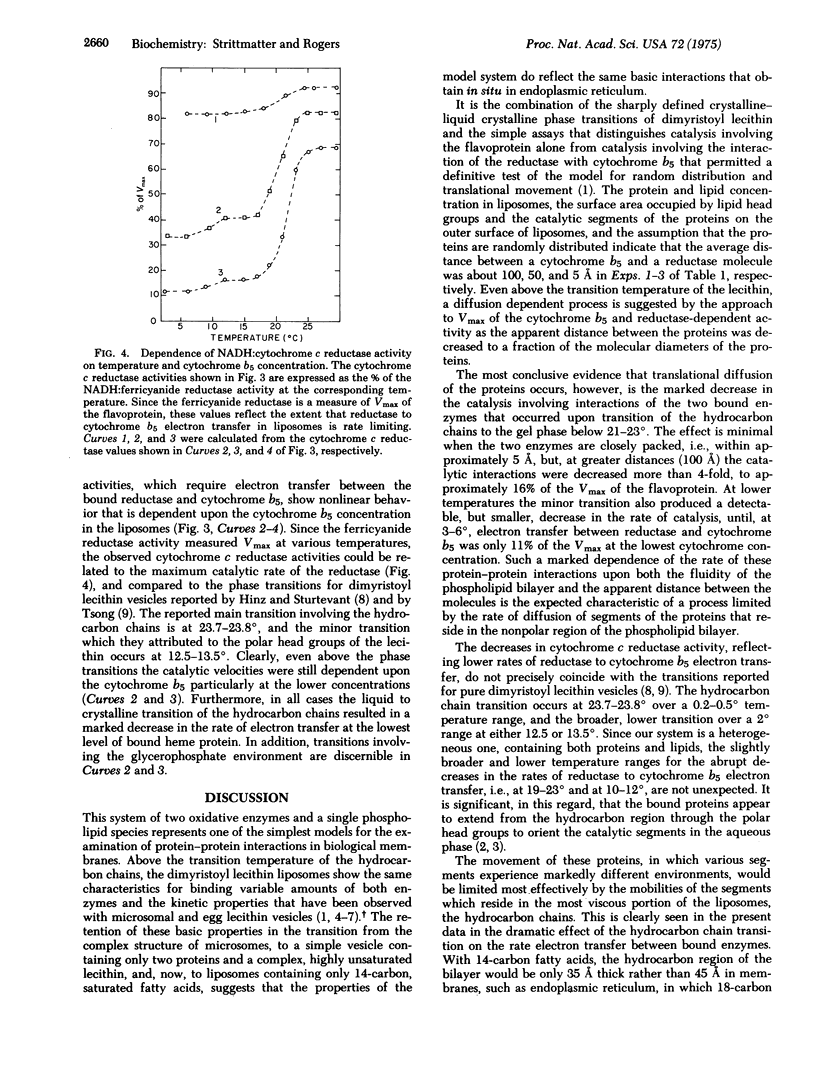
Abstract
Dimyristoyl lecithin liposomes, containing cytochrome b5 reductase (NADH:ferricytochrome b5 oxidoreductase, EC 1.6.2.2) and varying amounts of cytochrome b5, were used to measure flavoprotein catalysis alone and catalysis requiring electron transfer between the reductase and cytochrome as a function of temperature. Whereas flavoprotein catalysis showed a simple linear temperature dependence in an Arrhenius plot, the reaction involving electron transfer between the two bound enzymes showed a marked, 4-fold, change in rate at the crystalline-liquid crystalline phase transition of the hydrocarbon chains of the lecithin vesicles and a second, minor change involving the minor transition. These data represent strong evidence that protein-protein interactions in this membrane model system are dependent upon translational diffusion of nonpolar segments of the proteins in the hydrocarbon region of the phospholipid bilayer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hauser H. O. The effect of ultrasonic irradiation on the chemical structure of egg lecithin. Biochem Biophys Res Commun. 1971 Nov;45(4):1049–1055. doi: 10.1016/0006-291x(71)90443-8. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric studies of dilute aqueous suspensions of bilayers formed from synthetic L- -lecithins. J Biol Chem. 1972 Oct 10;247(19):6071–6075. [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Riveros-Moreno V., Wittenberg J. B. The self-diffusion coefficients of myoglobin and hemoglobin in concentrated solutions. J Biol Chem. 1972 Feb 10;247(3):895–901. [PubMed] [Google Scholar]
- Rogers M. J., Strittmatter P. Evidence for randon distribution and translational movement of cytochrome b5 in endoplasmic reticulum. J Biol Chem. 1974 Feb 10;249(3):895–900. [PubMed] [Google Scholar]
- Rogers M. J., Strittmatter P. Lipid-protein interactions in the reconstitution of the microsomal reduced nicotinamide adenine dinucleotide-cytochrome b 5 reductase system. J Biol Chem. 1973 Feb 10;248(3):800–806. [PubMed] [Google Scholar]
- Rogers M. J., Strittmatter P. The binding of reduced nicotinamide adenine dinucleotide-cytochrome b5 reductase to hepatic microsomes. J Biol Chem. 1974 Sep 10;249(17):5565–5569. [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of cytochrome b5 that contains an additional hydrophobic sequence of 40 amino acid residues. Proc Natl Acad Sci U S A. 1971 May;68(5):1042–1046. doi: 10.1073/pnas.68.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of reduced nicotinamide adenine dinucleotide-cytochrome b 5 reductase containing both the catalytic site and an additional hydrophobic membrane-binding segment. J Biol Chem. 1973 Feb 10;248(3):793–799. [PubMed] [Google Scholar]
- Sullivan M. R., Holloway P. W. The binding of cytochrome b5 to phosphatidylcholine vesicle. Biochem Biophys Res Commun. 1973 Sep 18;54(2):808–815. doi: 10.1016/0006-291x(73)91496-4. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Kinetics of the crystalline-liquid crystalline phase transition of dimyristoyl L-alpha-lecithin bilayers. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2684–2688. doi: 10.1073/pnas.71.7.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]




