Abstract
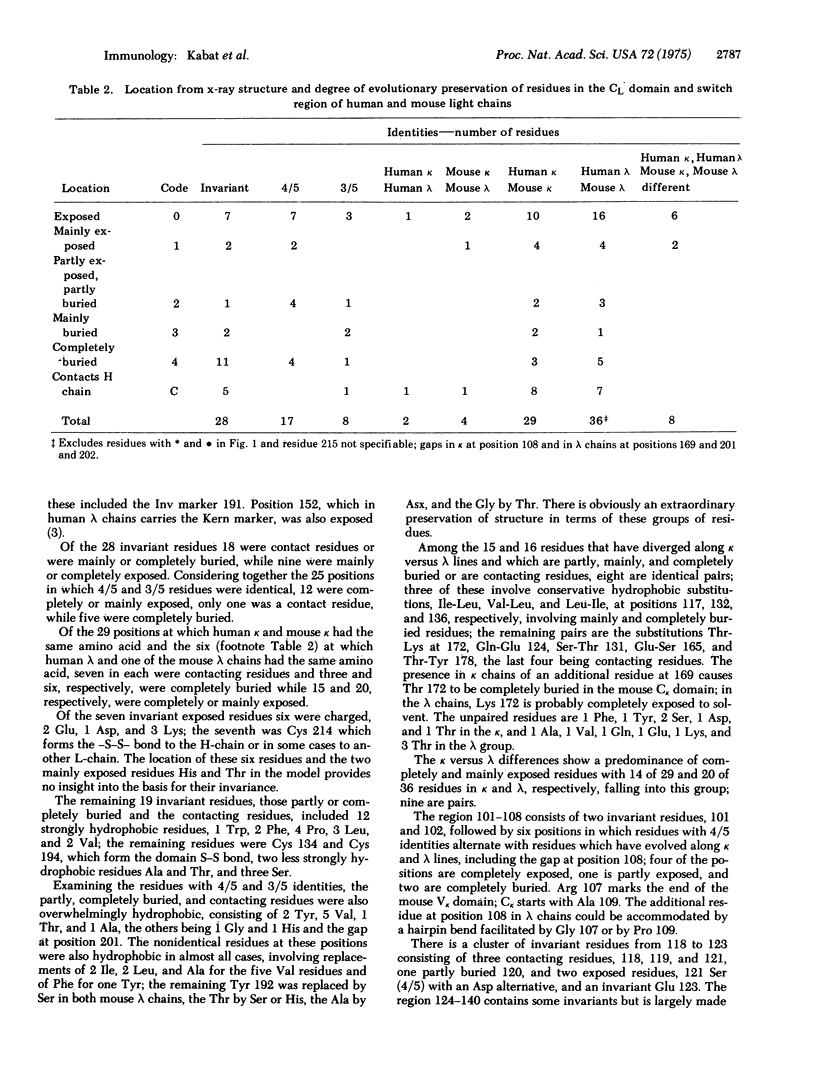
A comparison of five constant region sequences of human and mouse k and lambda immunoglobulin chains has been undertaken in order to reveal sequence homologies and evolutionary relationships. Simultaneously, a comparison with the three-dimensional structure of one mouse k-chain (McPC 603) has suggested structural reasons why many of the residues are invariant or conserved along k versus lambda lines. There are a number of residues that have remained invariant despite exposed positions for reasons that do not apppear to be connected with the folding of the CL domain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Poljak R. J., Amzel L. M., Chen B. L., Phizackerley R. P., Saul F. The three-dimensional structure of the fab' fragment of a human myeloma immunoglobulin at 2.0-angstrom resolution. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3440–3444. doi: 10.1073/pnas.71.9.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svasti J., Milstein C. The complete amino acid sequence of a mouse kappa light chain. Biochem J. 1972 Jun;128(2):427–444. doi: 10.1042/bj1280427. [DOI] [PMC free article] [PubMed] [Google Scholar]


