Abstract
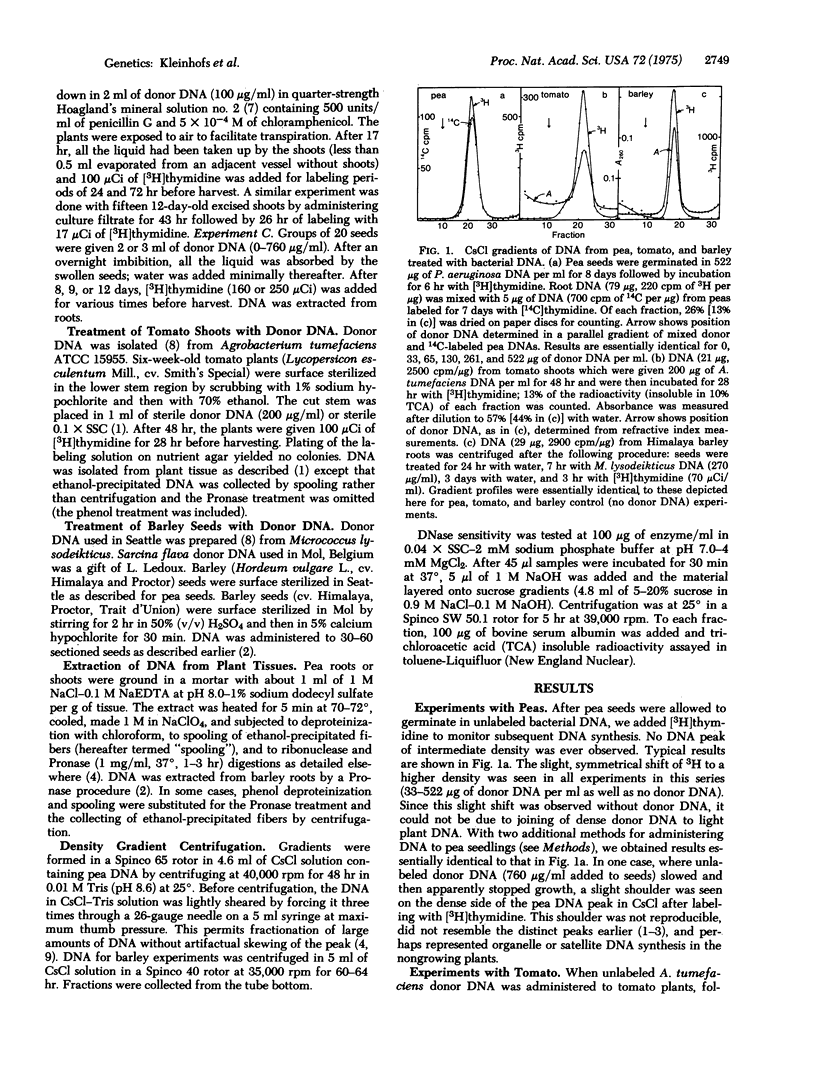
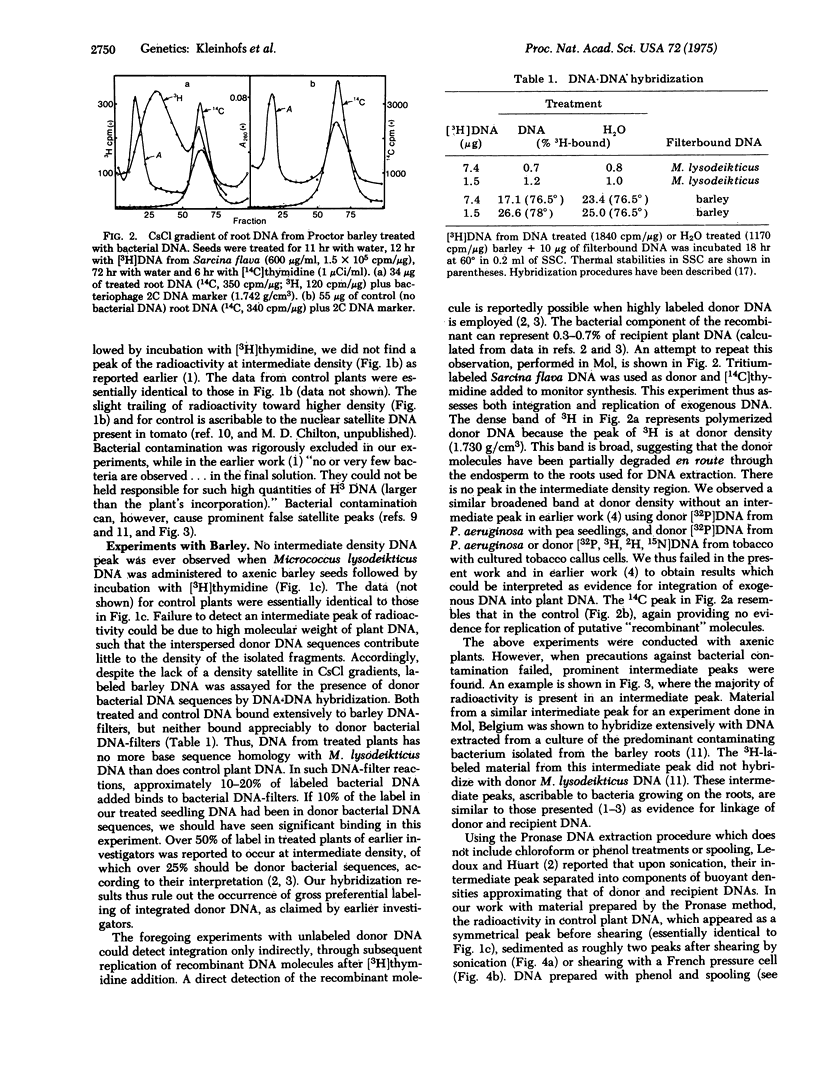
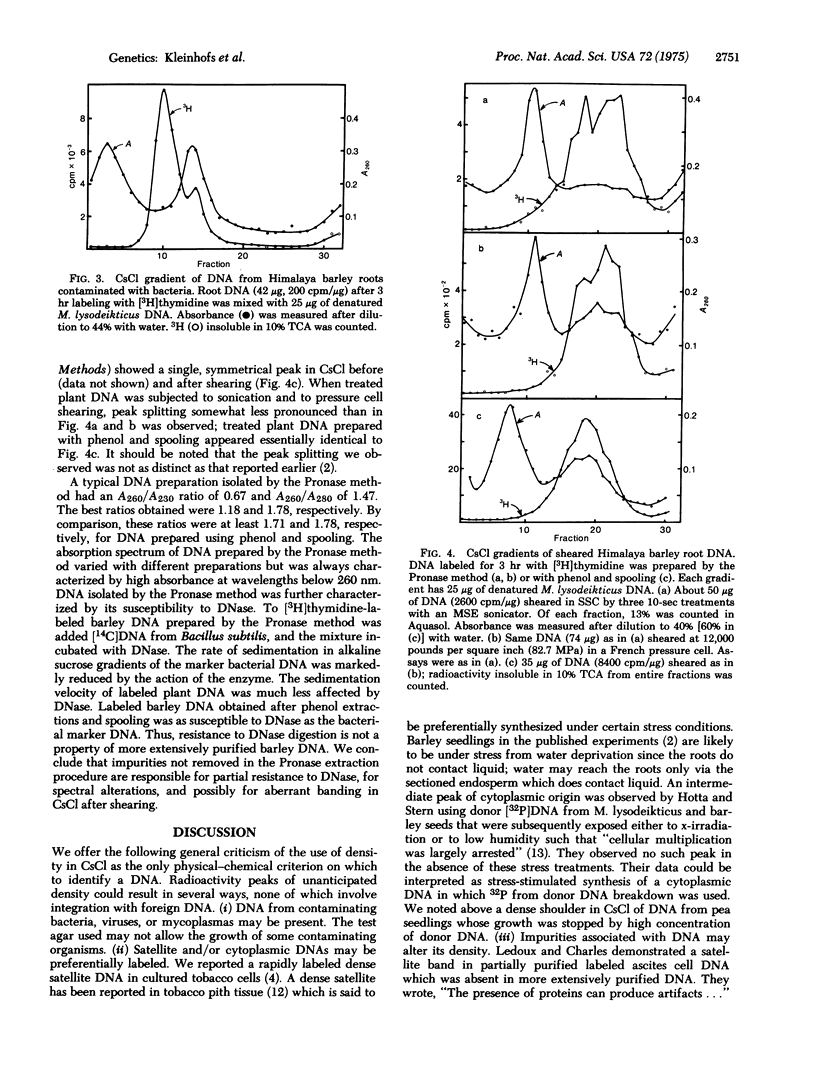
Extensive studies with pea, tomato, and barley failed to confirm the evidence presented by previous investigators for integration or replication of exogenously applied bacterial DNA in these plants. Labeled DNA of buoyant density in CsCl intermediate between that of high density donor bacterial DNA and of plant DNA was never observed with axenic plants. Intermediate peaks, similar to those used as evidence for recombination by earlier investigators, were observed only when the plants were contaminated with bacteria. Plant DNA prepared by a published procedure [Ledoux, L. & Huart, R. (1969) J. Mol. Biol. 43, 243-262] was found to be contaminated with unidentified impurities. Such DNA was partially protected from the action of DNase and produced aberrant banding patterns in CsCl after shearing. Much of the published evidence for integration of foreign DNA in plants is based upon experiments with plant DNA prepared by this procedure. We conclude that contamination is the likely explanation for what has been interpreted as evidence for integration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendich A. J. Effect of contaminating bacteria on the radiolabeling of nucleic acids from seedlings: false DNA "satellites". Biochim Biophys Acta. 1972 Jul 31;272(4):494–503. doi: 10.1016/0005-2787(72)90504-7. [DOI] [PubMed] [Google Scholar]
- Ingle J., Pearson G. G., Sinclair J. Species distribution and properties of nuclear satellite DNA in higher plants. Nat New Biol. 1973 Apr 18;242(120):193–197. doi: 10.1038/newbio242193a0. [DOI] [PubMed] [Google Scholar]
- Ledoux L., Huart R. Fate of exogenous bacterial deoxyribonucleic acids in barley seedlings. J Mol Biol. 1969 Jul 28;43(2):243–262. doi: 10.1016/0022-2836(69)90265-4. [DOI] [PubMed] [Google Scholar]
- Ledoux L., Huart R., Jacobs M. DNA-mediated genetic correction of thiamineless Arabidopsis thaliana. Nature. 1974 May 3;249(452):17–21. doi: 10.1038/249017a0. [DOI] [PubMed] [Google Scholar]
- Ledoux L., Huart R., Jacobs M. Fate of exogenous DNA in Arabidopsis thaliana. Translocation and integration. Eur J Biochem. 1971 Nov 11;23(1):96–108. doi: 10.1111/j.1432-1033.1971.tb01596.x. [DOI] [PubMed] [Google Scholar]
- McCarthy B. J., McConaughy B. L. Related base sequences in the DNA of simple and complex organisms. I. DNA-DNA duplex formation and the incidence of partially related base sequences in DNA. Biochem Genet. 1968 Jun;2(1):37–53. doi: 10.1007/BF01458450. [DOI] [PubMed] [Google Scholar]
- Parenti R., Guillé E., Grisvard J., Durante M., Giorgi L., Buiatti M. Transient DNA satellite in dedifferentiating pith tissue. Nat New Biol. 1973 Dec 19;246(155):237–239. doi: 10.1038/newbio246237a0. [DOI] [PubMed] [Google Scholar]
- Stroun M., Anker P., Gahan P., Rossier A., Greppin H. Agrobacterium tumefaciens ribonucleic acid synthesis in tomato cells and crown gall induction. J Bacteriol. 1971 May;106(2):634–639. doi: 10.1128/jb.106.2.634-639.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroun M., Anker P., Ledoux L. DNA replication in Solanum lycopersicum esc. after absorption of bacterial DNA. Curr Mod Biol. 1967 Aug;1(3):231–234. doi: 10.1016/0303-2647(67)90061-5. [DOI] [PubMed] [Google Scholar]


