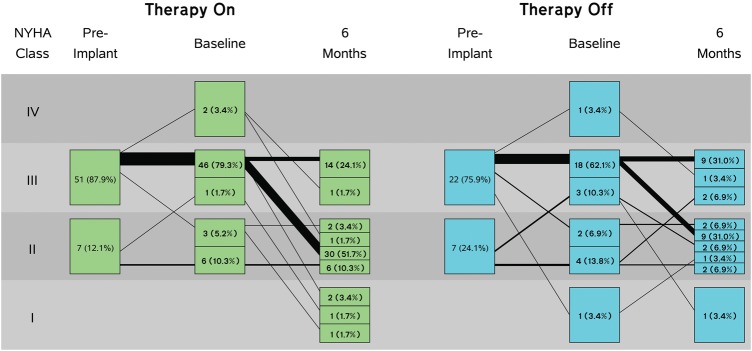
New York Heart Association class change from baseline to Month 6 (P = 0.032).
24-h Holter
As shown in Table 4, analysis of the 24-h Holter data did not reveal statistically significant differences in the changes from baseline to 6 months for minimum, maximum, or mean HR. Time domain measures of heart rate variability showed that SDANN was statistically increased in the active treatment group when compared with the control group, but not for RMSSD, SDNN, and the traditional frequency domain parameters.
Table 4.
Results of the 24-h Holter analysis
| Therapy (N = 55) |
Control (N = 28) |
P-value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 months | Δ therapy | Baseline | 6 months | Δ control | ||
| RMSSD (ms ± SD) | 78.8 ± 41.8 | 97.0 ± 40.2 | 18.2 ± 50.9 | 94.5 ± 31.5 | 89.9 ± 37.0 | −4.7 + 45.5 | 0.26 |
| SDNN (ms ± SD) | 146 ± 48.3 | 129.7 ± 52.1 | −16.3 + 60.3 | 146.3 ± 47.2 | 132.1 ± 41.3 | −14.2 + 60.9 | 0.83 |
| SDANN (ms ± SD) | 29.1 ± 2.1 | 29.4 ± 2.3 | 0.3 ± 2.0 | 29.6 ± 2.5 | 28.8 ± 2.2 | −0.8 + 2.1 | 0.03 |
| Mean HR (bpm ± SD) | 70.1 ± 10.4 | 70.7 ± 10.0 | 0.5 ± 8.2 | 69.2 ± 9.7 | 73.0 ± 10.7 | 3.8 ± 8.7 | 0.10 |
| Minimum HR (bpm ± SD) | 54.6 ± 9.2 | 53.2 ± 8.0 | −1.5 + 7.6 | 53.4 ± 8.6 | 54.6 ± 10.0 | 1.3 ± 6.8 | 0.14 |
| Maximum HR (bpm ± SD) | 102.4 ± 15.7 | 107.4 ± 19.2 | 4.9 ± 19.3 | 101.0 ± 15.9 | 109.0 ± 17.9 | 7.9 ± 17.2 | 0.56 |
Safety
Table 5 shows adverse events for the control and therapy groups. One patient died pre-randomization from 4 days post-operatively from a pulmonary embolism. There were three patient deaths that occurred between randomization and 6 months; two patients randomized to the control group died from heart failure complications 40 and 124 days after VNS implant, and one patient randomized to therapy died 127 days after VNS implant from worsening heart failure.
Table 5.
Summary of the serious adverse events and serious adverse device effects for the control and therapy patients
| Therapy (N = 63) |
Control (N = 32) |
|||||
|---|---|---|---|---|---|---|
| Events | Patients | % | Events | Patients | % | |
| Death and/or HF hospitalization | 11 | 7 | 11.1 | 11 | 5 | 15.6 |
| Death | 1 | 1 | 1.6 | 2 | 2 | 6.3 |
| HF hospitalization | 10 | 7 | 11.1 | 9 | 5 | 15.6 |
| Cardiovascular–Non-HF | 9 | 7 | 11.1 | 7 | 5 | 15.6 |
| Non-cardiovascular | 8 | 8 | 12.7 | 12 | 11 | 34.4 |
| Pulmonary | 0 | 0 | 0.0 | 3 | 3 | 9.4 |
| Genitourinary | 1 | 1 | 1.6 | 2 | 2 | 6.3 |
| Other Non-cardiovascular | 7 | 7 | 11.1 | 7 | 7 | 21.9 |
| Investigational system related* | 9 | 9 | 14.3 | 4 | 4 | 12.5 |
Data are reported for events ≥5%.
*Includes post-surgical infections of the lead and pulse generator, pulse generator failure leading to loss of therapy, and right recurrent laryngeal nerve injury.
An overall infection rate of 7.4% (7 infections) associated with the implanted system occurred in the entire cohort of 95 patients; three infections resulted in explant of the VNS system (two control, one therapy). Four infections were managed with antibiotics. One patient needed a pulse generator pocket revision because of problems recharging the device, and another patient underwent lead revision due to inappropriate lead movement. There were no significant differences in the occurrence of ICD shock delivery and/or anti-tachycardia pacing between the two groups (VNS-therapy 9.5%, control 6.5%; P = 0.71). All ICD shocks and anti-tachycardia pacing were adjudicated and determined not to be associated with interference from the investigational system.
Discussion
NECTAR-HF is the first randomized controlled trial to investigate a prescribed right-sided VNS protocol in an HF population. The study failed to demonstrate an improvement in LV remodelling parameters, LV function, or circulating biomarkers following 6 months of chronic VNS at the prescribed stimulation settings. NECTAR-HF did however demonstrate significant improvements in the subjective endpoints of NYHA functional class and heart failure related quality-of-life measures, although these findings should be interpreted with caution given the imperfect patient-level blinding (Table 2). The safety profile for this application of VNS appeared acceptable, with an overall infection rate comparable with that in patients implanted with a VNS system for the treatment of epilepsy.10 The small volume to implants per centre and the level of device implant experience likely contributed to an infection rate that was higher than is seen for other cardiac devices.
The lack of cardiac remodelling benefit was an unexpected finding given the results from pre-clinical experiments3,4,11,12 and the initial open-label pilot study.6 Several potential factors may have played a role in the apparent lack of translation from pre-clinical experiments to the randomized sham-controlled feasibility clinical study.
In the open-label pilot study of 32 systolic heart failure patients,6 the results suggested a beneficial effect on LV remodelling, LV function, 6-min hall walk, and NYHA class. The most important distinction between the two studies is the inclusion of a sham-treated control group in NECTAR-HF, which was absent in the previous pilot study. Although difficult to implement in device trials,13 the inclusion of a concurrent control group is of paramount importance, as recently underlined by the results of the Simplicity III trial.14 In the current study, patients receiving therapy reported significant improvements in heart failure related quality-of-life and NYHA functional class. This may be in part due to a placebo effect. Indeed, despite efforts to keep investigators and patients blinded to the delivered therapy, more patients in the therapy group correctly guessed their randomization assignment (Table 2). Titrating to the highest comfortable amplitude likely resulted in some patients detecting the sensation caused by chronic VNS. Control patients experienced these sensations only during titration. Thus, an assessment of appropriate blinding is important in randomized controlled device trials where proper blinding is challenging and often under-reported.15
Another potential reason for the observed lack of objective benefit in NECTAR-HF may be related to an incomplete understanding of appropriate dosing of VNS in humans. There are numerous stimulation parameters that can be deployed which impact the ‘dose’ of VNS a patient may receive: frequency, amplitude, duty cycle, timing to the cardiac cycle, and efferent/afferent nerve activation.16 The two parameters that are likely to be of most relevance for the activation of vagus nerves are the amplitude and the stimulation frequency. It is acknowledged from the large experience with VNS for epilepsy17 and by the experience of De Ferrari et al.6 that in most patients the titration of the stimulation to higher amplitudes is limited by side effects, which may impact the dose delivered.
The most appropriate technique of increasing ‘physiologic’ vagus nerve activation is still a matter of debate. In the present study, therapy patients experienced side effects (e.g. neck pain, coughing), which limited programming to low stimulation amplitudes. Since it appears that stimulation amplitude is inversely correlated with the stimulation frequency, the delivery of VNS at 20 Hz in NECTAR-HF may have reduced the maximum achievable current (mA). NECTAR-HF reached relatively low current (average 1.4 ± 0.8 mA) compared with a prior study investigating very low frequency (1–2 pulses per cardiac cycle, i.e. 1–3 Hz) that reached much higher current (4.1 ± 1.2 mA).6 By using a lower frequency, it may be possible to attain higher amplitudes of stimulation, which in turn allows the recruitment of a greater number of vagal fibres.18,19 However, it should be noted that pre-clinical studies in an established animal model of heart failure20 showed robust efficacy using NECTAR-HF stimulation parameters, and provided a strong rational for the use of a low amplitude, 20 Hz stimulation.12 Results were comparable with those using lower frequency and higher amplitudes.4 The preferential activation of efferent vs. afferent vagal fibres has also been proposed as an important therapeutic parameter. Efferent stimulation may be beneficial because of the direct innervation of the heart. However, afferent vagal stimulation may also contribute to the beneficial effects, leading to sympathetic withdrawal.21 The present study employed a helical bipolar electrode known to activate the nerve bi-directionally (i.e. both afferent and efferent vagal fibres).18 In contrast, the study by De Ferrari et al.6 employed an asymmetric bipolar multi-contact cuff electrode designed for preferential, but not exclusive, activation of vagal efferent fibres. Thus, stimulation characteristics may have contributed to the lack of significant benefit in NECTAR-HF, but on-going clinical trials are investigating alternative application parameters which may provide additional information. Autonomic Neural Regulation Therapy to Enhance Myocardial Function in Heart Failure is investigating 10 Hz VNS,22 and Increase of Vagal Tone in Chronic Heart Failure23 is investigating an asymmetric bipolar multi-contact cuff electrode delivering 1–2 pulses per cardiac cycle. Results of these studies may further provide insight into the ‘dosing’ dependencies of VNS for human heart failure.
Cardiac resynchronization is one of the few implantable devices for the treatment of chronic heart failure, and has been shown to induce a robust beneficial cardiac remodelling effect in the first 6 months and beyond.24 However, it may not be appropriate to compare CRT with VNS given the likely mechanistic differences, and thus the 6 month randomized period of this trial may have been too short to detect changes in cardiac remodelling. Remodelling will be further assessed at the scheduled 12- and 18-month follow-up visits. However, the lack of a concurrent control group after the 6-month randomization period will limit the interpretation of the findings.
Inappropriate patient selection may have also contributed to the neutral findings. The relatively low levels of NT-proBNP, the lack of cardiac remodelling progression, and the low levels of inflammatory markers suggest that patients were well managed and in a stabilized phase of their HF trajectory. The selection of patients with direct markers of autonomic imbalance could enhance the potential to respond to vagal stimulation. The regulation of systemic inflammation by the vagus nerve has been demonstrated in a series of acute experimental studies25–29 and although the mechanism by which this occurs is not well understood30 the selection of patients with markers of systemic inflammation may also be considered. Thus, compared with the pre-clinical models with active remodelling and evidence of inflammation, the relatively controlled heart failure progression in the NECTAR-HF patients may have limited the ability to demonstrate the benefit of VNS.
The importance of heart rate variability has been demonstrated previously.31 Results of the Holter data in the present study suggest that VNS had no effect in decreasing the mean, minimum or maximum HR, but showed a modest effect in modulating HR variability through an improvement in SDANN. There was also a non-significant improvement in RMSSD. Whether the magnitude of change observed in this study has a biological significance or not is unclear.
Although robust pre-clinical data showed the benefit of VNS, the NECTAR-HF trial failed to demonstrate a successful clinical translation of VNS therapy to the primary endpoint. There were statistically significant improvements seen in the quality-of-life measures, and there were no significant safety concerns. The specified stimulation protocol of VNS used in this randomized sham-controlled clinical trial was shown to be ineffective for the treatment of heart failure. Additional clinical research still needs to be performed to determine if alternative translation methods can become an effective heart failure therapy.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was funded fully by Boston Scientific Corporation, Marlborough, MA, USA. Funding to pay the Open Access publication charges for this article was provided by Boston Scientific Corporation.
Conflict of interest: F.Z. receives honoraria from Air Liquide, Bayer, Biomérieux, Biotronik, Boston Scientific, CVCRx, Janssen, Novartis, Pfizer, Resmed, Roche Diagnostics, Sanofi, Servier, St Jude, Takeda, speaker fees from Mitsubishi, and owns stocks at CVCT and CardioRenal diagnostics; G.M.D.F. receives honoraria from Amgen, Boston Scientific, Menarini and Merck; S.R., D.D., S.M., C.S., B.S., A.R., N.W., and K.S. are employees of Boston Scientific Corporation. S.D.S. has received research funding from Boston Scientific Corporation for service as the Echocardiography Core Laboratory. P.N. has received research funding from Boston Scientific Corporation. F.Z., G.M.D.F., H.K., A.T., D.W., J.B., and C.B. have received modest consulting fees from Boston Scientific Corporation for serving on the NECTAR-HF steering committee. N.W., S.B.R., S.M., D.D., C.S., A.R., K.M.S., and B.S. are employees of Boston Scientific Corporation. C.B., M.A.C., and A.D’O. have no conflicts of interest to report.
Acknowledgements
The authors thank Juan Hincapie, Brian Soltis, David Ternes, Jana Meschede, Kenny Wynants, David Carrero, Frances Pinero, and Federica Paltrinieri for providing valuable clinical support to physicians and patients. Additionally, the authors wish to thank Dr Hans van der Aa for his invaluable surgical support.
Appendix
Steering committee
Faiez Zannad (Chairman), Gaetano M. De Ferrari, Josep Brugada, Jay Wright, Helmut Klein, Anton Tuinenburg, Christian Butter, John McMurray, and Kenneth E. Stein.
Clinical events committee
Luigi Tavazzi, Daniel Gras, and Henk C.E. van Lambalgen.
Data safety monitoring committee
Henry J. Dargie, Erland Erdmann, Poul Erik Bloch Thomsen, Andreas Schulze-Bonhage, and Ian Ford.
Investigators
BELGIUM: Dr Jean-Benoit le Polain de Waroux, Cliniques Universitaires Saint-Luc A.S.B.L., Brussels; CZECH REPUBLIC: Prof. Petr Neuzil, Nemocnice Na Homolce, Prague; FRANCE: Prof. Salem Kacet, CHRU de Lille, Lille; Prof. Faiez Zannad, CHRU Nancy Brabois, Nancy; GERMANY: Dr Christian Butter, Immanuel Klinikum Bernau Herzzentrum Brandenburg, Bernau; Prof. Markus Zabel, Georg-August-Universitaet Goettingen, Goettingen; Dr Ali Aydin, Universitaetsklinik Eppendorf; Dr Christian Kühne Universitaetsklinikum Leipzig, Leipzig; Dr Christian Meyer, Universitätsklinikum Düsseldorf, Dusseldorf; ITALY: Dr Antonio D'Onofrio, ‘Azienda Ospedaliera Dei Colli’ Monaldi-Cotugno-CTO, Napoli; Dr Gaetano M. De Ferrari, IRCCS Policlinico S. Matteo, Pavia; Dr Maurizio Lunati, Azienda Ospedaliera Ospedale Niguarda Ca’ Granda, Milan; SPAIN: Dr Maria Ángeles Castel, Hospital Clinico de Barcelona, Barcelona; Dr Ignacio Fernandez Lozano, Hospital Puerta de Hierro, Madrid; Dr Juan José Gavira Gómez, Clinica Universitaria de Navarra, Pamplona; Dr María López Gil, Hospital 12 de Octubre, Madrid; Dr Luis Almenar Bonet, Hospital Universitario La Fe, Valencia; NETHERLANDS: Dr Cornelis Botman, Catharina Ziekenhuis; Dr Anton Tuinenburg, Universitair Medisch Centrum, Utrecth; UNITED KINGDOM: Dr Angus Nightingale, Bristol Royal Infirmary, Bristol; Dr Pier Lambiase, The Heart Hospital, London; Dr David Wright, Liverpool Heart and Chest Hospital, Liverpool; Dr Prapa Kanagaratnam, St. Mary's Hospital Imperial College, London; Dr Francis Murgatroy, King's College Hospital, London.
References
- 1.Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, et al. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation. 1997;96:3450–3458. doi: 10.1161/01.cir.96.10.3450. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Anker SD, Chua TP, Szelemej R, Piepoli M, Adamopoulos S, et al. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;79:1645–1650. doi: 10.1016/s0002-9149(97)00215-4. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 4.Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Failure Rev. 2011;16:171–178. doi: 10.1007/s10741-010-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz PJ, De Ferrari GM, Sanzo A, Landolina M, Rordorf R, Raineri C, et al. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Failure. 2008;10:884–891. doi: 10.1016/j.ejheart.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 6.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 7.Members ATF, McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 8.De Ferrari GM, Tuinenburg AE, Ruble S, Brugada J, Klein H, Butter C, et al. Rationale and study design of the NEuroCardiac TherApy foR Heart Failure Study: NECTAR-HF. Eur J Heart Failure. 2014;16:692–699. doi: 10.1002/ejhf.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Control Clin Trials. 2004;25:143–156. doi: 10.1016/j.cct.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 10.DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41:1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR, et al. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Failure. 2009;2:692–699. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- 12.Hamann JJ, Ruble SB, Stolen C, Wang M, Gupta RC, Rastogi S, et al. Vagus nerve stimulation improves left ventricular function in a canine model of chronic heart failure. Eur J Heart Failure. 2013;15:1319–1326. doi: 10.1093/eurjhf/hft118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zannad F, Stough WG, Pina IL, Mehran R, Abraham WT, Anker SD, et al. Current challenges for clinical trials of cardiovascular medical devices. Int J Cardiol. 2014;175:30–37. doi: 10.1016/j.ijcard.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. New Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 15.Schulz KF, Grimes DA, Altman DG, Hayes RJ. Blinding and exclusions after allocation in randomised controlled trials: survey of published parallel group trials in obstetrics and gynaecology. BMJ (Clin Res Ed) 1996;312:742–744. doi: 10.1136/bmj.312.7033.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Ferrari GM, Schwartz PJ. Vagus nerve stimulation: from pre-clinical to clinical application: challenges and future directions. Heart Fail Rev. 2011;16:195–203. doi: 10.1007/s10741-010-9216-0. [DOI] [PubMed] [Google Scholar]
- 17.Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg. 2011;115:1248–1255. doi: 10.3171/2011.7.JNS11977. [DOI] [PubMed] [Google Scholar]
- 18.Castoro MA, Yoo PB, Hincapie JG, Hamann JJ, Ruble SB, Wolf PD, et al. Excitation properties of the right cervical vagus nerve in adult dogs. Exp Neurol. 2011;227:62–68. doi: 10.1016/j.expneurol.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Yoo PB, Lubock NB, Hincapie JG, Ruble SB, Hamann JJ, Grill WM. High-resolution measurement of electrically-evoked vagus nerve activity in the anesthetized dog. J Neural Eng. 2013;10:026003. doi: 10.1088/1741-2560/10/2/026003. [DOI] [PubMed] [Google Scholar]
- 20.Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, et al. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;260:H1379–H1384. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 21.De Ferrari G, Schwartz P. Vagus nerve stimulation: from pre-clinical to clinical application: challenges and future directions. Heart Failure Rev. 2011;16:195–203. doi: 10.1007/s10741-010-9216-0. [DOI] [PubMed] [Google Scholar]
- 22.Dicarlo L, Libbus I, Amurthur B, Kenknight BH, Anand IS. Autonomic regulation therapy for the improvement of left ventricular function and heart failure symptoms: the ANTHEM-HF Study. J Cardiac Failure. 2013;19:655–660. doi: 10.1016/j.cardfail.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Hauptman PJ, Schwartz PJ, Gold MR, Borggrefe M, Van Veldhuisen DJ, Starling RC, et al. Rationale and study design of the increase of vagal tone in heart failure study: INOVATE-HF. Am Heart J. 2012;163:954–962. doi: 10.1016/j.ahj.2012.03.021. e1. [DOI] [PubMed] [Google Scholar]
- 24.Verhaert D, Grimm RA, Puntawangkoon C, Wolski K, De S, Wilkoff BL, et al. Long-term reverse remodeling with cardiac resynchronization therapy results of extended echocardiographic follow-up. J Am Coll Cardiol. 2010;55:1788–1795. doi: 10.1016/j.jacc.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu Rev Immunol. 2012;30:313–335. doi: 10.1146/annurev-immunol-020711-075015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 28.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 30.Martelli D, Yao ST, McKinley MJ, McAllen RM. Reflex control of inflammation by sympathetic nerves, not the vagus. J Physiol. 2014;592:1677–1686. doi: 10.1113/jphysiol.2013.268573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzzetti S, Rovere MTL, Pinna GD, Maestri R, Borroni E, Porta A, et al. Different spectral components of 24 h heart rate variability are related to different modes of death in chronic heart failure. Eur Heart J. 2005;26:357–362. doi: 10.1093/eurheartj/ehi067. [DOI] [PubMed] [Google Scholar]