Abstract
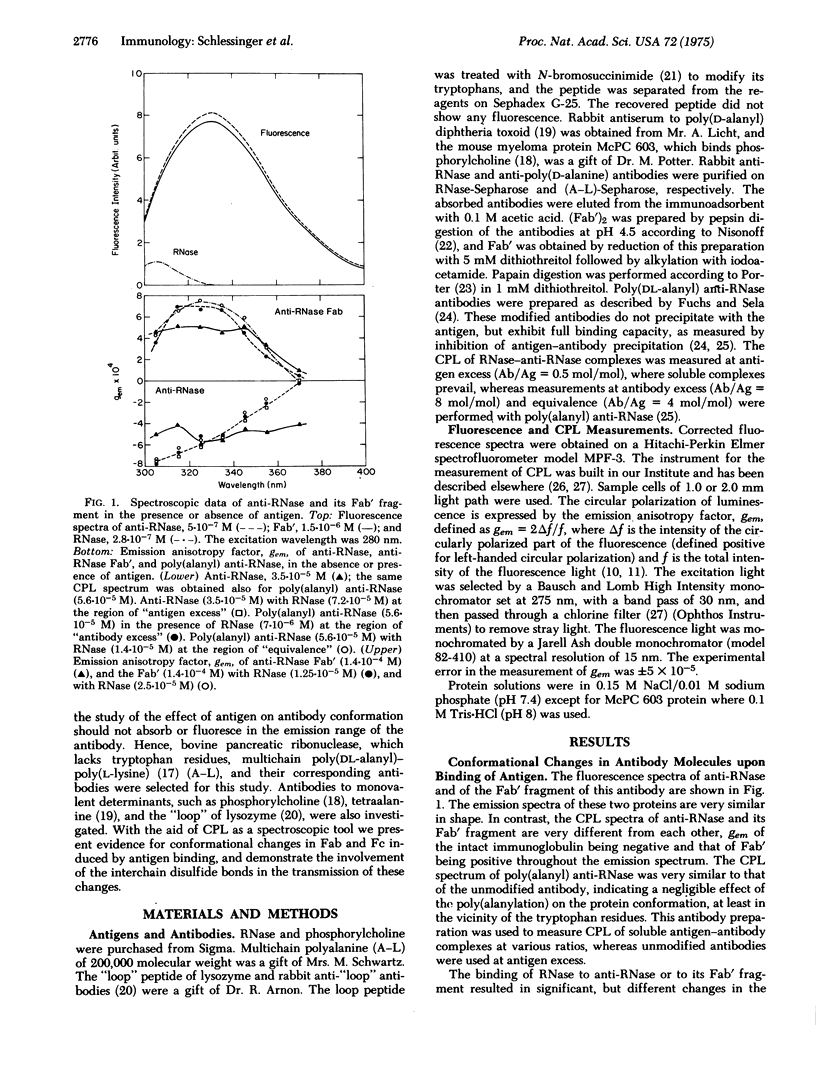
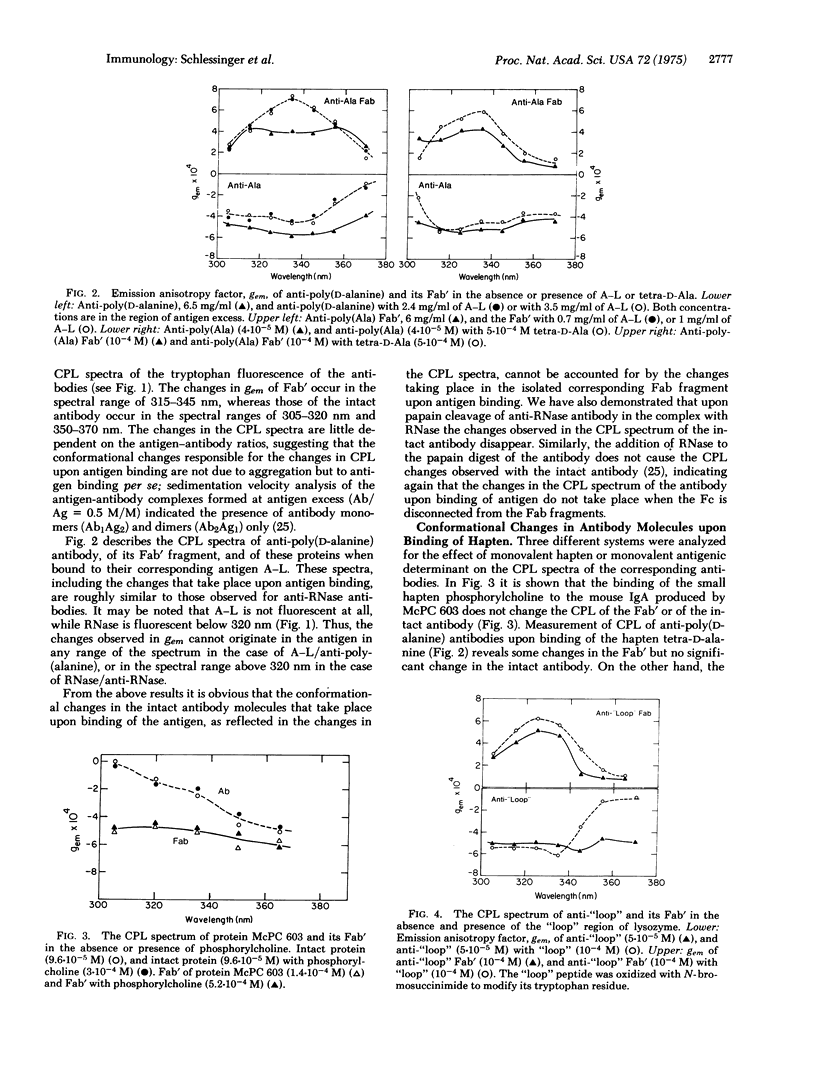
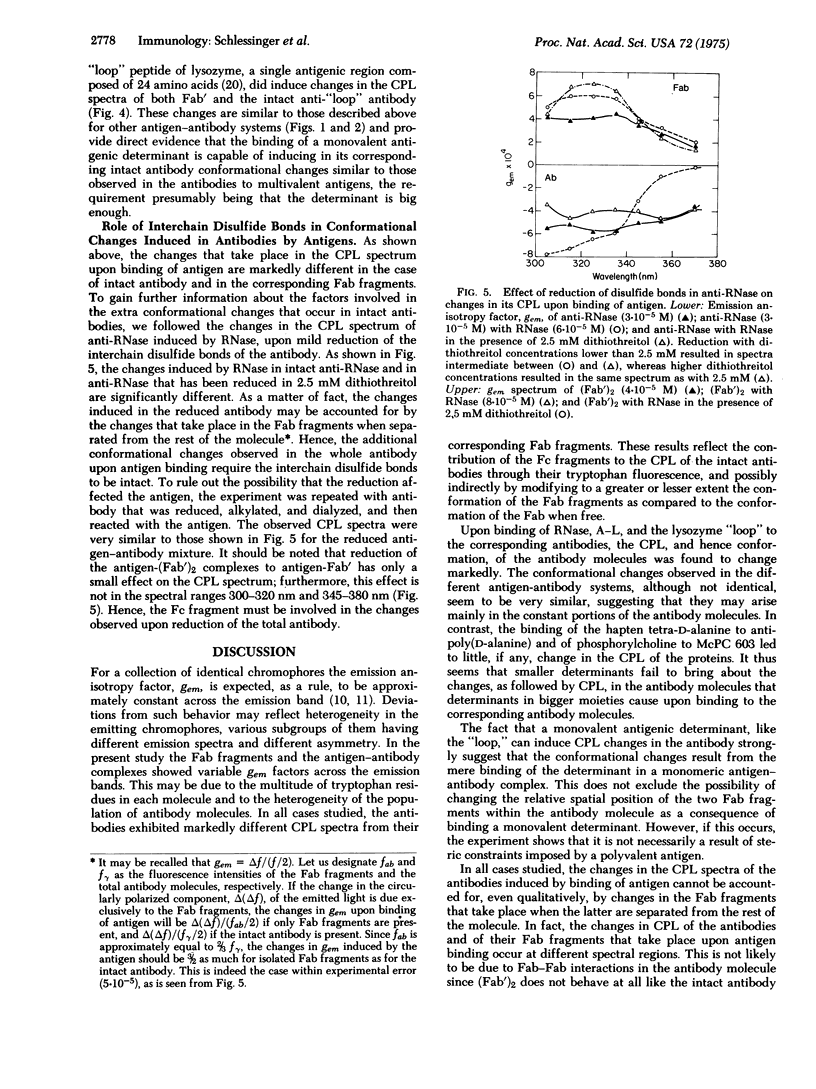
Conformational changes induced in antibody molecules and in their Fab fragments by binding of antigen were investigated by the circular polarization of the fluorescence emitted by the tryptophan residues. This property of the fluorescence is related to the asymmetry, and thus to the conformation and environment, of the emitting chromophore. Changes in the circular polarization of the fluorescence of the antibody were observed upon binding of RNase to anti-RNase, of poly(DL-alanyl)-poly(L-lysine) to antipoly(D-alanine), and of the "loop" of lysozyme, a monovalent antigenic determinant, to anti"loop." The spectral changes were observed at different antigen-antibody ratios, including high antigen excess, indicating that they are due to antigen binding and not to aggregation. The circular polarization of fluorescence also detects changes in conformation of the different Fab fragments upon binding of the corresponding antigens. These changes in conformation were, however, markedly different from those observed for the whole antibody molecules, and indicated an interaction between the Fc and Fab fragments in the antibody molecule, and probably a change in the conformation of Fc upon binding of antigen to the antibody. In contrast, the small hapten, phosphorylcholine, did not induce a change in the circular polarization of the fluorescence of its antibody or corresponding Fab fragments. Reduction of the interchain disulfide bonds of the antibodies abolished the antigen-induced spectral changes due to the presence of the Fc portion in the molecule, but not the changes observed in Fab, suggesting that the disulfide bonds at the hinge region of the antibody are required for the transmission of the conformational change from the Fab to the Fc.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon R., Sela M. Antibodies to a unique region in lysozyme provoked by a synthetic antigen conjugate. Proc Natl Acad Sci U S A. 1969 Jan;62(1):163–170. doi: 10.1073/pnas.62.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk H., Stemberger H., Wiedermann G., Eckerstorfer R., Tappeiner G. The influence of 2-mercaptoethanol (2-ME) treatment of IgG antibody on its ability to induce cytotoxicity in nonsensitized lymphocytes. Cell Immunol. 1974 Sep;13(3):489–492. doi: 10.1016/0008-8749(74)90268-8. [DOI] [PubMed] [Google Scholar]
- Fuchs S., Sela M. Preparation and characterization of poly-DL-alanyl rabbit gamma-globulin. J Biol Chem. 1965 Sep;240(9):3558–3567. [PubMed] [Google Scholar]
- Givol D. The cleavage of rabbit immunoglobulin G by trypsin after mild reduction and aminoethylation. Biochem J. 1967 Sep;104(3):39C–40C. doi: 10.1042/bj1040039c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowka D. A., Strosberg A. D., Kimball J. W., Haber E., Cathou R. E. Changes in intrinsic circular dichroism of several homogeneous anti-type 3 pneumococcal antibodies on binding of a small hapten. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3399–3403. doi: 10.1073/pnas.69.11.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht A., Schechter B., Sela M. Antibodies against polyalanyl determinants attached to negatively or positively charged carriers. Eur J Immunol. 1971 Nov;1(5):351–359. doi: 10.1002/eji.1830010510. [DOI] [PubMed] [Google Scholar]
- Metzger H. Effect of antigen binding on the properties of antibody. Adv Immunol. 1974;18:169–207. doi: 10.1016/s0065-2776(08)60310-7. [DOI] [PubMed] [Google Scholar]
- NISONOFF A. ENZYMATIC DIGESTION OF RABBIT GAMMA GLOBULIN AND ANTIBODY AND CHROMATOGRAPHY OF DIGESTION PRODUCTS. Methods Med Res. 1964;10:134–141. [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz I., Kratky O., Licht A., Sela M. Shape and volume of anti-poly(D-alanyl) antibodies in the presence and absence of tetra-D-alanine as followed by small-angle x-ray scattering. Biochemistry. 1973 Nov 20;12(24):4998–5005. doi: 10.1021/bi00748a028. [DOI] [PubMed] [Google Scholar]
- Pilz I., Kratky O., Licht A., Sela M. Shape and volume of fragments Fab' and (Fab')2 of anti-poly(D-alanyl) antibodies in the presence and absence of tetra-D-alanine as determined by small-angle x-ray scattering. Biochemistry. 1975 Mar 25;14(6):1326–1333. doi: 10.1021/bi00677a035. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Chen B. L., Phizackerley R. P., Saul F. Three-dimensional structure of the Fab' fragment of a human immunoglobulin at 2,8-A resolution. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3305–3310. doi: 10.1073/pnas.70.12.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollet R., Edelhoch H. Changes in optical parameters of myeloma proteins with phosphorylcholine binding. J Biol Chem. 1974 Aug 25;249(16):5188–5194. [PubMed] [Google Scholar]
- Potter M., Leon M. A. Three IgA myeloma immunoglobulins from the BALB/ mouse: precipitation with pneumococcal C polysaccharide. Science. 1968 Oct 18;162(3851):369–371. doi: 10.1126/science.162.3851.369. [DOI] [PubMed] [Google Scholar]
- SCHUR P. H., CHRISTIAN G. D. THE ROLE OF DISULFIDE BONDS IN THE COMPLEMENT-FIXING AND PRECIPITATING PROPERTIES OF 7S RABBIT AND SHEEP ANTIBODIES. J Exp Med. 1964 Oct 1;120:531–545. doi: 10.1084/jem.120.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELA M., FUCHS S., ARNON R. Studies on the chemical basis of the antigenicity of proteins. 5. Synthesis, characterization and immunogenicity of some multichain and linear polypeptides containing tyrosine. Biochem J. 1962 Oct;85:223–235. doi: 10.1042/bj0850223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M., Girling R. L., Ely K. R., Edmundson A. B. Structure of a lambda-type Bence-Jones protein at 3.5-A resolution. Biochemistry. 1973 Nov 6;12(23):4620–4631. doi: 10.1021/bi00747a013. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Roche R. S., Steinberg I. Z. A study of subtilisin types Novo and Carlsberg by circular polarization of fluorescence. Biochemistry. 1975 Jan 28;14(2):255–262. doi: 10.1021/bi00673a010. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Steinberg I. Z. Circular polarization of fluorescence of probes bound to chymotrypsin. Change in asymmetric environment upon electronic excitation. Proc Natl Acad Sci U S A. 1972 Mar;69(3):769–772. doi: 10.1073/pnas.69.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Steinberg I. Z., Pecht I. Antibody-hapten interactions: circular and linear polarization of the fluorescence of dansyl bound to anti-dansyl antibodies. J Mol Biol. 1974 Aug 25;87(4):725–740. doi: 10.1016/0022-2836(74)90081-3. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumerman L. A., Nezlin R. S., Zagyansky Y. A. Increase of the rotational relaxation time of antibody molecule after complex formation with dansyl-hapten. FEBS Lett. 1972 Jan 1;19(4):290–292. doi: 10.1016/0014-5793(72)80062-0. [DOI] [PubMed] [Google Scholar]
- Warner C., Schumaker V. Detection of a conformational change in an antihapten--antibody system upon interaction with divalent hapten. Biochemistry. 1970 Feb 3;9(3):451–459. doi: 10.1021/bi00805a001. [DOI] [PubMed] [Google Scholar]


