Abstract
OBJECTIVE
The purpose of this article is to assess the effects of various CT, patient, and renal cyst characteristics on the occurrence of pseudoenhancement in in vivo renal mass CT examinations using subtraction MRI as the reference standard.
MATERIALS AND METHODS
Adult patients imaged with 120-kVp standard kernel biphasic renal mass protocol CT and dynamic contrast-enhanced MRI of the abdomen from January 1, 2005, through May 4, 2012, were identified. Those with nonenhancing Bosniak categories I and II cysts on MRI were selected (n = 33 patients; 110 cysts). By treating measured cyst enhancement (nephrographic CT attenuation minus unenhanced CT attenuation) as either a continuous or categoric outcome variable, a variety of CT, patient-level, and renal cyst characteristics were assessed using mixed effect multivariate models.
RESULTS
On univariate assessment, cysts that exhibited pseudoenhancement (> 10 HU) were significantly more endophytic (p = 0.02), significantly smaller (p = 0.0004), and adjacent to significantly higher attenuation renal parenchyma in the nephrographic phase (p = 0.02). On multivariate assessment, cyst diameter (p < 0.0001) and background nephrographic phase parenchymal attenuation (p = 0.003) were the strongest in vivo predictors of pseudoenhancement. The odds of pseudoenhancement occurring increased by 2.14 (95% CI, 1.41–3.23) for every 5-mm decrease in renal cyst diameter and increased by 2.45 (95% CI, 1.41–4.26) for every 25-HU increase in enhanced renal parenchymal attenuation. Endophytic growth was not significant in the multivariate analyses (p = 0.07).
CONCLUSION
Renal cyst size and enhanced renal parenchymal attenuation are better in vivo predictors of pseudoenhancement than is endophytic growth pattern.
Keywords: beam hardening, enhancement, multivariate, pseudoenhancement, renal cyst
Pseudoenhancement was first described by Rao and Alfidi in 1981 [1]. It represents an erroneous increase in CT number within a nonenhancing structure that occurs when the structure is adjacent to higher-attenuation material. This effect is accentuated with MDCT [2–7], a phenomenon that has been hypothesized to relate to a computational error resulting from inadequate correction of beam-hardening artifact [2]. This artifactual increase in attenuation has clinical relevance because it can mimic solid enhancement within a non-enhancing cyst and can lead to a change in management [8, 9].
Studies have been performed in an attempt to determine the effect of various renal cyst characteristics (e.g., size [2–7, 10–13], unenhanced attenuation [6], and location [6, 11]), CT parameters (e.g., number of detectors [3– 7], tube current [3], peak kilovoltage [5, 11], kernel [3]), and background renal parenchymal attenuation [2–5, 7, 11–14] on the occurrence of renal cyst pseudoenhancement. However, because of the difficulty in establishing a suitable reference standard to confirm the simple nature of in vivo renal cysts (simple renal cysts typically are not resected), these studies have primarily used ex vivo phantoms to simulate what might occur in an in vivo environment. Although a few in vivo studies have been performed, most of these have been limited by either univariate analysis [10] or a self-referential reference standard (e.g., unenhanced CT characteristics) [6, 11]. Only one other study to our knowledge has incorporated a multivariate analysis using MRI as an in vivo reference standard [7].
Dynamic contrast-enhanced MRI with subtraction capability is an accepted confirmatory or exclusion test for evaluating the presence of internal enhancement within a small renal mass that has indeterminate CT or sonographic imaging features [9, 15, 16]. This is because MRI is much more sensitive to the paramagnetic effect of gadolinium-based contrast media than is CT to the attenuating effects of iodinated contrast media. MRI is also not subject to the acoustic attenuating effects in larger patients that impair the characterization of small cysts with ultrasound. Therefore, contrast-enhanced MRI with subtraction can act as an excellent reference standard for the study of in vivo effects on renal cyst pseudoenhancement, particularly for small renal lesions (< 2 cm) that may be difficult to characterize definitively with ultrasound [9].
The hypothesis of our study was that in a fixed 120-kVp standard kernel renal mass CT examination, a variety of features can influence the occurrence of in vivo renal cyst pseudoenhancement, including renal cyst diameter, number of CT detectors, renal cyst location, abdominal cross-sectional area, and background renal parenchymal attenuation. The purpose of our study was to assess the effect of various CT, patient, and renal cyst characteristics on the occurrence of renal cyst pseudoenhancement in a 120-kVp standard kernel in vivo renal mass protocol CT examination using subtraction contrast-enhanced MRI as a reference standard.
Materials and Methods
Before the initiation of this investigation, institutional review board approval was obtained. The study was performed in compliance with the HIPAA. Patient informed consent was not required on the basis of institutional policy and the retrospective nature of this investigation.
Subjects
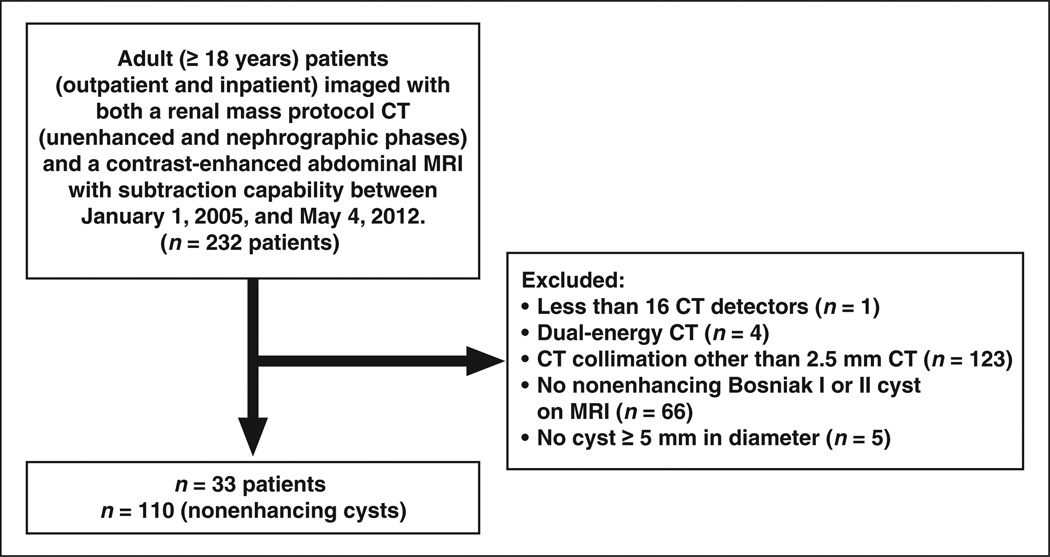
The study population consisted of all adult (≥ 18 years old) patients (outpatient and inpatient) imaged with both a renal mass protocol CT (unenhanced and nephrographic phase) and a contrast-enhanced abdominal MRI with subtraction capability, both of which were obtained between January 1, 2005, and May 4, 2012. Patients meeting the inclusion criteria (n = 232) were identified through a search of the radiology information system. We excluded patients imaged with fewer than 16 CT detectors (n = 1), patients imaged with dual-energy CT (n = 4), patients with a CT reconstruction slice thickness other than 2.5 mm (n = 123), patients without an unequivocally nonenhancing (i.e., no enhancement and no subtraction artifact) Bosniak category I or II cyst [8, 9] on MRI (n = 66), and patients without a renal cyst measuring 5 mm or larger (i.e., at least twice the CT slice thickness, n = 5). This resulted in 33 patients with 110 nonenhancing cysts (Fig. 1), including 19 women (mean age, 59 years; range, 45–82 years) and 14 men (mean age, 60 years; range, 24–79 years).
Fig. 1.
Study population flowchart.
Renal Mass Protocol CT
The unenhanced and nephrographic phase CT images were acquired and reconstructed using the same parameters: 120 kVp, variable tube current (ranging from 99 to 576 mA), “standard” MDCT kernel (GE Healthcare), 2.5-mm reconstructed slice thickness, and 1:1 pitch. Scanner types included 16-MDCT (LightSpeed 16, GE Healthcare) and 64-MDCT (LightSpeed VCT, GE Healthcare). Nephrographic phase CT was acquired 150 seconds after the bolus IV power injection (2–3 mL/s) of 100–175 mL (mean, 117 mL) of low osmolality iodinated contrast material (n = 24, iopamidol 300 [Isovue, Bracco Diagnostics]; n = 5, iopromide 300 [Ultravist, Bayer Healthcare]; n = 1, iohexol 300 [Omnipaque, GE HealthCare]; n = 3, unknown).
Reference Standard
Dynamic contrast-enhanced MRI of the abdomen with subtraction capability was used as the reference standard for each renal cyst in the study group. Determination of absent internal enhancement was visual or subjective and was made by one fellowship-trained abdominal radiologist with 2 years of experience who was blinded to the CT number determinations of the target cysts. Renal cysts were defined as round or oval masses arising from the renal parenchyma that were devoid of internal enhancement on contrast-enhanced MRI (with and without subtraction). Scan parameters are provided in Table 1 and the scan indications are provided in Table 2. Contrast-enhanced imaging was acquired following the IV power injection (1–2 mL/s) of one of the following gadolinium- based contrast media: gadobenate dimeglumine (n = at least 21 examinations; mean dose, 17 mL; MultiHance, Bracco Diagnostics), gadopentetate dimeglumine (unknown number of examinations; Magnevist, Bayer Healthcare), or gadoteridol (n = at least 2 examinations; mean dose, 20 mL; ProHance, Bracco Diagnostics). Ten examinations were associated with an unknown gadolinium-based contrast agent, but it was one of the three listed above. Subtraction imaging was performed on a workstation (Advantage, GE HealthCare), and all other image review was performed on a PACS workstation (Horizon Medical Imaging PACS, McKesson). The median time between the CT and MRI studies was 0.5 month (range, 65 months before the CT to 56 months after the CT).
TABLE 1.
Scan Parameters for Dynamic Contrast-Enhanced 3D T1-Weighted Spoiled Gradient-Echo MRI Used to Confirm Nonenhancement of All Cysts in Study Group, by Phase
| Parameter | Unenhanced | Arterial | Venous or Corticomedullary | Delayed or Nephrographic |
|---|---|---|---|---|
| TR (ms) | 3.6 | 3.6 | 3.6 | 3.6 |
| TE (ms) | 1.3 | 1.3 | 1.3 | 1.3 |
| FOV | Variable | Variable | Variable | Variable |
| Flip angle (°) | 12 | 12 | 12 | 12 |
| Matrix (frequency) | 256–320 | 256–320 | 256–320 | 256–320 |
| Matrix (phase) | 128–192 | 128–192 | 128–192 | 128–192 |
| Frequency direction | Right to left | Right to left | Right to left | Right to left |
| Section thickness (mm) | 4 | 4 | 4 | 4 |
| Receiver bandwidth (Hz) | 31.25–41.67 | 31.25–41.67 | 31.25–41.67 | 31.25–41.67 |
| Sensitivity-encoding factor | 2 | 2 | 2 | 2 |
| Acquisition time (s) | 18–22 | 18–22 | 18–22 | 18–22 |
| Delay (s) | NA | 20–30 | 60–90 | 120–150 |
| Subtraction capability | NA | Yes | Yes | Yes |
Note—Hz = hertz, NA = not applicable.
TABLE 2.
Clinical Indication for Multiphasic Contrast-Enhanced Abdominal MRI Examinations With Subtraction Capability Used to Confirm Nonenhancing Nature of the Study Group Renal Cysts (n = 110)
| Indication | No. of Cysts |
|---|---|
| Evaluate equivocally enhancing study population cyst | 13 |
| Evaluate different indeterminate lesion(s) in ipsilateral kidney | 36 |
| Evaluate different indeterminate lesion(s) in contralateral kidney | 37 |
| Nonrenal indication | 24 |
Image Review
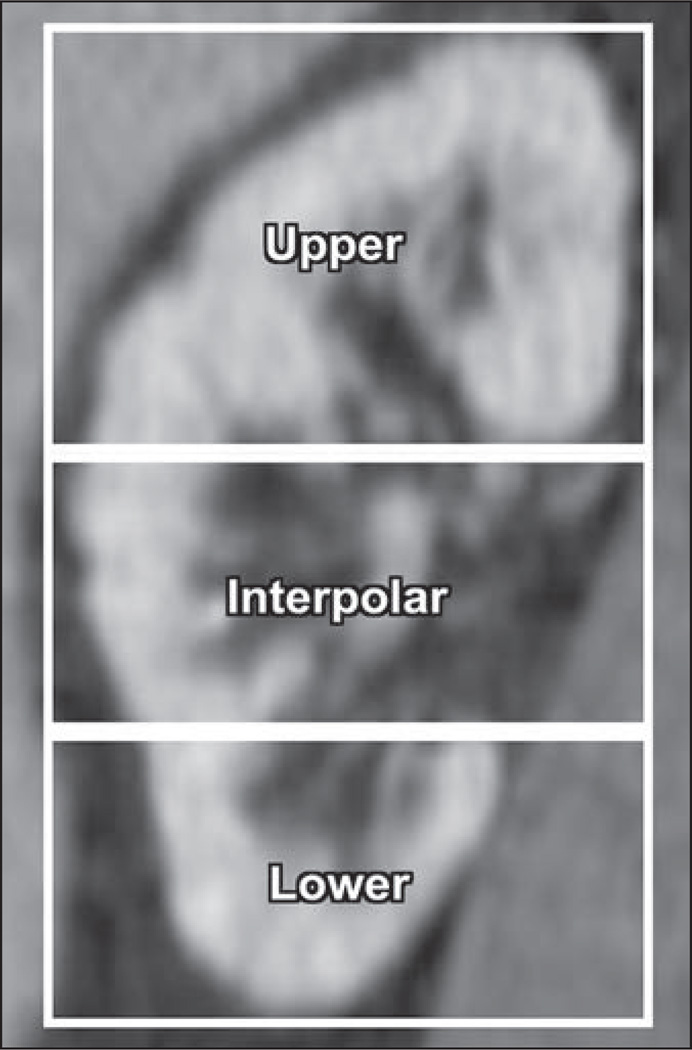
All images were reviewed by a subspecialty-trained abdominal radiologist. Morphologic features of each cyst were recorded (Table 3). Endophytic was defined as greater than 75% of the cyst margin encompassed by normal renal parenchyma. Exophytic was defined as greater than 75% of the cyst margin external to the renal parenchyma. Mesophytic was defined as neither endophytic nor exophytic. Upper, lower, and interpolar designations were made on the basis of the renal polar lines (Fig. 2). Size was recorded as the largest axial diameter in millimeters. Attenuation measurements were made within each cyst on the unenhanced and nephrographic CT images, and the difference (nephrographic attenuation minus unenhanced attenuation, in Hounsfield units) was calculated. Regions of interest were placed to encompass at least 75% of the axial surface area while avoiding partial volume effects. A nephrographic phase region of interest using similar principles 10 mm or more in diameter was also placed in the closest adjacent renal cortex to each cyst in the study group.
TABLE 3.
Study Population Characteristics Stratified by Presence of Measured Pseudoenhancement
| Characteristic | Patients | Cysts Without Pseudoenhancement |
Cysts With Pseudoenhancement |
pa |
|---|---|---|---|---|
| Patient sex | 0.2 | |||
| Male | 42 (14/33) | 51 (35/68) | 36 (15/42) | |
| Female | 58 (19/33) | 49 (33/68) | 64 (27/42) | |
| Cyst location | 0.2 | |||
| Right kidney | NA | 47 (32/68) | 33 (14/42) | |
| Left kidney | NA | 53 (36/68) | 67 (28/42) | |
| Growth patternb | 0.02c | |||
| Exophytic | NA | 32 (22/68) | 7 (3/42) | |
| Mesophytic | NA | 31 (21/68) | 29 (12/42) | |
| Endophytic | NA | 37 (25/68) | 64 (27/42) | |
| Position | 0.2 | |||
| Upper pole | NA | 28 (19/68) | 45 (19/42) | |
| Interpolar | NA | 41 (28/68) | 36 (15/42) | |
| Lower pole | NA | 31 (21/68) | 19 (8/42) | |
| Tube current (mA), mean (SD)d | NA | 345 (88) | 320 (121) | 0.5 |
| CT detectors | 0.3 | |||
| 16-MDCT scannerd | 15 (5/33) | 15 (10/68) | 7 (3/42) | |
| 64-MDCT scannerd | 85 (28/33) | 85 (58/68) | 93 (39/42) | |
| Abdominal cross-sectional area (cm2), mean (SD) | NA | 734 (165) | 711 (175) | 0.5 |
| Cyst diameter (mm), mean (SD) | NA | 25 (16) | 14 (6) | 0.0004c |
| Parenchymal attenuation (HU), mean (SD)e | NA | 140 (20) | 152 (28) | 0.02c |
| Measured enhancement (HU) | —f | |||
| Mean (SD) | NA | 4 (4) | 15 (4) | |
| ≤ 10 | NA | 100 (68/68) | 0 (0/42) | |
| 11–19 | NA | 0 (0/68) | 93 (39/42) | |
| ≥ 20 | NA | 0 (0/68) | 7 (3/42) | |
Note—Except where noted otherwise, data are percentage of patients or cysts (no./total). Pseudoenhancement is defined as nephrographic phase CT attenuation minus unenhanced CT attenuation >10 HU.
NA = not applicable (i.e., the characteristic is a cyst-level characteristic and varied within patients).
p values refer to univariate comparisons between characteristics of cysts with vs without pseudoenhancement while controlling for repeated measures within patients.
Endophytic cysts had a significantly higher pseudoenhancement rate than did exophytic cysts in univariate analysis (p = 0.02; odds ratio, 7.90; 95% CI, 1.35–46.32). This finding lost significance in the multivariate model.
Statistically significant (p < 0.05).
All CT studies used 120 kVp, variable tube current, 2.5 mm collimation, and standard kernel.
Parenchymal attenuation in the nephrographic phase.
Not assessed, definitional.
Fig. 2.
Designation of upper pole, interpolar region, and lower pole on basis of renal polar lines.
The anteroposterior and transverse dimensions of the patient’s abdomen in centimeters were recorded for each cyst, and abdominal cross-sectional area was calculated (π × [anteroposterior diameter / 2] × [transverse diameter / 2]). Measurements were made on the same slice as the attenuation measurements.
Pseudoenhancement
Pseudoenhancement was defined as a measured CT enhancement (nephrographic phase CT number minus unenhanced CT number) of more than 10 HU in a renal cyst devoid of internal enhancement on subtraction MRI.
Data Analysis
Measured CT enhancement was analyzed as an outcome variable (both as a continuous and as a binary variable). To account for repeated measures within patients (i.e., more than one cyst per patient), a univariate repeated measures analysis with a linear mixed-effect model was used to examine the independent effect of various CT parameters (number of CT detectors, milliamperes [fixed peak kilovoltage and fixed standard kernel]), patient characteristics (sex, abdominal cross-sectional area, and nephrographic phase renal parenchymal attenuation), and renal cyst characteristics (sidedness, growth pattern [endophytic, mesophytic, or exophytic], position [upper, lower, or interpolar], cyst diameter, cyst area, and cyst attenuation unenhanced) on measured CT enhancement. A multivariate mixed-effect model was used to identify the significant predictors of measured CT enhancement in the presence of other factors. To examine the association between the above independent variables and rate of pseudoenhancement (binary variable, measured enhancement > 10 or ≤ 10 HU), univariate and multivariate generalized linear mixed models were used with binomial distribution. Odds ratios (ORs) and 95% CIs were calculated to show the degree of significant associations. Posthoc analyses were performed using the Tukey- Kramer method to adjust for pairwise comparison of categoric variables. Variables with a p value less than 1.5 in the univariate analysis were included in the multivariate model. A p value of 0.05 or smaller was considered significant for all hypothesis tests. The above procedures were done in SAS version 9.3 (SAS Institute).
Results
The mean cyst size (n = 110 cysts) was 21 mm (range, 5–78 mm). The mean unenhanced and nephrographic phase cyst attenuations were 10 HU (range, −6 to 79 HU) and 18 HU (range, −2 to 91 HU), respectively. There were 15 cysts with an unenhanced attenuation 20 HU or higher and 35 cysts with an unenhanced attenuation greater than 10 HU. Other detailed patient, CT, and cyst-level characteristics are provided in Table 3.
Of the 110 MRI-confirmed nonenhancing renal cysts in our study group, 42 (38%) had measured enhancement on CT compatible with pseudoenhancement (> 10 HU; Fig. 3). Only three (2.7%) had measured enhancement 20 HU or higher. Of the cysts that exhibited pseudoenhancement, 26% (11/42) had an unenhanced attenuation greater than 10 HU. On univariate assessment, the cysts that exhibited pseudoenhancement (> 10 HU) were significantly more endophytic (overall p = 0.02; endophytic cysts were significantly more likely to exhibit pseudoenhancement than were exophytic cysts ([p = 0.02; OR, 7.90; 95% CI, 1.35–46.32]), were significantly smaller (p = 0.0004), and were adjacent to significantly higher attenuation renal parenchyma in the nephrographic phase (p = 0.02). Unenhanced attenuation did not predict the likelihood of pseudoenhancement (p = 0.96; OR, 1.0; 95% CI, 0.97–1.04), and endophytic growth lost significance as an independent predictor in the multivariate analyses (p = 0.07).
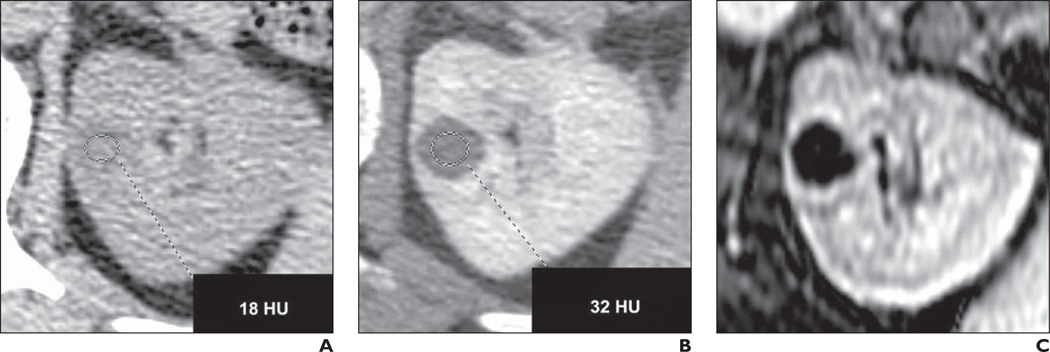
Fig. 3.
54-year-old woman with history of hepatitis C, cirrhosis, and 15-mm incidental left renal lesion.
A, Unenhanced CT shows attenuation of 18 HU.
B, Nephrographic phase CT shows attenuation of 32 HU (nephrographic minus unenhanced = 14 HU).
C, Fat-saturated dynamic contrast-enhanced T1-weighted gradient-echo MRI with subtraction shows absent internal enhancement, compatible with pseudoenhancement.
When measured enhancement was treated as a continuous variable (Table 4), multivariate analysis revealed that both cyst diameter in millimeters (p ≤ 0.0001; point estimate, −0.20; 95% CI, −0.30 to −0.12) and nephrographic phase renal parenchymal attenuation in Hounsfield units (p = 0.003; point estimate, 0.07; 95% CI, 0.03–0.12) were significant predictors of the enhancement measurement. The point estimates indicate the magnitude of effect. Specifically, for every 1-mm increase in cyst diameter, the measured enhancement was predicted to decrease by 0.20 HU (e.g., all other things being equal, a 5-mm cyst would be predicted to have a measured enhancement 4 HU greater than that of a 25-mm cyst). Likewise, for every CT number increase in adjacent renal parenchymal attenuation, the measured enhancement was predicted to increase by 0.07 HU (e.g., all other things being equal, the measured enhancement of a cyst adjacent to 140 HU parenchyma would be predicted to be approximately 4 HU greater if the adjacent parenchyma instead measured ≈ 200 HU).
TABLE 4.
Analysis of Effects on Measured Enhancement Detected by Contrast-Enhanced CT Within Nonenhancing Renal Cysts, Controlling for Repeated Measures Within Patients
| Covariate | Measured Enhancement (Continuous) | Measured Enhancement (Categoric, > or ≤ 10 HU) | ||||
|---|---|---|---|---|---|---|
| Univariate p | Multivariate p | OR (95% CI) | Univariate p | Multivariate p | OR (95% CI) | |
| Patient sex | 0.5 | — | — | 0.2 | — | — |
| Abdomen cross-sectional area (cm2) | 0.9 | — | — | 0.5 | — | — |
| Kidney (left or right) | 0.3 | — | — | 0.2 | — | — |
| Growth (exophytic, endophytic, or mesophytic)a | 0.03b | NS | — | 0.02b | NS | — |
| Position (upper, mid, or lower pole)c | 0.2 | — | — | 0.2 | — | — |
| CT detectors (16 or 64) | 0.4 | — | — | 0.3 | — | — |
| Tube current (mA) | 0.5 | — | — | 0.2 | — | — |
| Cyst diameter (mm) | < 0.0001b | < 0.0001d | −0.20 (−0.30 to −0.12) | 0.0004b | < 0.0004d | 0.86 (0.79–0.93) |
| Cyst area (mm2) | 0.0004b | NS | — | 0.009b | NS | — |
| Cyst attenuation unenhanced (HU) | 0.2 | — | — | 0.9 | — | — |
| Parenchymal attenuation (HU)e | 0.03b | 0.003d | 0.07 (0.03–0.12) | 0.02b | 0.002d | 1.04 (1.01–1.06) |
Note—Measured enhancement equals cyst nephrographic phase attenuation minus cyst unenhanced attenuation. Measured enhancement was treated as either a continuous or a categoric dichotomous variable. Dashes indicate that the covariate was not assessed.
OR = odds ratio, NS = not significant in the multivariate model.
Cyst growth pattern is a three-level categoric variable.
Included in multivariate analysis (p < 0.15).
Cyst position in the kidney is a three-level categoric variable.
Significant (p < 0.05) in multivariate analysis.
Parenchymal attenuation refers to nephrographic phase CT number of the closest normal renal parenchyma adjacent to the reference cyst.
Similar multivariate results are seen when measured enhancement was considered not as a continuous variable but as a dichotomous variable stratified by the presence of pseudoenhancement. For every 1-mm increase in cyst diameter, the odds of pseudoenhancement occurring decreased by 0.86 (p = 0.0004); for every 5-mm decrease in renal cyst diameter, the odds of pseudoenhancement occurring increased by an OR of 2.14 (95% CI, 1.41–3.23; p = 0.0004). For every CT number increase in enhanced renal parenchymal attenuation, the odds of pseudoenhancement occurring increased by 1.04 (p = 0.002); for every 25-HU increase in enhanced parenchymal attenuation, the odds of pseudoenhancement occurring increased by an OR of 2.45 (95% CI, 1.41–4.26; p = 0.002).
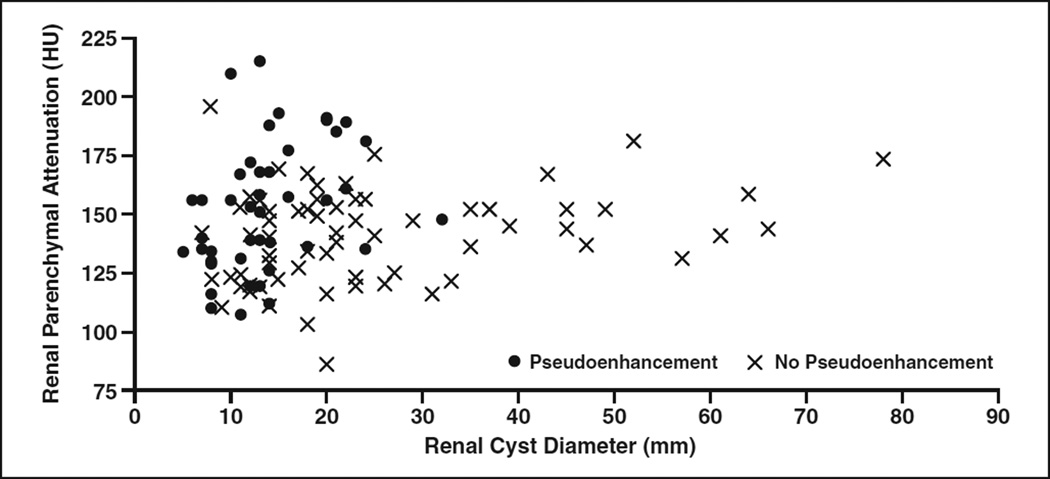
The relationship between renal cyst diameter and renal parenchymal attenuation on the occurrence of pseudoenhancement is displayed in Figure 4. Pseudoenhancement was prevalent among small renal cysts 5–20 mm in diameter (50% [36/72]) and in cysts where the background nephrographic phase parenchymal attenuation was greater than 155 HU (56% [20/36]). It was less common in cysts larger than 20 mm in diameter (16% [6/38]) and in those where the background nephrographic phase parenchymal attenuation was 155 HU or less (30% [22/74]). Pseudoenhancement was uncommon in renal cysts that were both larger than 20 mm in diameter and adjacent to a background parenchyma with attenuation 155 HU or less (8% [2/25]).
Fig. 4.
Relationship of renal cyst diameter (millimeters) and nephrographic phase renal parenchymal attenuation (Hounsfield units) on occurrence of renal cyst pseudoenhancement (measured enhancement >10 HU in renal cyst without internal enhancement on subtraction contrast-enhanced MRI).
The three renal cysts with a measured enhancement 20 HU or greater had diameters of 21, 20, and 8 mm, respectively; parenchymal attenuation of 185, 190, and 134 HU, respectively; and measured enhancement of 22 HU (unenhanced, 2 HU; nephrographic, 24 HU), 21 HU (unenhanced, 2 HU; nephrographic, 23 HU), and 34 HU (unenhanced, −6 HU; nephrographic, 29 HU), respectively. Each would have been classified as a simple cyst on the basis of the unenhanced attenuation alone.
Other covariates that would be expected from prior experiments to influence pseudoenhancement did not have significant effects in our study: number of CT detectors (16 vs 64) [3–7], abdominal cross-sectional area (centimeters squared) [2], and growth pattern [6, 11]. Although growth pattern (endophytic, mesophytic, or exophytic) was a significant predictor in our univariate analysis, it lost significance in our multivariate analysis.
Discussion
Enhancement on dual-phase CT has classically been defined as an increase in attenuation of more than 10 HU between unenhanced and enhanced phases [8]. However, because of the greater frequency of pseudoenhancement effects seen with MDCT compared with single-detector CT, this threshold has lost specificity, and 20 HU or greater is now used frequently [9]. Measured enhancement of 11–19 HU is considered by many to be equivocal [9], and masses exhibiting equivocal enhancement create management dilemmas. The management of a homogeneous renal mass with equivocal enhancement is often left to the individual practitioner (e.g., ignore, follow up, or characterize by ultrasound or MRI) [9, 17]. Knowledge about which factors most strongly influence in vivo pseudoenhancement might improve management of equivocally enhancing renal lesions. Specifically, if a particular combination of cystand patient-level factors was highly associated with pseudoenhancement, the presence of those factors in an individual clinical setting may lead to less-aggressive management recommendations. Similarly, if a particular combination of cyst- and patient-level factors was extremely unlikely to be associated with pseudoenhancement, more-aggressive management might be pursued.
Our results showed that at a fixed peak kilovoltage and standard reconstruction kernel, renal cyst diameter (p < 0.0001) and background nephrographic phase renal parenchymal attenuation (p = 0.003) were the strongest in vivo predictors of pseudoenhancement. As renal cyst diameter decreased or enhanced renal parenchymal attenuation increased, the odds of pseudoenhancement increased. The magnitude of this effect was small for small changes in renal cyst size (millimeters) and parenchymal attenuation (Hounsfield units), but was clinically meaningful for larger deviations that are often encountered in clinical practice. Specifically, the odds of pseudoenhancement occurring increased by 2.14 (95% CI, 1.41–3.23) for every 5-mm decrease in renal cyst diameter and increased by 2.45 (95% CI, 1.41–4.26) for every 25-HU increase in enhanced renal parenchymal attenuation.
Pseudoenhancement is common among renal cysts that lack enhancement with MRI (38% [42/110]), but uncommon (8% [2/25]) among the subset larger than 20 mm that are adjacent to a background enhanced parenchymal attenuation of 155 HU or less. Therefore, the false-positive rate for solid enhancement within equivocally enhancing renal lesions that are both larger than 20 mm and adjacent to relatively low-enhanced-attenuation parenchyma is much lower than that of smaller (≤ 20 mm) equivocally enhancing lesions that are adjacent to higher-attenuation background parenchyma (> 155 HU). All three cysts in our series with measured enhancement 20 HU or higher (7%; 3/42 cysts with pseudoenhancement) were either small (8 mm diameter; n = 1) or adjacent to higher attenuation enhanced renal parenchyma (185–190 HU; n = 2).
Although radiologists cannot control the size of the cysts they encounter, they can modulate the attenuation of the renal parenchyma. For example, the use of earlier-time-point contrast-enhanced renal imaging (e.g., early nephrographic), the use of higher-concentration contrast media (e.g., 370 mg I/mL instead of 300 mg I/mL), and the use of a higher dose of iodinated contrast media will result in a relatively greater concentration of contrast material within the kidneys, increasing the attenuation of the background parenchyma and potentially increasing the incidence of pseudoenhancement. Paradoxically, lower-peak-kilovoltage imaging, which is used to decrease CT radiation dose and increases the attenuation of iodinated contrast material, has been shown in at least one phantom study to decrease the occurrence and magnitude of pseudoenhancement [5]. This may be because the lesser beam-hardening effects of lower-peak-kilovoltage imaging overrode the increased attenuation effects within the parenchyma.
Although many ex vivo phantom studies have sought to quantify covariates affecting the occurrence of pseudoenhancement, few in vivo studies have been performed [6, 7, 10, 11]. In 2000, Bae et al. [10] used ultrasound as a reference standard to analyze 24 consecutive renal cysts 0.6–10.8 cm. Of all univariate predictors the authors assessed (cyst size, phase of contrast-enhanced imaging, growth pattern, cyst location, and body habitus), only renal cyst size was significant (p = 0.03); the authors did not perform a multivariate analysis. Coulam et al. in 2000 [11] performed both an ex vivo and in vivo assessment; in the in vivo arm (n = 60 presumed simple cysts), benign features on unenhanced CT and lack of growth over a 3-month period were used as the reference standards. These authors similarly performed only univariate analyses and found that renal cyst size and endophytic growth had a significant effect on pseudoenhancement. Tappouni et al. in 2012 [6] also used unenhanced CT as the reference standard and performed only univariate analyses (n = 233 presumed simple cysts), finding that renal cyst size smaller than 1 cm and endophytic growth pattern were significant predictors of pseudoenhancement. Only one other study to our knowledge has assessed in vivo pseudoenhancement using subtraction MRI as the reference standard [7]. In that study, Sai et al. [7] found that for cysts 1 cm or larger, cyst diameter, number of CT detectors, and renal parenchymal attenuation were significant independent predictors of pseudoenhancement, though the magnitude of effect for those variables in the multivariate analysis was not reported. Our multivariate analysis builds on these prior in vivo studies by allowing us to identify and quantify the dominant predictors of pseudoenhancement while controlling for the confounding effect of other covariates (e.g., growth pattern).
There were some unexpected results from our study. Three factors hypothesized to affect renal cyst pseudoenhancement—endophytic growth, abdominal cross-sectional area, and number of CT detectors—were not significant predictors in our final multivariate model. All three have hypothetical associations with pseudoenhancement because of their effect on beam hardening [2–7, 11]. Endophytic growth was a significant predictor in the univariate analysis, but lost significance in the multivariate analysis. This was likely because renal cyst diameter and renal parenchymal attenuation better explained the variance seen with endophytic growth. A similar phenomenon was seen with renal cyst diameter and renal cyst area, with renal cyst area losing significance in the multivariate model as a result of the dominant influence of renal cyst diameter. Because abdominal cross-sectional area and number of CT detectors are relatively fixed patient-level effects (with minor within-patient variation for abdominal cross-sectional area), our study may have been underpowered to assess them.
Our study has some limitations. Although we included 110 renal cysts in our analysis, they were present in only 33 patients. We accounted for this bias by using statistical tests that minimize the effects of repeated measures within patients, but our study may have been underpowered to detect the effects of patientlevel characteristics (e.g., number of CT detectors). We did not have a large number of cysts that both exhibited pseudoenhancement and also had an unenhanced attenuation greater than 10 HU (the subpopulation of greatest clinical relevance, n = 11 of 42 cysts with pseudoenhancement). However, unenhanced attenuation was not a significant predictor of pseudoenhancement in our study (p = 0.96). Therefore, our results are likely generalizable to cysts of all unenhanced attenuation levels. Although we lacked histologic confirmation for the renal cysts in our study, contrast- enhanced MRI is an accepted reference standard for the detection of enhancement in a renal lesion [9]. Some of the MRI confirmatory studies were performed a long time before the CT examinations. It is possible (albeit unlikely) that some of the pseudoenhancement effects we observed were related to new true enhancement within a previously nonenhancing cyst. We did not assess the effect of peak kilovoltage or reconstruction kernel, both of which have been shown in phantom studies to affect pseudoenhancement. We intentionally fixed peak kilovoltage and reconstruction kernel because of sample size considerations to minimize the number of covariates in our study. Finally, our multivariate model assumes a linear relationship between each covariate and the occurrence of pseudoenhancement, which is likely an oversimplification.
In conclusion, renal cyst size and adjacent enhanced renal parenchymal attenuation are independent significant in vivo predictors of pseudoenhancement. These factors are better predictors of pseudoenhancement than growth pattern. Therefore, an endophytic lesion that is exhibiting equivocal enhancement but is both larger than 20 mm and adjacent to relatively low-attenuation enhanced parenchyma (≤ 155 HU) should be considered with more caution than a similar lesion that is less than or equal to 20 mm in size and adjacent to relatively high-attenuation enhanced renal parenchyma (> 155 HU). Because lesser enhancement of the renal parenchyma leads to less pseudoenhancement, modifying contrast media dose or administration technique might be beneficial to minimize pseudoenhancement effects, subject to considering how these changes might alter renal mass conspicuity and detection. The results of this study can be used to inform clinical decision making for equivocally enhancing renal lesions and may be relevant for renal mass CT protocol development.
Acknowledgments
Financial support for statistical analysis was provided by the National Institutes of Health (grant UL1TR000433). No industry support was provided for this study.
Footnotes
R. H. Cohan is a consultant to a law firm representing GE Healthcare regarding ongoing nephrogenic systemic fibrosis litigation. J. H. Ellis consults with GE Healthcare regarding contrast media–related issues. M. S. Davenport and S. Khalatbari had control of all data that might represent a conflict of interest for the other authors.
References
- 1.Rao PS, Alfidi RJ. The environmental density artifact: a beam-hardening effect in computed tomography. Radiology. 1981;141:223–227. doi: 10.1148/radiology.141.1.7291529. [DOI] [PubMed] [Google Scholar]
- 2.Maki DD, Birnbaum BA, Chakraborty DP, et al. Renal cyst pseudoenhancement: beam-hardening effects on CT numbers. Radiology. 1999;213:468–472. doi: 10.1148/radiology.213.2.r99nv33468. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaum BA, Hindman N, Lee J, et al. Renal cyst pseudoenhancement: influence of multidetector CT reconstruction algorithm and scanner type in phantom model. Radiology. 2007;244:767–775. doi: 10.1148/radiol.2443061537. [DOI] [PubMed] [Google Scholar]
- 4.Heneghan JP, Spielmann AL, Sheafor DH, et al. Pseudoenhancement of simple renal cysts: a comparison of single and multidetector helical CT. J Comput Assist Tomogr. 2002;26:90–94. doi: 10.1097/00004728-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Wang ZJ, Coakley FV, Fu Y, et al. Renal cyst pseudoenhancement at multidetector CT: what are the effects of number of detectors and peak tube voltage? Radiology. 2008;248:910–916. doi: 10.1148/radiol.2482071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tappouni R, Kissane J, Sarwani N, et al. Pseudoenhancement of renal cysts: influence of lesion size, lesion location, slice thickness, and number of MDCT detectors. AJR. 2012;198:133–137. doi: 10.2214/AJR.10.6057. [DOI] [PubMed] [Google Scholar]
- 7.Sai V, Rakow-Pener R, Yeh BM, et al. Renal cyst pseudoenhancement at 16- and 64-detector row MDCT. Clin Imaging. 2013;37:520–525. doi: 10.1016/j.clinimag.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Bosniak MA. The current radiological approach to renal cysts. Radiology. 1986;158:1–10. doi: 10.1148/radiology.158.1.3510019. [DOI] [PubMed] [Google Scholar]
- 9.Israel GM, Bosniak MA. How I do it: evaluating renal masses. Radiology. 2005;236:441–450. doi: 10.1148/radiol.2362040218. [DOI] [PubMed] [Google Scholar]
- 10.Bae KT, Heiken JP, Siegel CL, et al. Renal cysts: is attenuation artificially increased on contrast-enhanced CT images? Radiology. 2000;216:792–796. doi: 10.1148/radiology.216.3.r00se14792. [DOI] [PubMed] [Google Scholar]
- 11.Coulam CH, Sheafor DH, Leder RA, et al. Evaluation of pseudoenhancement of renal cysts during contrast-enhanced CT. AJR. 2000;174:493–498. doi: 10.2214/ajr.174.2.1740493. [DOI] [PubMed] [Google Scholar]
- 12.Abdulla C, Kalra MK, Saini S, et al. Pseudoenhancement of simulated renal cysts in a phantom using different multidetector CT scanners. AJR. 2002;179:1473–1476. doi: 10.2214/ajr.179.6.1791473. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum BA, Maki DD, Chakraborty DP, et al. Renal cyst pseudoenhancement: evaluation with an anthropomorphic body CT phantom. Radiology. 2002;225:83–90. doi: 10.1148/radiol.2251010930. [DOI] [PubMed] [Google Scholar]
- 14.Gokan T, Ohgiya Y, Munechika H, et al. Renal cyst pseudoenhancement with beam hardening effect on CT attenuation. Radiat Med. 2002;20:187–190. [PubMed] [Google Scholar]
- 15.Hecht EM, Israel GM, Krinsky GA, et al. Renal masses: analysis of enhancement with signal intensity measurements versus qualitative analysis of enhancement with image subtraction for diagnosing malignancy at MR imaging. Radiology. 2004;232:373–378. doi: 10.1148/radiol.2322031209. [DOI] [PubMed] [Google Scholar]
- 16.Ho VB, Allen SF, Hood MN, Choyke PL. Renal masses: quantitative assessment of enhancement with dynamic MR imaging. Radiology. 2002;224:695–700. doi: 10.1148/radiol.2243011048. [DOI] [PubMed] [Google Scholar]
- 17.Silverman SG, Israel GM, Herts BR, et al. Management of the incidental renal mass. Radiology. 2008;249:16–31. doi: 10.1148/radiol.2491070783. [DOI] [PubMed] [Google Scholar]