Abstract
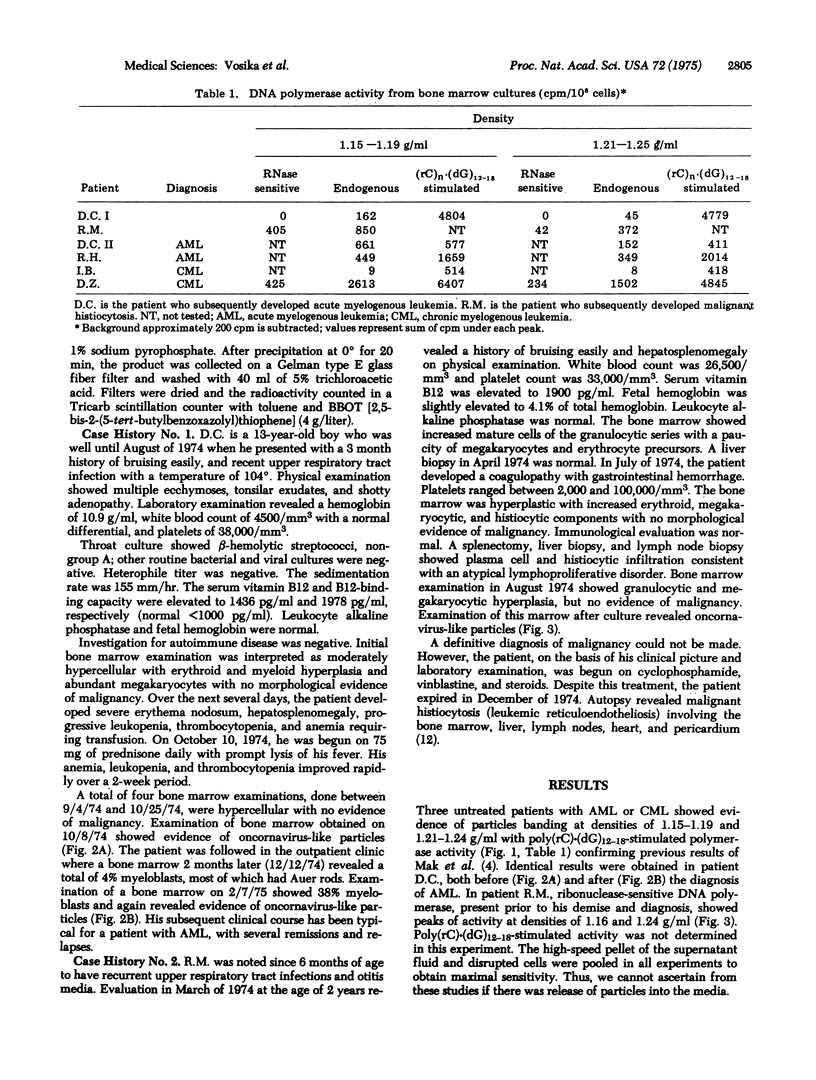
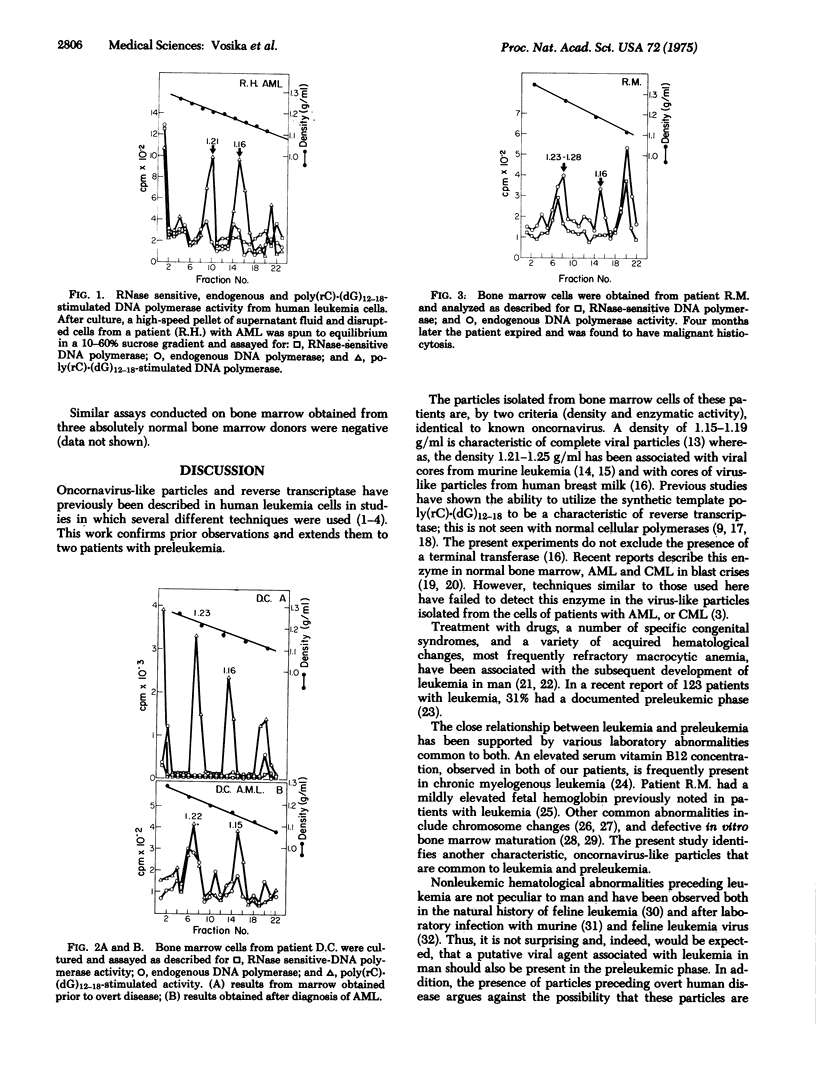
Particles with the density and enzymatic activity characteristic of known oncornavirus have been previously described in bone marrow cells from patients with leukemia in relapse and in remission. We have confirmed these findings and studied two patients in whom preleukemia was among the diagnostic considerations. Following cultivation of bone marrow from these patients for 1 week in conditioned media with dexamethasone, a high-speed pellet of the supernatant fluid and disrupted cells was prepared and analyzed on a sucrose gradient for enzymatic activity characteristic of RNA-directed DNA polymerase (reverse transcriptase). Peaks of endogenous DNA polymerase activity showing ribonuclease sensitivity and/or stimulation with the synthetic template poly(rC)-(dG)12-18 were demonstrated in both patients at densities of 1.15 to 1.19 and 1.21 to 1.24 g/ml. Subsequently, diagnosis 2 and 4 months after initial evaluation revealed acute myelogenous leukemia and malignant histiocytosis, respectively. Prior studies have suggested a possible etiological significance of such particles in human leukemia. The demonstration of similar particles preceding clinically overt disease in these patients supports this hypothesis and offers the possibility of early diagnosis and treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOCK M., JACOBSON L. O., BETHARD W. F. Preleukemic acute human leukemia. J Am Med Assoc. 1953 Jul 11;152(11):1018–1028. doi: 10.1001/jama.1953.03690110032010. [DOI] [PubMed] [Google Scholar]
- Baxt W. G. Sequences present in both human leukemic cell nuclear DNA and Rauscher leukemia virus. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2853–2857. doi: 10.1073/pnas.71.7.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxt W., Hehlmann R., Spiegelman S. Human leukaemic cells contain reverse transcriptase associated with a high molecular weight virus-related RNA. Nat New Biol. 1972 Nov 15;240(98):72–75. doi: 10.1038/newbio240072a0. [DOI] [PubMed] [Google Scholar]
- Brodsky I., Kahn S. B., Ross E. M., Petkov G., Braverman S. D. Prelymphoid leukemia phase of Rauscher virus infection. J Natl Cancer Inst. 1967 May;38(5):779–787. [PubMed] [Google Scholar]
- De-Thé G., O'Connor T. E. Structure of a murine leukemia virus after disruption with tween-ether and comparison with two myxoviruses. Virology. 1966 Apr;28(4):713–728. doi: 10.1016/0042-6822(66)90256-x. [DOI] [PubMed] [Google Scholar]
- Feldman S. P., Schlom J., Spiegelman S. Further evidence for oncornaviruses in human milk: the production of cores. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1976–1980. doi: 10.1073/pnas.70.7.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R. E., Gallo R. C. Type C RNA tumor virus isolated from cultured human acute myelogenous leukemia cells. Science. 1975 Jan 31;187(4174):350–353. doi: 10.1126/science.46123. [DOI] [PubMed] [Google Scholar]
- Gallagher R. E., Todaro G. J., Smith R. G., Livingston D. M., Gallo R. C. Relationship between RNA-directed DNA polymerase (reverse transcriptase) from human acute leukemic blood cells and primate type-C viruses. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1309–1313. doi: 10.1073/pnas.71.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Miller N. R., Saxinger W. C., Gillespie D. Primate RNA tumor virus-like DNA synthesized endogenously by RNA-dependent DNA polymerase in virus-like particles from fresh human acute leukemic blood cells. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3219–3224. doi: 10.1073/pnas.70.11.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde D. W., Cline M. J. Human preleukemia. Identification of a maturation defect in vitro. N Engl J Med. 1973 May 24;288(21):1083–1086. doi: 10.1056/NEJM197305242882101. [DOI] [PubMed] [Google Scholar]
- Hehlmann R., Kufe D., Spiegelman S. RNA in human leukemic cells related to the RNA of a mouse leukemia virus (leukocytes-RNA-DNA hybridization-rauscher virus-polysomal RNA). Proc Natl Acad Sci U S A. 1972 Feb;69(2):435–439. doi: 10.1073/pnas.69.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B. J., Abrell J. W., Smith R. G., Gallo R. C. DNA polymerases in human lymphoblastoid cells infected with simian sarcoma virus. Biochim Biophys Acta. 1974 May 17;349(2):148–160. doi: 10.1016/0005-2787(74)90076-8. [DOI] [PubMed] [Google Scholar]
- Lisker R., Cobo de Gutiérrez A., Velázquez-Ferrari M. Longitudinal bone marrow chromosome studies in potential leukemic myeloid disorders. Cancer. 1973 Mar;31(3):509–515. doi: 10.1002/1097-0142(197303)31:3<509::aid-cncr2820310304>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Mackey L., Jarrett W., Jarrett O., Laird H. Anemia associated with feline leukemia virus infection in cats. J Natl Cancer Inst. 1975 Jan;54(1):209–217. doi: 10.1093/jnci/54.1.209. [DOI] [PubMed] [Google Scholar]
- Mak T. W., Aye M. T., Messner H., Sheinin R., Till J. E., McCulloch E. A. Reverse transcriptase activity: increase in marrow cultures from leukaemic patients in relapse and remission. Br J Cancer. 1974 Jun;29(6):433–437. doi: 10.1038/bjc.1974.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak T. W., Kurtz S., Manaster J., Housman D. Viral-related information in oncornavirus-lik particles isolated from cultures of marrow cells from leukemic patients in relapse and remission. Proc Natl Acad Sci U S A. 1975 Feb;72(2):623–627. doi: 10.1073/pnas.72.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak T. W., Manaster J., Howatson A. F., McCulloch E. A., Till J. E. Particles with characteristics of leukoviruses in cultures of marrow cells from leukemic patients in remission and relapse. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4336–4340. doi: 10.1073/pnas.71.11.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer H. S., Vida L. N., Honig G. R. Similarities of the erythrocytes in juvenile chronic myelogenous leukemia to fetal erythrocytes. Blood. 1972 Jun;39(6):778–784. [PubMed] [Google Scholar]
- McCaffrey R., Harrison T. A., Parkman R., Baltimore D. Terminal deoxynucleotidyl transferase activity in human leukemic cells and in normal human thymocytes. N Engl J Med. 1975 Apr 10;292(15):775–780. doi: 10.1056/NEJM197504102921504. [DOI] [PubMed] [Google Scholar]
- Miller N. R., Saxinger W. C., Reitz M. S., Gallagher R. E., Wu A. M., Gallo R. C., Gillespie D. Systematics of RNA tumor viruses and virus-like particles of human origin. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3177–3181. doi: 10.1073/pnas.71.8.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Pierre R. V. Preleukemic states. Semin Hematol. 1974 Jan;11(1):73–92. [PubMed] [Google Scholar]
- Rowley J. D., Blaisdell R. K., Jacobson L. O. Chromosome studies in preleukemia. I. Aneuploidy of group C chromosomes in three patients. Blood. 1966 Jun;27(6):782–799. [PubMed] [Google Scholar]
- Saarni M. I., Linman J. W. Preleukemia. The hematologic syndrome preceding acute leukemia. Am J Med. 1973 Jul;55(1):38–48. doi: 10.1016/0002-9343(73)90148-4. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Sarin P. S., Reitz M. S., Gallo R. C. Reverse transcriptase activity of human acute leukaemic cells: purification of the enzyme, response to AMV 70S RNA, and characterization of the DNA product. Nat New Biol. 1972 Nov 15;240(98):67–72. doi: 10.1038/newbio240067a0. [DOI] [PubMed] [Google Scholar]
- Senn J. S., Pinkerton P. H. Defective in vitro colony formation by human bone marrow preceding overt leukaemia. Br J Haematol. 1972 Sep;23(3):277–281. doi: 10.1111/j.1365-2141.1972.tb08873.x. [DOI] [PubMed] [Google Scholar]
- Skaff G., Lowman J. T., Brunning R., Krivit W. Vitamin B12 studies in myelogenous leukemia of childhood. J Pediatr. 1966 Jun;68(6):890–894. doi: 10.1016/s0022-3476(66)80207-x. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Gallo R. C. Immunological relationship of DNA polymerase from human acute leukaemia cells and primate and mouse leukaemia virus reverse transcriptase. Nature. 1973 Jul 27;244(5413):206–209. doi: 10.1038/244206a0. [DOI] [PubMed] [Google Scholar]


