Abstract
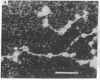
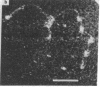
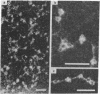
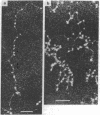
Dark field scanning electron microscopy of unstained, unfixed samples of chromatin, histone-1-depleted chromatin, and nucleohistone has been used to identify an apparent subunit of chromatin, namely a disk-shaped structure we term the unit particle, which is probably about 135 A wide and 50 A thick in the hydrated state. The unit particles are found at rather uniform intervals along thin DNA-like fibers. Histone 1 depletion leads to a bimodal distribution of these spacings. Our observations suggest that the unit particle consists of a loop of nucleoprotein, perhaps around a histone core.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., LITTAU V. C., MIRSKY A. E. METHODS FOR THE PURIFICATION OF THYMUS NUCLEI AND THEIR APPLICATION TO STUDIES OF NUCLEAR PROTEIN SYNTHESIS. J Cell Biol. 1964 May;21:213–231. doi: 10.1083/jcb.21.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bram S., Butler-Browne G., Baudy P., Ibel K. Quaternary structure of chromatin. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1043–1045. doi: 10.1073/pnas.72.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bram S., Ris H. On the structure of nucleohistone. J Mol Biol. 1971 Feb 14;55(3):325–336. doi: 10.1016/0022-2836(71)90321-4. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Schlehuber C., Bonner J. Properties of formaldehyde-treated nucleohistone. Biochemistry. 1969 Aug;8(8):3214–3218. doi: 10.1021/bi00836a013. [DOI] [PubMed] [Google Scholar]
- Clark R. J., Felsenfeld G. Chemical probes of chromatin structure. Biochemistry. 1974 Aug 13;13(17):3622–3628. doi: 10.1021/bi00714a034. [DOI] [PubMed] [Google Scholar]
- Crewe A. V., Wall J. A scanning microscope with 5 A resolution. J Mol Biol. 1970 Mar;48(3):375–393. doi: 10.1016/0022-2836(70)90052-5. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. Interactions of histone LAK (f2a2) with histones KAS (f2b) and GRK (f2a1). Biochemistry. 1974 May 7;13(10):2098–2104. doi: 10.1021/bi00707a016. [DOI] [PubMed] [Google Scholar]
- HART R. G. Electron microscopy of the 50-S ribosomes of Escherichia coli. Biochim Biophys Acta. 1962 Jul 16;60:629–637. doi: 10.1016/0006-3002(62)90881-8. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Ilyin Y. V., Varshavsky A. Y., Mickelsaar U. N., Georgiev G. P. Studies on deoxyribonucleoprotein structure. Redistribution of proteins in mixtures of deoxyribonucleoproteins, DNA and RNA. Eur J Biochem. 1971 Sep 24;22(2):235–245. doi: 10.1111/j.1432-1033.1971.tb01537.x. [DOI] [PubMed] [Google Scholar]
- KLUG A., FINCH J. T. STRUCTURE OF VIRUSES OF THE PAPILLOMA-POLYOMA TYPE. I. HUMAN WART VIRUS. J Mol Biol. 1965 Feb;11:403–423. doi: 10.1016/s0022-2836(65)80066-3. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Thomas J. O. Chromatin structure; oligomers of the histones. Science. 1974 May 24;184(4139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Martinson H. G., McCarthy B. J. Histone-histone associations within chromatin. Cross-linking studies using tetranitromethane. Biochemistry. 1975 Mar 11;14(5):1073–1078. doi: 10.1021/bi00676a030. [DOI] [PubMed] [Google Scholar]
- Noll M. Subunit structure of chromatin. Nature. 1974 Sep 20;251(5472):249–251. doi: 10.1038/251249a0. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E. Spheroid chromatin units (v bodies). Science. 1974 Jan 25;183(4122):330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. The heterogeneity of histones. I. A quantitative analysis of calf histones in very long polyacrylamide gels. Biochemistry. 1969 Oct;8(10):3972–3979. doi: 10.1021/bi00838a013. [DOI] [PubMed] [Google Scholar]
- Pardon J. F., Richards B. M., Cotter R. I. X-ray diffraction studies on oriented nucleohistone gels. Cold Spring Harb Symp Quant Biol. 1974;38:75–81. doi: 10.1101/sqb.1974.038.01.010. [DOI] [PubMed] [Google Scholar]
- Rill R., Van Holde K. E. Properties of nuclease-resistant fragments of calf thymus chromatin. J Biol Chem. 1973 Feb 10;248(3):1080–1083. [PubMed] [Google Scholar]
- Ris H., Kubai D. F. Chromosome structure. Annu Rev Genet. 1970;4:263–294. doi: 10.1146/annurev.ge.04.120170.001403. [DOI] [PubMed] [Google Scholar]
- SLAYTER H. S., WARNER J. R., RICH A., HALL C. E. THE VISUALIZATION OF POLYRIBOSOMAL STRUCTURE. J Mol Biol. 1963 Dec;7:652–657. doi: 10.1016/s0022-2836(63)80112-6. [DOI] [PubMed] [Google Scholar]
- Slayter H. S., Shih T. Y., Adler A. J., Fasman G. D. Electron microscopy and circular dichroism studies on chromatin. Biochemistry. 1972 Aug 1;11(16):3044–3054. doi: 10.1021/bi00766a016. [DOI] [PubMed] [Google Scholar]
- Unwin P. N. Electron microscopy of the stacked disk aggregate of tobacco mosaic virus protein. II. The influence of electron irradiation of the stain distribution. J Mol Biol. 1974 Aug 25;87(4):657–670. doi: 10.1016/0022-2836(74)90076-x. [DOI] [PubMed] [Google Scholar]
- Wall J., Langmore J., Isaacson M., Crewe A. V. Scanning transmission electron microscopy at high resolution. Proc Natl Acad Sci U S A. 1974 Jan;71(1):1–5. doi: 10.1073/pnas.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Fisher H. W. Electron microscopy of tobacco mosaic virus under conditions of minimal beam exposure. J Mol Biol. 1970 Aug 28;52(1):121–123. doi: 10.1016/0022-2836(70)90181-6. [DOI] [PubMed] [Google Scholar]