Abstract
The reaction of phenylglyoxal with two enzymes in which ATP plays a complex role has been studied. Both ovine brain glutamine synthetase and Escherichia coli carbamyl phosphate synthetase [carbamoyl-phosphate synthase (glutamine); ATP:carbamate phosphotransferase (dephosphorylating, amido-transferring); EC 2.7.2.9]were inactivated by phenylglyoxal. The specificity of this reagent for arginyl residues of the two proteins was confirmed by amino acid analysis. ATP, but not the other substrates, protected these enzymes against inactivation by phenylglyoxal. Carbamyl phosphate synthetase was also protected by IMP and ornithine, positive allosteric effectors that alter the enzymatic activity be increasing the affinity for ATP. UMP, a negative allosteric effector that decreases the affinity for ATP, did not protect against inactivation. Differential labeling experiments with [14C]phenylglyoxal showed that the number of arginyl residues protected by ATP corresponded quite well to the known number of ATP catalytic sites for each protein. These data indicate that arginyl residues at the active sites of glutamine synthetase and carbamyl phosphate synthetase are involved in the binding of ATP. This phenylglyoxal inactivation study also provided information about the mechanistic role of ATP in the two synthetases. The data obtained on glutamine synthetase support the theory that ATP is attached to the enzyme as a portion of the catalytic site, and that its presence is essential for the binding of glutamate and glutamine. The data obtained on carbamyl phosphate synthetase are consistent with the previous proposal that carbonyl phosphate is an intermediate in the ATP-dependent activation of bicarbonate by this enzyme. It is also of interest that, with both glutamine synthetase and carbamyl phosphate synthetase, only a small portion of the total arginyl population of these enzymes reacted with phenylglyoxal. A summary of previous studies on the modification of enzyme arginyl residues is presented.
Full text
PDF
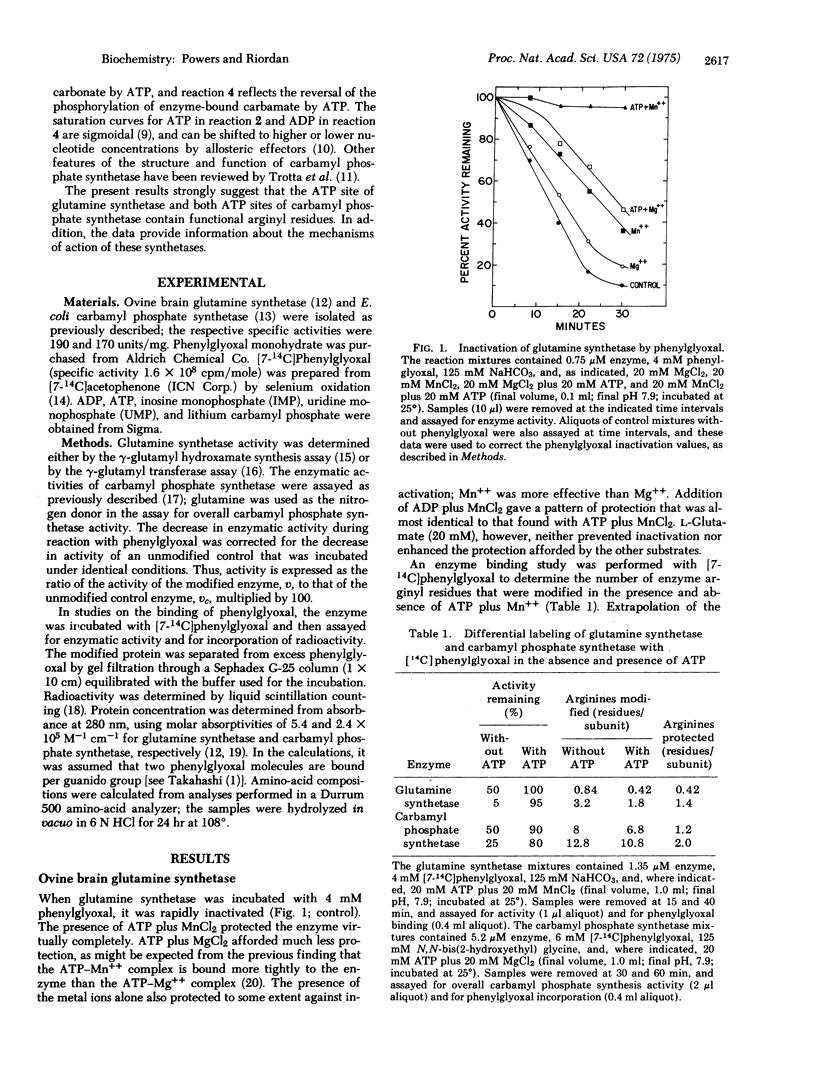
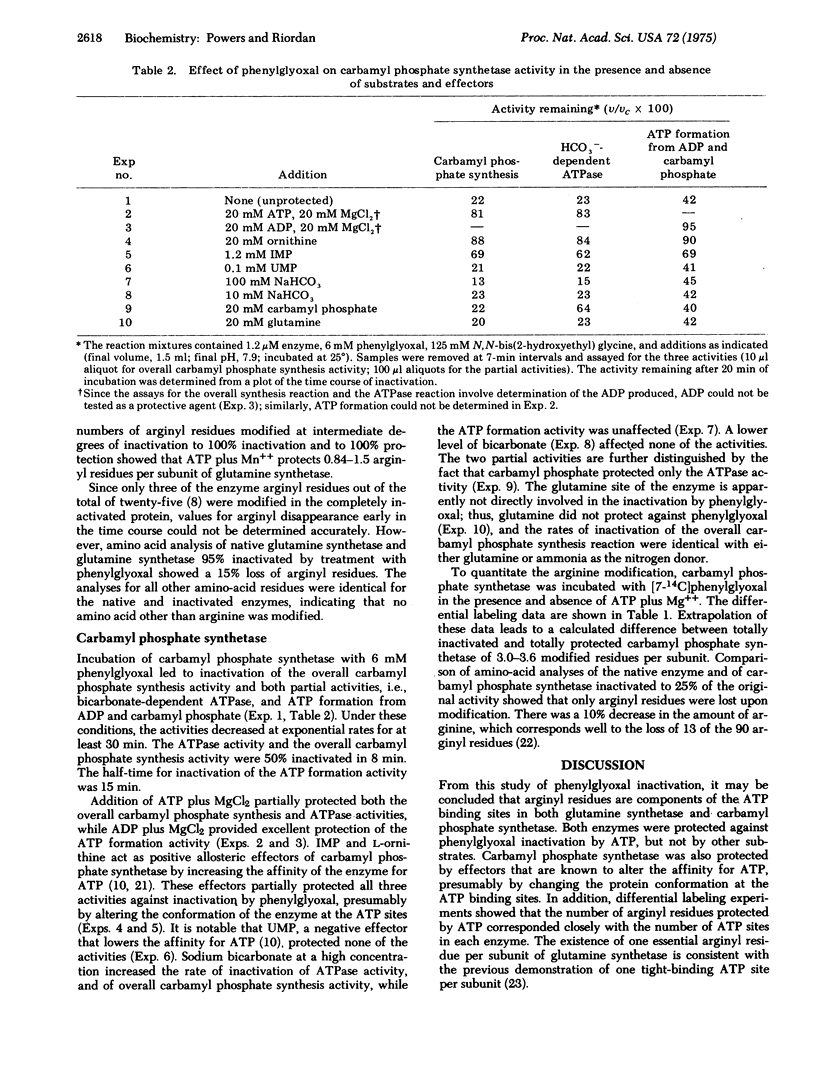


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Marvin S. V. Effect of ornithine, IMP, and UMP on carbamyl phosphate synthetase from Escherichia coli. Biochem Biophys Res Commun. 1968 Sep 30;32(6):928–934. doi: 10.1016/0006-291x(68)90116-2. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Bicarbonate-dependent cleavage of adenosine triphosphate and other reactions catalyzed by Escherichia coli carbamyl phosphate synthetase. Biochemistry. 1966 Oct;5(10):3157–3163. doi: 10.1021/bi00874a012. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Control of Escherichia coli carbamyl phosphate synthetase by purine and pyrimidine nucleotides. Biochemistry. 1966 Oct;5(10):3164–3169. doi: 10.1021/bi00874a013. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Evidence for an activated form of carbon dioxide in the reaction catalyzed by Escherichia coli carbamyl phosphate synthetase. Biochemistry. 1965 Dec;4(12):2803–2809. doi: 10.1021/bi00888a034. [DOI] [PubMed] [Google Scholar]
- Arnone A., Bier C. J., Cotton F. A., Day V. W., Hazen E. E., Jr, Richardson D. C., Yonath A., Richardson J. S. A high resolution structure of an inhibitor complex of the extracellular nuclease of Staphylococcus aureus. I. Experimental procedures and chain tracing. J Biol Chem. 1971 Apr 10;246(7):2302–2316. [PubMed] [Google Scholar]
- Cotton F. A., Day V. W., Hazen E. E., Jr, Larsen S. Structure of methylguanidinium dihydrogenorthophosphate. A model compound for arginine-phosphate hydrogen bonding. J Am Chem Soc. 1973 Jul 25;95(15):4834–4840. doi: 10.1021/ja00796a012. [DOI] [PubMed] [Google Scholar]
- Daemen F. J., Riordan J. F. Essential arginyl residues in Escherichia coli alkaline phosphatase. Biochemistry. 1974 Jul 2;13(14):2865–2871. doi: 10.1021/bi00711a014. [DOI] [PubMed] [Google Scholar]
- Foster M., Harrison J. H. Selective chemical modification of arginine residues in mitochondrial malate dehydrogenase. Biochem Biophys Res Commun. 1974 May 7;58(1):263–267. doi: 10.1016/0006-291x(74)90921-8. [DOI] [PubMed] [Google Scholar]
- KAZIRO Y., HASS L. F., BOYER P. D., OCHOA S. Mechanism of the propionyl carboxylase reaction. II. Isotopic exchange and tracer experiments. J Biol Chem. 1962 May;237:1460–1468. [PubMed] [Google Scholar]
- LEVINTOW L. The glutamyltransferase activity of normal and neoplastic tissues. J Natl Cancer Inst. 1954 Oct;15(2):347–352. [PubMed] [Google Scholar]
- Lange L. G., 3rd, Riordan J. F., Vallee B. L. Functional arginyl residues as NADH binding sites of alcohol dehydrogenases. Biochemistry. 1974 Oct 8;13(21):4361–4370. doi: 10.1021/bi00718a019. [DOI] [PubMed] [Google Scholar]
- Patthy L., Smith E. L. Identification of functional arginine residues in ribonuclease A and lysozyme. J Biol Chem. 1975 Jan 25;250(2):565–569. [PubMed] [Google Scholar]
- Polakis S. E., Guchhait R. B., Lane M. D. On the possible involvement of a carbonyl phosphate intermediate in the adenosine triphosphate-dependent carboxylation of biotin. J Biol Chem. 1972 Feb 25;247(4):1335–1337. [PubMed] [Google Scholar]
- Riordan J. F. Functional arginyl residues in carboxypeptidase A. Modification with butanedione. Biochemistry. 1973 Sep 25;12(20):3915–3923. doi: 10.1021/bi00744a020. [DOI] [PubMed] [Google Scholar]
- Ronzio R. A., Rowe W. B., Meister A. Studies on the mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochemistry. 1969 Mar;8(3):1066–1075. doi: 10.1021/bi00831a038. [DOI] [PubMed] [Google Scholar]
- Takahashi K. The reaction of phenylglyoxal with arginine residues in proteins. J Biol Chem. 1968 Dec 10;243(23):6171–6179. [PubMed] [Google Scholar]
- Trotta P. P., Burt M. E., Haschemeyer R. H., Meister A. Reversible dissociation of carbamyl phosphate synthetase into a regulated synthesis subunit and a subunit required for glutamine utilization. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2599–2603. doi: 10.1073/pnas.68.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta P. P., Estis L. F., Meister A., Haschemeyer R. H. Self-association and allosteric properties of glutamine-dependent carbamyl phosphate synthetase. Reversible dissociation to monomeric species. J Biol Chem. 1974 Jan 25;249(2):482–489. [PubMed] [Google Scholar]
- Trotta P. P., Pinkus L. M., Haschemeyer R. H., Meister A. Reversible dissociation of the monomer of glutamine-dependent carbamyl phosphate synthetase into catalytically active heavy and light subunits. J Biol Chem. 1974 Jan 25;249(2):492–499. [PubMed] [Google Scholar]
- Wellner V. P., Anderson P. M., Meister A. Interaction of Escherichia coli carbamyl phosphate synthetase with glutamine. Biochemistry. 1973 May 22;12(11):2061–2066. doi: 10.1021/bi00735a006. [DOI] [PubMed] [Google Scholar]
- Wellner V. P., Meister A. Binding of adenosine triphosphate and adenosine diphosphate by glutamine synthetase. Biochemistry. 1966 Mar;5(3):872–879. doi: 10.1021/bi00867a010. [DOI] [PubMed] [Google Scholar]


