Abstract
Aims
Although active-controlled trials with renin–angiotensin inhibitors are ethically mandated in heart failure with reduced ejection fraction, clinicians and regulators often want to know how the experimental therapy would perform compared with placebo. The angiotensin receptor-neprilysin inhibitor LCZ696 was compared with enalapril in PARADIGM-HF. We made indirect comparisons of the effects of LCZ696 with putative placebos.
Methods and results
We used the treatment-arm of the Studies Of Left Ventricular Dysfunction (SOLVD-T) as the reference trial for comparison of an ACE inhibitor to placebo and the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity-Alternative trial (CHARM-Alternative) as the reference trial for comparison of an ARB to placebo. The hazard ratio of LCZ696 vs. a putative placebo was estimated through the product of the hazard ratio of LCZ696 vs. enalapril (active-control) and that of the historical active-control (enalapril or candesartan) vs. placebo. For the primary composite outcome of cardiovascular death or heart failure hospitalization in PARADIGM-HF, the relative risk reduction with LCZ696 vs. a putative placebo from SOLVD-T was 43% (95%CI 34–50%; P < 0.0001) with similarly large effects on cardiovascular death (34%, 21–44%; P < 0.0001) and heart failure hospitalization (49%, 39–58%; P < 0.0001). For all-cause mortality, the reduction compared with a putative placebo was 28% (95%CI 15–39%; P < 0.0001). Putative placebo analyses based on CHARM-Alternative gave relative risk reductions of 39% (95%CI 27–48%; P < 0.0001) for the composite outcome of cardiovascular death or heart failure hospitalization, 32% (95%CI 16–45%; P < 0.0001) for cardiovascular death, 46% (33–56%; P < 0.0001) for heart failure hospitalization, and 26% (95%CI 11–39%; P < 0.0001) for all-cause mortality.
Conclusion
These indirect comparisons of LCZ696 with a putative placebo show that the strategy of combined angiotensin receptor blockade and neprilysin inhibition led to striking reductions in cardiovascular and all-cause mortality, as well as heart failure hospitalization. These benefits were obtained even though LCZ696 was added to comprehensive background beta-blocker and mineralocorticoid receptor antagonist therapy.
Keywords: Heart failure, Angiotensin II, Natriuretic peptides
See page 410 for the editorial comment on this article (doi:10.1093/eurheartj/ehu501)
Introduction
Ethically, new antagonists of the renin–angiotensin system such as angiotensin-receptor blockers (ARBs) and direct renin inhibitors have to be tested in heart failure with reduced ejection fraction (HF-REF) in active-controlled comparisons with an angiotensin converting enzyme (ACE) inhibitor.1–4 Recently, LCZ696 which both blocks the angiotensin II type 1 receptor as well as inhibits neprilysin, the enzyme responsible for degradation of natriuretic and other vasoactive peptides, was also compared with enalapril in patients with HF-REF.5 LCZ696 was superior to enalapril, reducing the primary composite outcome of cardiovascular death or heart failure hospitalization (and both components of this composite), as well as all-cause mortality. Although active-controlled trials such as this may be ethically mandated, clinicians and regulators often want to know how the experimental therapy would have performed had it been compared directly with placebo. Well-developed statistical approaches allow such indirect comparisons to be made, assuming that a comparison of the active-control (standard therapy) and placebo is available, which is the case in HF-REF.6–8 We describe putative placebo analyses for LCZ696 using both a placebo-controlled ACE inhibitor and a similar ARB trial in HF-REF.
Methods
We used the treatment-arm of the Studies Of Left Ventricular Dysfunction (SOLVD-T) as the reference trial for comparison of an ACE inhibitor to placebo.9 We used the Candesartan in Heart failure; Assessment of Reduction in Mortality and morbidity-Alternative trial (CHARM-Alternative) as the reference trial for comparison of an ARB to placebo.10 These are the only large randomized trials comparing a renin–angiotensin system inhibitor to placebo in patients with predominantly New York Heart Association (NYHA) Class II and III heart failure with a reduced ejection fraction and the only trials reporting long-term clinical outcomes. As such, they are well suited for comparison with PARADIGM-HF.
SOLVD-T
Patients in NYHA functional class II–IV with an LVEF ≤ 35% were eligible for SOLVD-T if aged ≤80 years and not treated with an ACE inhibitor.9 There were a number of exclusion criteria including a serum creatinine >177 µmol/l (>2.0 mg/dL). Patients entered a single-blind run-in period of 2–7 days treatment with enalapril 2.5 mg bid followed by a period of 14–17 days treatment with placebo. Thereafter, the 2569 remaining patients were randomly assigned to enalapril titrated to a target dose of 10 mg bid or matching placebo. The primary endpoint was all-cause mortality and the mean follow-up was 41.4 months.
CHARM-alternative
Patients in NYHA functional class II–IV with an LVEF ≤ 40% were eligible for CHARM-Alternative if not receiving an ACE inhibitor due to previous intolerance.10 There were a number of exclusion criteria including a serum creatinine ≥265 µmol/l (≥3.0 mg/dL). Patients received other standard medical therapy including diuretics, beta-blockers, and spironolactone. A total of 2028 patients were randomized to candesartan, titrated as tolerated up to a maximum daily dose of 32 mg or matching placebo. There was no run-in period. The primary endpoint was the composite of cardiovascular mortality or heart failure hospitalization and the median follow-up was 33.7 months.
PARADIGM-HF
Patients in NYHA functional class II–IV, previously treated with an ACE inhibitor or ARB and with an LVEF ≤ 40% (changed to ≤35% by amendment dated 15 December 2010), and a plasma B-type natriuretic peptide (BNP) of at least 150 pg/mL [or N-terminal proBNP (NT-proBNP) of at least 600 pg/mL] or a BNP of at least 100 pg/mL (or NT-proBNP of at least 400 pg/mL) if hospitalized for heart failure within the last 12 months were eligible for PARADIGM-HF.5 Patients were required to be treated with a beta-blocker if tolerated and mineralocorticoid receptor antagonist (MRA) therapy was recommended in accordance with major international guidelines. There were a number of exclusion criteria including an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 body-surface area. Patients entered a single-blind run-in period of 2 weeks treatment with enalapril 10 mg bid followed by a period of 2–4 weeks treatment with LCZ696 100 mg twice daily, increasing to 200 mg twice daily. Thereafter, the 8442 remaining patients were randomly assigned to LCZ696 200 mg bid or matching enalapril 10 mg bid. The primary endpoint was cardiovascular death or heart failure hospitalization and the median follow-up was 27 months.
Figure 1 shows in schematic format the comparisons made. The hazard ratio of LCZ696 vs. a putative placebo was estimated through the product of the hazard ratio of LCZ696 vs. enalapril (active-control) and that of the historical active-control (enalapril or candesartan) vs. placebo with the assumption that the hazard ratio in the active-control vs. placebo is the same as would have been obtained had a placebo arm been possible in the trial with LCZ696. The standard error, used in the derivation of the 95% confidence interval of the product, is derived from the square root of the sum of the two squared standard errors of the logarithmic hazard ratios. The hazard ratio and its standard error of the logarithmic hazard ratio for the active control vs. placebo are obtained from the published historical data. The method used here was similar to that used in Fisher, Gent, and Büller using odds ratios.8 In this analysis, we made the assumption that the effect of LCZ696 compared with enalapril would be the same as that of LCZ696 compared with candesartan.
Figure 1.
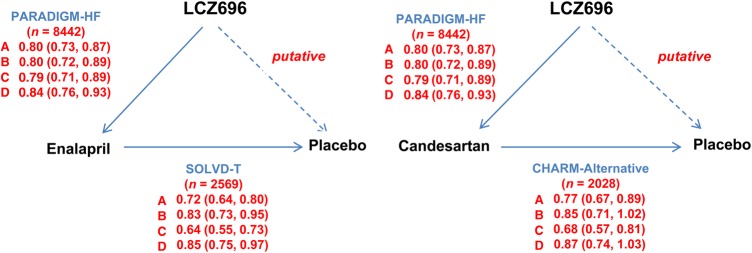
Schematic of the trials and comparisons used in the putative placebo analysis. SOLVD-T = treatment arm of the Studies Of Left Ventricular Dysfunction (comparing enalapril with placebo). CHARM-Alternative = Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity-Alternative trial (comparing candesartan with placebo). PARADIGM-HF = Prospective comparison of Angiotensin Receptor Neprilysin inhibitor (ARNI) with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (comparing LCZ696 with enalapril). For enalapril, solid arrows indicate directly performed comparison in the trials specified (direction of arrow indicates comparison of experimental treatment to the reference treatment); the interrupted arrow indicates the indirect, putative, placebo comparison (direction of arrow indicates comparison of experimental treatment to the putative placebo). For candesartan, the figure structure is the same except that it is assumed that the comparison in PARADIGM-HF was LCZ696 vs. candesartan (as opposed to enalapril in reality). The hazard ratios shown are those measured in the trials specified; the indirectly calculated hazard ratios for LCZ696 against placebo are shown in Table 3. (a) Cardiovascular death or heart failure hospitalization, (b) cardiovascular death, (c) heart failure hospitalization, and (d) all-cause mortality.
Results
Table 1 provides a summary of patient characteristics and baseline treatment in SOLVD-T, CHARM-Alternative, and PARADIGM-HF.
Table 1.
Key baseline characteristics of patients in trials compared
| SOLVD-T (n = 2569) | CHARM-Alternative (n = 2028) | PARADIGM-HF (n = 8399) | |
|---|---|---|---|
| Age, years | 61 (10) | 67 (11) | 64 (11) |
| Female sex, % | 20 | 32 | 22 |
| NYHA class, % | |||
| I | 11 | 0 | 5 |
| II | 57 | 48 | 70 |
| III | 30 | 49 | 24 |
| IV | 2 | 4 | 1 |
| History | |||
| MI | 66 | 61 | 43 |
| Hypertension | 42 | 50 | 71 |
| Diabetes mellitus | 26 | 27 | 35 |
| Systolic BP, mmHg | 125 (18) | 130 (19) | 121 (19) |
| LVEF, % | 25 (7) | 30 (7.4) | 29 (6.2) |
| Background therapy (%) | |||
| Diuretic | 85 | 85 | 80 |
| Digoxin | 67 | 45 | 30 |
| Beta-blocker | 8 | 55 | 93 |
| MRA | NR | 24 | 56 |
BP, blood pressure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; NR = not reported.
The subjects enrolled in PARADIGM-HF were similar to those enrolled in SOLVD-T in terms of the age, sex, NYHA functional class distribution, LVEF, and eGFR. Hypertension was more common in PARADIGM-HF and prior myocardial infarction was more common in SOLVD-T. Beta-blocker use was much more common, and digoxin use less common, in PARADIGM-HF, compared with SOLVD-T. Mineralocorticoid use was not reported in SOLVD-T.
The patients enrolled in PARADIGM-HF (and SOLVD-T) were younger and less often female compared with those in CHARM-Alternative. The NYHA class distribution was different with more patients in NYHA class II in PARADIGM-HF (and SOLVD-T) and fewer in NYHA class III and IV, than in CHARM-Alternative. Systolic blood pressure was lower in PARADIGM-HF (and SOLVD-T) than in CHARM-Alternative. Beta-blocker and MRA use was more common in PARADIGM-HF than in CHARM-Alternative.
Table 2 shows the number of events and event rates in the three trials for the clinical outcomes of interest. Table 3 summarizes the treatment effects for these outcomes of interest in the three trials. In SOLVD-T and CHARM-Alternative, active therapy was superior to placebo, leading to statistically significant risk-reductions in the composite of cardiovascular death or heart failure hospitalization (and heart failure hospitalization alone) with both treatments tested. However, while the reductions in cardiovascular and all-cause mortality with enalapril were statistically significant in SOLVD-T, they were not in CHARM-Alternative, although they were qualitatively and quantitatively similar to SOLVD-T. In PARADIGM-HF, all outcomes were reduced significantly by LCZ696 compared with enalapril.
Table 2.
Number of events and event rates (per 100 patient-years) in trials compared
| Outcome number (ratea) | SOLVD-T |
CHARM-Alternative |
PARADIGM-HF |
|||
|---|---|---|---|---|---|---|
| Placebo (n = 1284) | Enalapril (n = 1285) | Placebo (n = 1015) | Candesartan (n = 1013) | Enalapril (n = 4212) | LCZ696 (n = 4187) | |
| CV death or HF hospitalization | 707 (26.2) | 573 (18.5) | 406 (18.2) | 334 (13.8) | 1117 (13.2) | 914 (10.5) |
| CV death | 461 (13.7) | 399 (11.2) | 252 (9.8) | 219 (8.2) | 693 (7.5) | 558 (6.0) |
| HF hospitalization | 470 (17.2) | 332 (10.9) | 286 (12.8) | 207 (8.6) | 658 (7.7) | 537 (6.2) |
| All-cause mortality | 510 (15.1) | 452 (12.8) | 296 (11.5) | 265 (10.0) | 835 (9.0) | 711 (7.6) |
CV, cardiovascular; HF, heart failure.
aRate per 100 patient-years.
Table 3.
Treatment comparisons and hazard ratios for the clinical outcomes analysed
| SOLVD-T (n = 2569) | CHARM-Alternative (n = 2028) | PARADIGM-HF (n = 8399) | |
|---|---|---|---|
| Study treatments | |||
| Reference treatment | Placebo | Placebo | Enalapril 10 mg bid |
| Experimental treatment | Enalapril 10 mg bid | Candesartan 32 mg qd | LCZ696 200 mg bid |
| Clinical outcomes (HR, 95% CI) | |||
| CV death or HF hospitalization | 0.72 (0.64, 0.80) | 0.77 (0.67, 0.89) | 0.80 (0.73, 0.87) |
| CV death | 0.83 (0.73, 0.95) | 0.85 (0.71, 1.02) | 0.80 (0.71, 0.89) |
| HF hospitalization | 0.64 (0.55, 0.73) | 0.68 (0.57, 0.81) | 0.79 (0.71, 0.89) |
| All-cause mortality | 0.85 (0.75, 0.97) | 0.87 (0.74, 1.03) | 0.84 (0.76, 0.93) |
CV, cardiovascular; HF, heart failure; HR, hazard ratio.
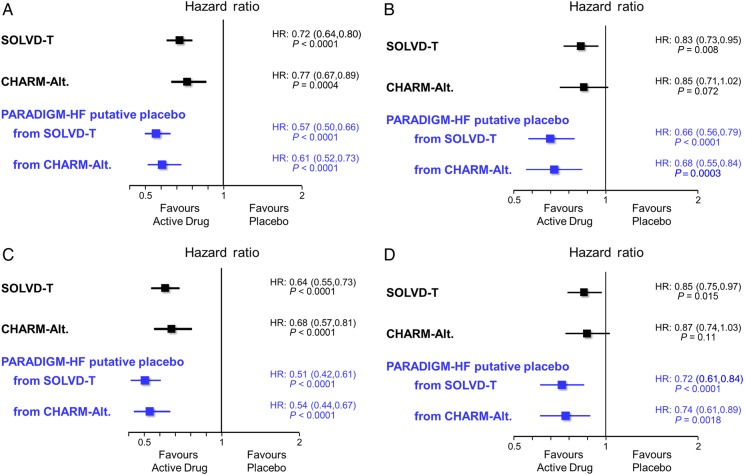
Figure 2A–D shows the putative placebo analyses for LCZ696. For the primary composite outcome of cardiovascular death or heart failure hospitalization in PARADIGM-HF, the relative risk reduction with LCZ696 vs. a putative placebo from SOLVD-T was 43% (95%CI 34–50%; P < 0.0001) with similarly large effects on cardiovascular death (34%, 21–44%; P < 0.0001) and heart failure hospitalization (49%, 39–58%; P < 0.0001). For all-cause mortality, the reduction compared with a putative placebo was 28% (95%CI 16–39%; P < 0.0001). Qualitatively and quantitatively, very similar effects were seen when LCZ696 was compared with a putative placebo from CHARM-Alternative (Figure 2).
Figure 2.
Putative placebo analysis based upon the Studies Of Left Ventricular Dysfunction (SOLVD-T) as the reference trial for comparison of an angiotensin converting enzyme inhibitor to placebo and the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity-Alternative trial (CHARM-Alt.) as the reference trial for comparison of an angiotensin receptor blocker to placebo. (A) Composite of death from cardiovascular causes or heart failure hospitalization, (B) cardiovascular death, (C) heart failure hospitalization, and (D) all-cause mortality.
Using the analysis based on CHARM-Alternative, compared with a putative placebo, LCZ696 would have avoided or postponed 71 composite cardiovascular death or heart failure hospitalization events, 31 cardiovascular deaths, 59 first heart failure hospitalizations, and 18 deaths from any cause per 1000 patient-years of treatment.
Discussion
Because it is unethical to withhold an ACE inhibitor or ARB in patients with HF-REF, LCZ696 could not be compared directly with placebo. Therefore, we made indirect comparisons, using putative placebos. Employing a standard statistical approach, we quantified the effect of LCZ696 on a number of key cardiovascular outcomes, and on all-cause mortality. Compared in this way, LCZ696 was shown to have striking effects on all outcomes examined, with the relative risk reduction for cardiovascular mortality was 32–34% (for cardiovascular morbidity and mortality it was 39–43%) There were even larger effects on heart failure hospitalization, with relative risk reductions of 46–49%. These indirectly estimated effects are convincing because they were highly statistically significant. They are also highly clinically relevant as they are similar to the largest effects obtained when other proven pharmacological therapies were compared directly to placebo (i.e. to the effect of beta-blockers on cardiovascular mortality and MRAs on heart failure hospitalization). Importantly, in PARADIGM-HF, these benefits were obtained when LCZ696 was ‘added’ to background beta-blocker and MRA therapy.
Although the analytical approach we used is well recognized and has been employed in regulatory assessments of new drugs, it relies on certain assumptions that often cannot be met as fully as is desirable.6–8 These include the conditions that the placebo-controlled trial(s) and the active-controlled trial(s) used the same reference drug and in the same dose, that same outcomes were evaluated, the populations studied were similar, and that background therapy did not change between the older and newer trial(s).6–8,11,12 Only in this way can the effect of the reference compound (in this case enalapril) against placebo be considered to be constant. Our analysis meets some but not all of these requirements. We used the same ACE inhibitor as SOLVD-T, the same dosing-schedule and achieved a similar average daily dose (16.6 mg SOLVD-T and 18.9 mg in PARADIGM-HF). All the outcomes examined were similar although in SOLVD-T the cause of death and hospitalization was determined by the local site investigator rather than by a central committee as in CHARM-Alternative and PARADIGM-HF.9 The patients enrolled in PARADIGM-HF and SOLVD-T were perhaps more similar than might be expected given the 23-year gap between these trials. However, there were major differences in background pharmacological therapy between SOLVD-T and PARADIGM-HF, reflecting the accrual of new evidence and evolution of guidelines over that period.13,14 Beta-blocker use was much more common in PARADIGM-HF and there was probably a similar difference in MRA use, although we do not know for certain as use of MRAs was not reported in SOLVD-T. These differences might raise concerns that we cannot be sure about the constancy of the effect of enalapril. However, CHARM-Alternative provides reassurance in this respect.10 As documented in Table 3, the effect of candesartan, compared with placebo, in CHARM-Alternative was qualitatively and quantitatively remarkably similar to the effect of enalapril in SOLVD-T on all outcomes examined. This was despite the more frequent use of beta-blockers in CHARM-Alternative and probable greater MRA use as well (and, importantly, the treatment-effect of candesartan was not modified by either of these background therapies in CHARM-Alternative). In a similar way, CHARM-Alternative also provides reassurance with respect to the other relatively small differences in the patient populations enrolled in SOLVD-T and PARADIGM-HF. This is because the effect of candesartan was similar to that of enalapril, despite more notable difference between the characteristics of patients enrolled in CHARM-Alternative compared with those enrolled in SOLVD-T (and PARADIGM-HF). This uniformity of treatment-effect makes population differences less of a concern. In fact, although a different inhibitor of the renin–angiotensin system was tested in CHARM-Alternative, in a somewhat different population which, as mentioned earlier, was receiving more-contemporary therapy than in SOLVD-T, the putative placebo analysis using CHARM-Alternative gave very similar findings to the same analytical approach using SOLVD-T. For these reasons, we believe that our study is as robust as it is possible to be with analyses of this type.
Although our analysis had the strengths outlined earlier, it also had the fundamental limitation of all analyses of this type which is that they are indirect and not direct comparisons. Although early termination of trials can theoretically overestimate the magnitude of a treatment effect, we believe that this is unlikely in PARADIGM-HF because we achieved the projected number of cardiovascular deaths. We also did not know the proportion of patients in SOLVD-T treated with an MRA. Only patients intolerant of an ACE inhibitor were enrolled in CHARM-Alternative. Conversely, only patients able to tolerate enalapril 2.5 mg bid and 10 mg bid were included in SOLVD-T and in PARADIGM-HF, respectively. In addition, in PARADIGM-HF, patients switched from established neurohumoral blockade with an ACE inhibitor or ARB, whereas in SOLVD-T and CHARM-Alternative this was not the case. All of these factors create uncertainty about the exact size of the treatment effect of LCZ696 compared with placebo and affect the generalizability of our findings. There are only two large-scale, long-term, ACE inhibitor-placebo or ARB-placebo comparisons in a broad spectrum of patients with HF-REF. The duration of follow-up differed between these two trials and from PARADIGM-HF. We made the assumption that the effect of LCZ696 compared with enalapril would be the same as that of LCZ696 compared with candesartan.
In summary, these indirect comparisons of LCZ696 with putative placebos show that the strategy of combined angiotensin receptor blockade and neprilysin inhibition leads to striking relative risk reductions in cardiovascular and all-cause mortality, as well as in heart failure hospitalization. These estimated benefits were obtained despite LCZ696 being added to excellent background beta-blocker and MRA therapy.
Funding
The PARADIGM-HF trial with LCZ696 (ClinicalTrials.gov number, NCT01035255) was funded by Novartis. Funding to pay the Open Access publication charges for this article was provided by University of Glasgow.
Conflict of interest: All authors or their institutions received funding from Novartis in relation to the PARADIGM-HF trial.
References
- 1.Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, Konstam MA, Riegger G, Klinger GH, Neaton J, Sharma D, Thiyagarajan B. Effect of losartancompared with captopril on mortality in patients with symptomatic heart failure: randomised trial—the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000;355:1582–1587. doi: 10.1016/s0140-6736(00)02213-3. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. doi: 10.1056/NEJMoa032292. Erratum in N Engl J Med 2004;350:203. [DOI] [PubMed] [Google Scholar]
- 3.Dickstein K, Kjekshus J OPTIMAAL Steering Committee of the OPTIMAAL Study Group. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet. 2002;360:752–760. doi: 10.1016/s0140-6736(02)09895-1. [DOI] [PubMed] [Google Scholar]
- 4.Krum H, Massie B, Abraham WT, Dickstein K, Kober L, McMurray JJ, Desai A, Gimpelewicz C, Kandra A, Reimund B, Rattunde H, Armbrecht J ATMOSPHERE Investigators. Direct renin inhibition in addition to or as an alternative to angiotensin converting enzyme inhibition in patients with chronic systolic heart failure: rationale and design of the Aliskiren Trial to Minimize OutcomeS in Patients with HEart failuRE (ATMOSPHERE) study. Eur J Heart Fail. 2011;13:107–114. doi: 10.1093/eurjhf/hfq212. [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR PARADIGM-HF Investigators and Committees. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N Engl J med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 6.Hasselblad V, Kong DF. Statistical methods for comparison to placebo in active-control trials. Drug Info J. 2001;35:435–449. [Google Scholar]
- 7.Durrleman S, Chaikin P. The use of putative placebo in active control trials: two applications in a regulatory setting. Stat Med. 2003;22:941–952. doi: 10.1002/sim.1454. [DOI] [PubMed] [Google Scholar]
- 8.Fisher LD, Gent M, Büller HR. Active-control trials: how would a new agent compare with placebo? A method illustrated with clopidogrel, aspirin, and placebo. Am Heart J. 2001;141:26–32. doi: 10.1067/mhj.2001.111262. [DOI] [PubMed] [Google Scholar]
- 9.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 10.Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 11.James Hung HM, Wang SJ, Tsong Y, Lawrence J, O'Neil RT. Some fundamental issues with non-inferiority testing in active controlled trials. Stat Med. 2003;22:213–225. doi: 10.1002/sim.1315. [DOI] [PubMed] [Google Scholar]
- 12.Kaul S, Diamond GA, Weintraub WS. Trials and tribulations of non-inferiority: the ximelagatran experience. J Am Coll Cardiol. 2005;46:1986–1995. doi: 10.1016/j.jacc.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 13.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL WRITING COMMITTEE MEMBERS, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]