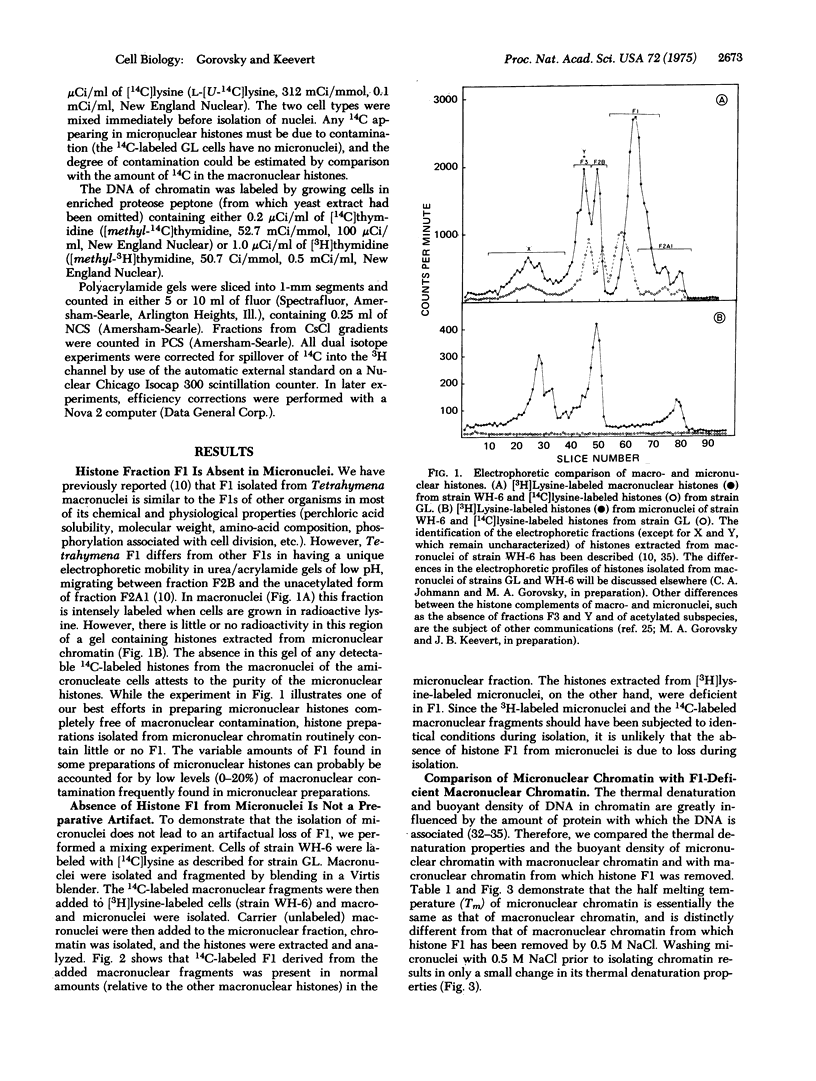
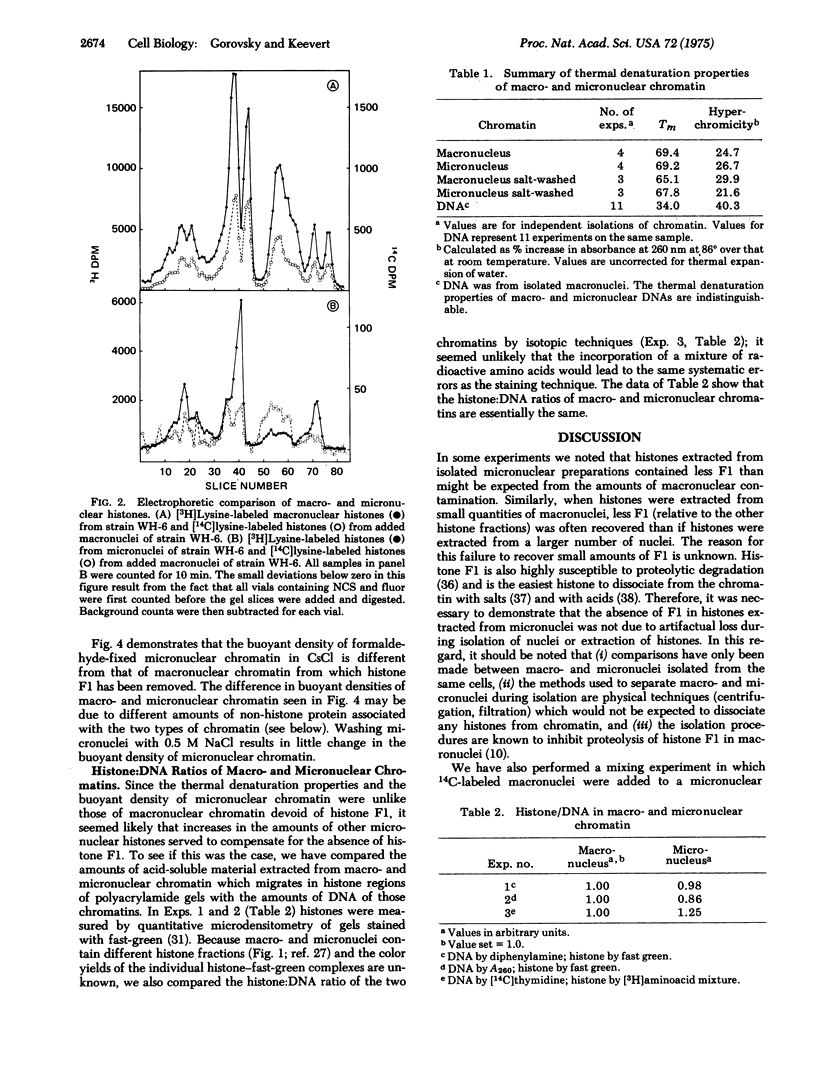
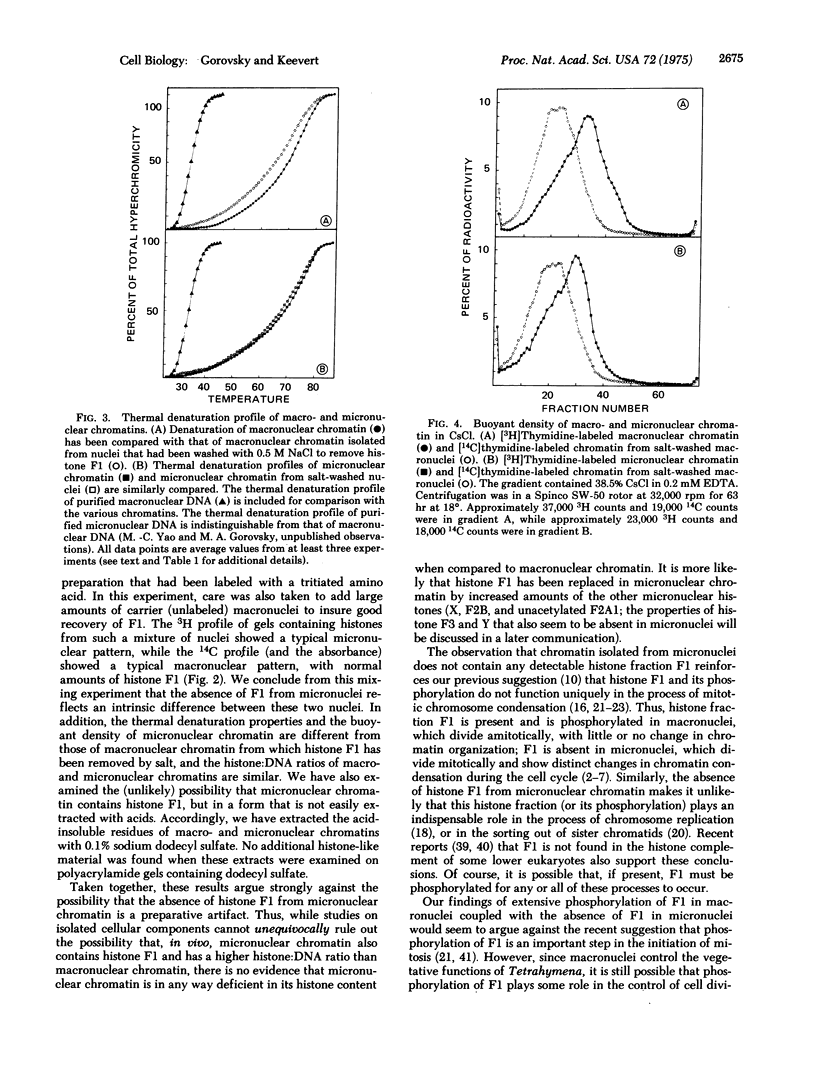
Abstract
Histones were extracted from macro- and micronuclear chromatin of the ciliated protozoan Tetrahymena pyriformis. Conditions that resulted in macronuclear chromatin containing large amounts of histone F1 yielded micronuclear chromatin in which this histone was absent. Evidence is presented indicating that the absence of F1 from micronuclei is not a preparative artifact and that histone F1 is replaced by other histone fractions. Since micronuclei divide mitotically, while macronuclei divide amitotically, these results suggest that histone F1 and its phosphorylation do not play an indispensable role in the process of mitotic chromosome condensation, in chromosome replication, or in the separation of newly synthesized chromatids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansevin A. T., Brown B. W. Specificity in the association of histones with deoxyribonucleic acid. Evidence from derivative thermal denaturation profiles. Biochemistry. 1971 Mar 30;10(7):1133–1142. doi: 10.1021/bi00783a006. [DOI] [PubMed] [Google Scholar]
- Balhorn R., Balhorn M., Chalkley R. Lysine-rach histone phosphorylation and hyperplasia in the developing rat. Dev Biol. 1972 Oct;29(2):199–203. doi: 10.1016/0012-1606(72)90056-5. [DOI] [PubMed] [Google Scholar]
- Balhorn R., Chalkley R., Granner D. Lysine-rich histone phosphorylation. A positive correlation with cell replication. Biochemistry. 1972 Mar 14;11(6):1094–1098. doi: 10.1021/bi00756a023. [DOI] [PubMed] [Google Scholar]
- Balhorn R., Rieke W. O., Chalkley R. Rapid electrophoretic analysis for histone phosphorylation. A reinvestigation of phosphorylation of lysine-rich histone during rat liver regeneration. Biochemistry. 1971 Oct 12;10(21):3952–3959. doi: 10.1021/bi00797a024. [DOI] [PubMed] [Google Scholar]
- Bartley J., Chalkley R. Further studies of a thymus nucleohistone-associated protease. J Biol Chem. 1970 Sep 10;245(17):4286–4292. [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R. Control of cell division by very lysine rich histone (F1) phosphorylation. Nature. 1974 Feb 1;247(5439):257–261. doi: 10.1038/247257a0. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R., Langan T. A. Molecular basis of control of mitotic cell division in eukaryotes. Nature. 1974 Jun 7;249(457):553–556. doi: 10.1038/249553a0. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R., Sarner N. Phosphorylation of very-lysine-rich histone in Physarum polycephalum. Correlation with chromosome condensation. Eur J Biochem. 1973 Feb 15;33(1):131–139. doi: 10.1111/j.1432-1033.1973.tb02664.x. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Schlehuber C., Bonner J. Properties of formaldehyde-treated nucleohistone. Biochemistry. 1969 Aug;8(8):3214–3218. doi: 10.1021/bi00836a013. [DOI] [PubMed] [Google Scholar]
- Flickinger C. J. The fine structure of the nuclei of Tetrahymena pyriformis throughout the cell cycle. J Cell Biol. 1965 Dec;27(3):519–529. doi: 10.1083/jcb.27.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco L., Johns E. W., Navlet J. M. Histones from baker's yeast. Isolation and fractionation. Eur J Biochem. 1974 Jun 1;45(1):83–89. doi: 10.1111/j.1432-1033.1974.tb03532.x. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Carlson K., Rosenbaum J. L. Simple method for quantitive densitometry of polyacrylamide gels using fast green. Anal Biochem. 1970 Jun;35(2):359–370. doi: 10.1016/0003-2697(70)90196-x. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A. Macro- and micronuclei of Tetrahymena pyriformis: a model system for studying the structure and function of eukaryotic nuclei. J Protozool. 1973 Feb;20(1):19–25. doi: 10.1111/j.1550-7408.1973.tb05995.x. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Pleger G. L., Keevert J. B., Johmann C. A. Studies on histone fraction F2A1 in macro- and micronuclei of Tetrahymena pyriformis. J Cell Biol. 1973 Jun;57(3):773–781. doi: 10.1083/jcb.57.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A. Studies on nuclear structure and function in Tetrahymena pyriformis. II. Isolation of macro- and micronuclei. J Cell Biol. 1970 Dec;47(3):619–630. doi: 10.1083/jcb.47.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. Cell cycle-specific changes in histone phosphorylation associated with cell proliferation and chromosome condensation. J Cell Biol. 1974 Feb;60(2):356–364. doi: 10.1083/jcb.60.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Cernosek R. M., Hnilica L. S. Histone synthesis and phosphorylation in regenerating rat liver. Biochim Biophys Acta. 1971 Oct 14;247(2):348–354. doi: 10.1016/0005-2787(71)90682-4. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Weintraub H., Mayne R., Mochan B. The cell cycle, cell lineages, and cell differentiation. Curr Top Dev Biol. 1972;7:229–256. doi: 10.1016/s0070-2153(08)60073-3. [DOI] [PubMed] [Google Scholar]
- Hsiang M. W., Cole R. D. The isolation of histone from Neurospora crassa. J Biol Chem. 1973 Mar 25;248(6):2007–2013. [PubMed] [Google Scholar]
- Ilyin Y. V., Georgiev G. P. Heterogeneity of deoxynucleoprotein particles as evidencec by ultracentrifugation of cesium chloride density gradient. J Mol Biol. 1969 Apr;41(2):299–303. doi: 10.1016/0022-2836(69)90395-7. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphoprotein metabolism in isolated lymphocyte nuclei. Proc Natl Acad Sci U S A. 1966 May;55(5):1182–1189. doi: 10.1073/pnas.55.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake R. S., Goidl J. A., Salzman N. P. F1-histone modification at metaphase in Chinese hamster cells. Exp Cell Res. 1972 Jul;73(1):113–121. doi: 10.1016/0014-4827(72)90108-5. [DOI] [PubMed] [Google Scholar]
- Lake R. S., Salzman N. P. Occurrence and properties of a chromatin-associated F1-histone phosphokinase in mitotic Chinese hamster cells. Biochemistry. 1972 Dec 5;11(25):4817–4826. doi: 10.1021/bi00775a027. [DOI] [PubMed] [Google Scholar]
- Marks D. B., Paik W. K., Borun T. W. The relationship of histone phosphorylation to deoxyribonucleci acid replication and mitosis during the HeLa S-3 cell cycle. J Biol Chem. 1973 Aug 25;248(16):5660–5667. [PubMed] [Google Scholar]
- Meisler M. H., Langan T. A. Characterization of a phosphatase specific for phosphorylated histones and protamine. J Biol Chem. 1969 Sep 25;244(18):4961–4968. [PubMed] [Google Scholar]
- Murray K. Stepwise removal of histones from native deoxyribonucleoprotein by titration with acid at low temperature and some properties of the resulting partial nucleoproteins. J Mol Biol. 1969 Jan 14;39(1):125–144. doi: 10.1016/0022-2836(69)90338-6. [DOI] [PubMed] [Google Scholar]
- Nilsson J. R. Suggestive structural evidence for macronuclear "subnuclei" in Tetrahymena pyriformis GL. J Protozool. 1970 Nov;17(4):539–548. doi: 10.1111/j.1550-7408.1970.tb04724.x. [DOI] [PubMed] [Google Scholar]
- Ohlenbusch H. H., Olivera B. M., Tuan D., Davidson N. Selective dissociation of histones from calf thymus nucleoprotein. J Mol Biol. 1967 Apr 28;25(2):299–315. doi: 10.1016/0022-2836(67)90143-x. [DOI] [PubMed] [Google Scholar]
- Oliver D., Balhorn R., Granner D., Chalkley R. Molecular nature of F 1 histone phosphorylation in cultured hepatoma cells. Biochemistry. 1972 Oct 10;11(21):3921–3925. doi: 10.1021/bi00771a014. [DOI] [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Further studies on phosphorylation and the thiol-disulphide ratio of histones in growth and development. Biochem J. 1969 Mar;112(1):81–89. doi: 10.1042/bj1120081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Bilek D., Chalkley R. An electrophoretic comparison of vertebrate histones. J Biol Chem. 1971 Jul 10;246(13):4206–4215. [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Sherod D., Johnson G., Chalkley R. Phosphorylation of mouse ascites tumor cell lysine-rich histone. Biochemistry. 1970 Nov 10;9(23):4611–4615. doi: 10.1021/bi00825a022. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Bonner J. Thermal denaturation and template properties of DNA complexes with purified histone fractions. J Mol Biol. 1970 Mar;48(3):469–487. doi: 10.1016/0022-2836(70)90059-8. [DOI] [PubMed] [Google Scholar]
- Stevely W. S., Stocken L. A. Variations in the phosphate content of histone F1 in normal and irradiated tissues. Biochem J. 1968 Nov;110(2):187–191. doi: 10.1042/bj1100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson I., Lloyd F. P. Ultrastructure of nuclear division in Paramecium aurelia. II. Amitosis of the macronucleus. Aust J Biol Sci. 1971 Oct;24(5):977–987. doi: 10.1071/bi9710977. [DOI] [PubMed] [Google Scholar]
- Stevenson I., Lloyd F. P. Ultrastructure of nuclear division in Paramecium aurella. I. Mitosis in the micronucleus. Aust J Biol Sci. 1971 Oct;24(5):963–975. doi: 10.1071/bi9710963. [DOI] [PubMed] [Google Scholar]



