Abstract
Molecular determinants of biological activity of gangliosides are generally believed to be carbohydrate in nature. However, our studies of immunomodulation by highly purified naturally occurring tumor gangliosides provide another perspective: while the immunosuppressive activity of gangliosides requires the intact molecule (both carbohydrate and ceramide moieties), ceramide structure strikingly influences ganglioside immunosuppressive activity. Molecular species of human neuroblastoma GD2 ganglioside in which the ceramide contains a shorter fatty acyl chain (C16:0, C18:0) were 6- to 10-fold more active than those with a longer fatty acyl chain (C22:0/C24:1, C24:0). These findings were confirmed in studies of ceramide species of human leukemia sialosylparagloboside and murine lymphoma GalNAcGM1b. Gangliosides that contain shorter-chain fatty acids (and are most immunosuppressive) are known to be preferentially shed by tumor cells. Therefore, the results suggest that the tumor cell is optimized to protect itself from host immune destruction by selective shedding of highly active ceramide species of gangliosides.
Full text
PDF
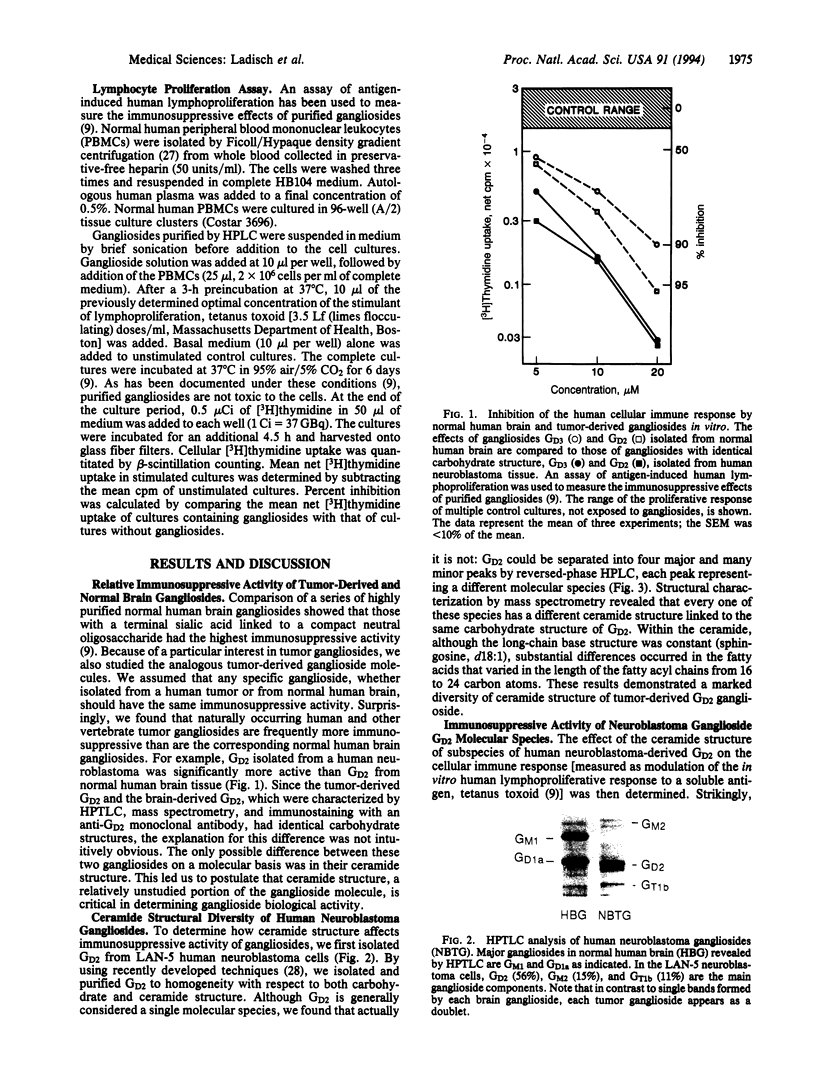
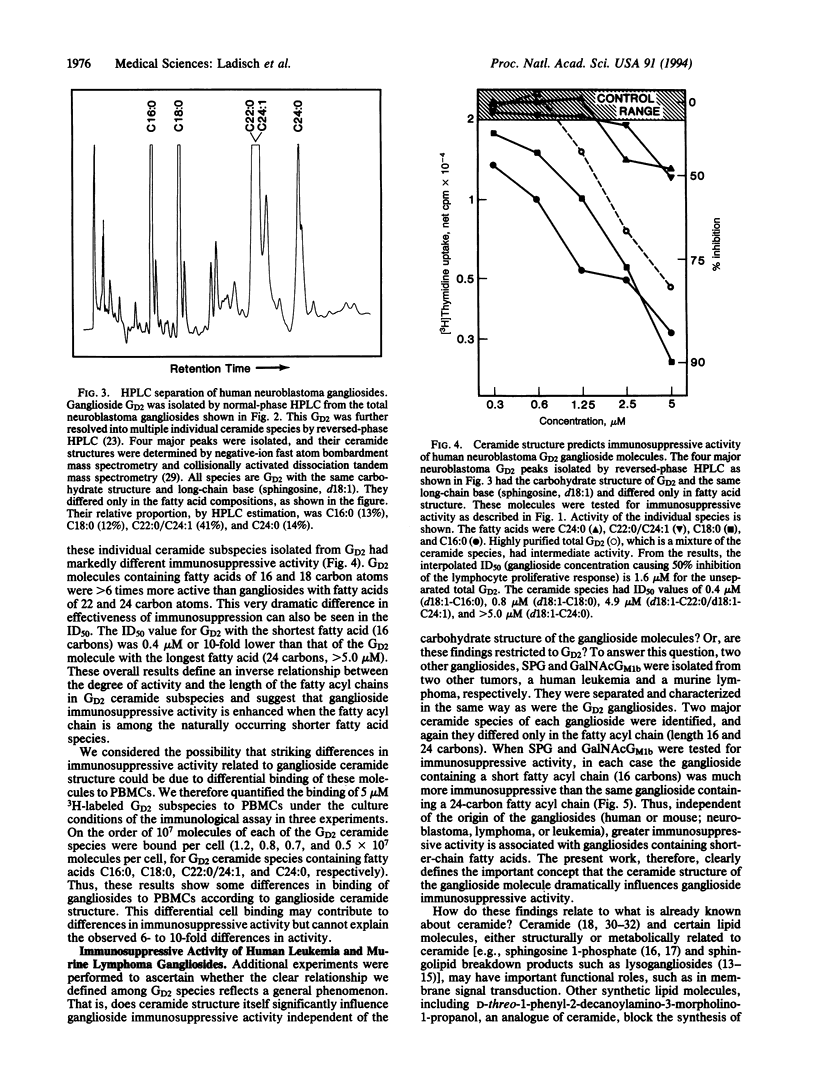
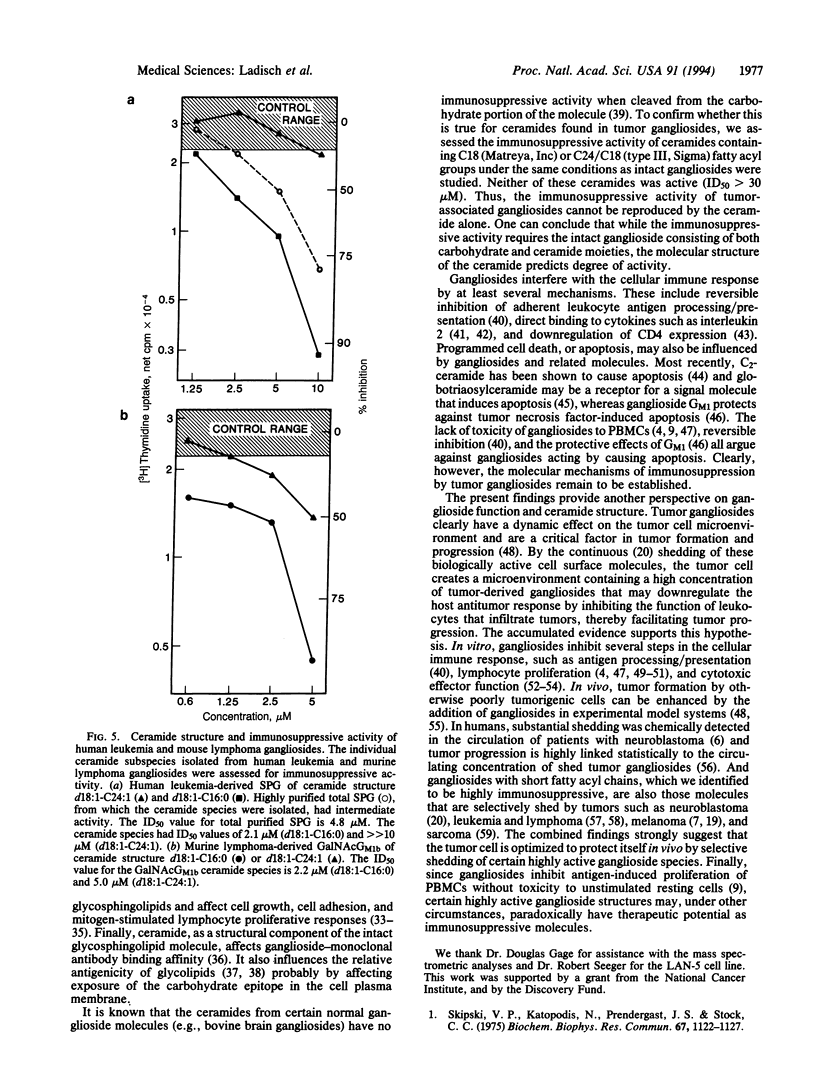

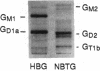
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessandri G., Filippeschi S., Sinibaldi P., Mornet F., Passera P., Spreafico F., Cappa P. M., Gullino P. M. Influence of gangliosides on primary and metastatic neoplastic growth in human and murine cells. Cancer Res. 1987 Aug 15;47(16):4243–4247. [PubMed] [Google Scholar]
- Ando I., Hoon D. S., Suzuki Y., Saxton R. E., Golub S. H., Irie R. F. Ganglioside GM2 on the K562 cell line is recognized as a target structure by human natural killer cells. Int J Cancer. 1987 Jul 15;40(1):12–17. doi: 10.1002/ijc.2910400104. [DOI] [PubMed] [Google Scholar]
- Barbour S., Edidin M., Felding-Habermann B., Taylor-Norton J., Radin N. S., Fenderson B. A. Glycolipid depletion using a ceramide analogue (PDMP) alters growth, adhesion, and membrane lipid organization in human A431 cells. J Cell Physiol. 1992 Mar;150(3):610–619. doi: 10.1002/jcp.1041500322. [DOI] [PubMed] [Google Scholar]
- Bergelson L. D., Dyatlovitskaya E. V., Klyuchareva T. E., Kryukova E. V., Lemenovskaya A. F., Matveeva V. A., Sinitsyna E. V. The role of glycosphingolipids in natural immunity. Gangliosides modulate the cytotoxicity of natural killer cells. Eur J Immunol. 1989 Nov;19(11):1979–1983. doi: 10.1002/eji.1830191102. [DOI] [PubMed] [Google Scholar]
- Bernhard H., Meyer zum Büschenfelde K. H., Dippold W. G. Ganglioside GD3 shedding by human malignant melanoma cells. Int J Cancer. 1989 Jul 15;44(1):155–160. doi: 10.1002/ijc.2910440127. [DOI] [PubMed] [Google Scholar]
- Black P. H. Shedding from the cell surface of normal and cancer cells. Adv Cancer Res. 1980;32:75–199. doi: 10.1016/s0065-230x(08)60361-9. [DOI] [PubMed] [Google Scholar]
- Bremer E. G., Schlessinger J., Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1986 Feb 15;261(5):2434–2440. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cheresh D. A., Pierschbacher M. D., Herzig M. A., Mujoo K. Disialogangliosides GD2 and GD3 are involved in the attachment of human melanoma and neuroblastoma cells to extracellular matrix proteins. J Cell Biol. 1986 Mar;102(3):688–696. doi: 10.1083/jcb.102.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J. W., Sharom F. J. Interleukin-2 binds to gangliosides in micelles and lipid bilayers. Biochim Biophys Acta. 1990 Oct 19;1028(3):205–214. doi: 10.1016/0005-2736(90)90168-n. [DOI] [PubMed] [Google Scholar]
- Dobrowsky R. T., Hannun Y. A. Ceramide stimulates a cytosolic protein phosphatase. J Biol Chem. 1992 Mar 15;267(8):5048–5051. [PubMed] [Google Scholar]
- Domon B., Costello C. E. Structure elucidation of glycosphingolipids and gangliosides using high-performance tandem mass spectrometry. Biochemistry. 1988 Mar 8;27(5):1534–1543. doi: 10.1021/bi00405a021. [DOI] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B., Igarashi Y., Fenderson B. A., Park L. S., Radin N. S., Inokuchi J., Strassmann G., Handa K., Hakomori S. A ceramide analogue inhibits T cell proliferative response through inhibition of glycosphingolipid synthesis and enhancement of N,N-dimethylsphingosine synthesis. Biochemistry. 1990 Jul 3;29(26):6314–6322. doi: 10.1021/bi00478a028. [DOI] [PubMed] [Google Scholar]
- Floutsis G., Ulsh L., Ladisch S. Immunosuppressive activity of human neuroblastoma tumor gangliosides. Int J Cancer. 1989 Jan 15;43(1):6–9. doi: 10.1002/ijc.2910430103. [DOI] [PubMed] [Google Scholar]
- Gazzotti G., Sonnino S., Ghidoni R., Kirschner G., Tettamanti G. Analytical and preparative high-performance liquid chromatography of gangliosides. J Neurosci Res. 1984;12(2-3):179–192. doi: 10.1002/jnr.490120206. [DOI] [PubMed] [Google Scholar]
- Gazzotti G., Sonnino S., Ghidoni R. Normal-phase high-performance liquid chromatographic separation of non-derivatized ganglioside mixtures. J Chromatogr. 1985 Dec 4;348(2):371–378. doi: 10.1016/s0021-9673(01)92475-6. [DOI] [PubMed] [Google Scholar]
- Grayson G., Ladisch S. Immunosuppression by human gangliosides. II. Carbohydrate structure and inhibition of human NK activity. Cell Immunol. 1992 Jan;139(1):18–29. doi: 10.1016/0008-8749(92)90096-8. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Tumor-associated carbohydrate antigens. Annu Rev Immunol. 1984;2:103–126. doi: 10.1146/annurev.iy.02.040184.000535. [DOI] [PubMed] [Google Scholar]
- Hanai N., Nores G. A., MacLeod C., Torres-Mendez C. R., Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 and lyso-GM3 in tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1988 Aug 5;263(22):10915–10921. [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science. 1987 Feb 6;235(4789):670–674. doi: 10.1126/science.3101176. [DOI] [PubMed] [Google Scholar]
- Kan C. C., Kolesnick R. N. A synthetic ceramide analog, D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol, selectively inhibits adherence during macrophage differentiation of human leukemia cells. J Biol Chem. 1992 May 15;267(14):9663–9667. [PubMed] [Google Scholar]
- Kannagi R., Nudelman E., Hakomori S. Possible role of ceramide in defining structure and function of membrane glycolipids. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3470–3474. doi: 10.1073/pnas.79.11.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R., Stroup R., Cochran N. A., Urdal D. L., Young W. W., Jr, Hakomori S. Factors affecting expression of glycolipid tumor antigens: influence of ceramide composition and coexisting glycolipid on the antigenicity of gangliotriaosylceramide in murine lymphoma cells. Cancer Res. 1983 Oct;43(10):4997–5005. [PubMed] [Google Scholar]
- Karlsson G., Månsson J. E., Wikstrand C., Bigner D., Svennerholm L. Characterization of the binding epitope of the monoclonal antibody DMAb-1 to ganglioside GM2. Biochim Biophys Acta. 1990 Apr 17;1043(3):267–272. doi: 10.1016/0005-2760(90)90026-t. [DOI] [PubMed] [Google Scholar]
- Kim M. Y., Linardic C., Obeid L., Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991 Jan 5;266(1):484–489. [PubMed] [Google Scholar]
- Kloppel T. M., Keenan T. W., Freeman M. J., Morré D. J. Glycolipid-bound sialic acid in serum: increased levels in mice and humans bearing mammary carcinomas. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3011–3013. doi: 10.1073/pnas.74.7.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T., Fehsel K., Zielasek J., Kolb H., Burkart V. Gangliosides protect from TNF alpha-induced apoptosis. Immunol Lett. 1993 Mar;35(3):207–212. doi: 10.1016/0165-2478(93)90184-4. [DOI] [PubMed] [Google Scholar]
- Ladisch S., Becker H., Ulsh L. Immunosuppression by human gangliosides: I. Relationship of carbohydrate structure to the inhibition of T cell responses. Biochim Biophys Acta. 1992 Apr 23;1125(2):180–188. doi: 10.1016/0005-2760(92)90043-u. [DOI] [PubMed] [Google Scholar]
- Ladisch S., Gillard B. A solvent partition method for microscale ganglioside purification. Anal Biochem. 1985 Apr;146(1):220–231. doi: 10.1016/0003-2697(85)90419-1. [DOI] [PubMed] [Google Scholar]
- Ladisch S., Gillard B., Wong C., Ulsh L. Shedding and immunoregulatory activity of YAC-1 lymphoma cell gangliosides. Cancer Res. 1983 Aug;43(8):3808–3813. [PubMed] [Google Scholar]
- Ladisch S., Kitada S., Hays E. F. Gangliosides shed by tumor cells enhance tumor formation in mice. J Clin Invest. 1987 Jun;79(6):1879–1882. doi: 10.1172/JCI113031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladisch S., Sweeley C. C., Becker H., Gage D. Aberrant fatty acyl alpha-hydroxylation in human neuroblastoma tumor gangliosides. J Biol Chem. 1989 Jul 15;264(20):12097–12105. [PubMed] [Google Scholar]
- Ladisch S., Ulsh L., Gillard B., Wong C. Modulation of the immune response by gangliosides. Inhibition of adherent monocyte accessory function in vitro. J Clin Invest. 1984 Dec;74(6):2074–2081. doi: 10.1172/JCI111631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladisch S., Wu Z. L. Detection of a tumour-associated ganglioside in plasma of patients with neuroblastoma. Lancet. 1985 Jan 19;1(8421):136–138. doi: 10.1016/s0140-6736(85)91906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Lengle E. E. Increased levels of lipid-bound sialic acid in thymic lymphocytes and plasma from leukemic AKR/J mice. J Natl Cancer Inst. 1979 Jun;62(6):1565–1567. [PubMed] [Google Scholar]
- Lengle E. E., Krishnaraj R., Kemp R. G. Inhibition of the lectin-induced mitogenic response of thymocytes by glycolipids. Cancer Res. 1979 Mar;39(3):817–822. [PubMed] [Google Scholar]
- Li R. X., Ladisch S. Shedding of human neuroblastoma gangliosides. Biochim Biophys Acta. 1991 Apr 24;1083(1):57–64. doi: 10.1016/0005-2760(91)90124-z. [DOI] [PubMed] [Google Scholar]
- Li R., Gage D., Ladisch S. Biosynthesis and shedding of murine lymphoma gangliosides. Biochim Biophys Acta. 1993 Nov 3;1170(3):283–290. doi: 10.1016/0005-2760(93)90011-w. [DOI] [PubMed] [Google Scholar]
- Mangeney M., Richard Y., Coulaud D., Tursz T., Wiels J. CD77: an antigen of germinal center B cells entering apoptosis. Eur J Immunol. 1991 May;21(5):1131–1140. doi: 10.1002/eji.1830210507. [DOI] [PubMed] [Google Scholar]
- Novikov A. M., Kozlov A. M., Bassalyk L. S. Izbiratel'noe sbrasyvanie gangliozidov kletkami astsitnoi sarkoma-37 myshei. Biull Eksp Biol Med. 1986 Aug;102(8):229–231. [PubMed] [Google Scholar]
- Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. Programmed cell death induced by ceramide. Science. 1993 Mar 19;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Offner H., Thieme T., Vandenbark A. A. Gangliosides induce selective modulation of CD4 from helper T lymphocytes. J Immunol. 1987 Nov 15;139(10):3295–3305. [PubMed] [Google Scholar]
- Okazaki T., Bell R. M., Hannun Y. A. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989 Nov 15;264(32):19076–19080. [PubMed] [Google Scholar]
- Okazaki T., Bielawska A., Bell R. M., Hannun Y. A. Role of ceramide as a lipid mediator of 1 alpha,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1990 Sep 15;265(26):15823–15831. [PubMed] [Google Scholar]
- Robb R. J. The suppressive effect of gangliosides upon IL 2-dependent proliferation as a function of inhibition of IL 2-receptor association. J Immunol. 1986 Feb 1;136(3):971–976. [PubMed] [Google Scholar]
- Ryan J. L., Shinitzky M. Possible role for glycosphingolipids in the control of immune responses. Eur J Immunol. 1979 Feb;9(2):171–175. doi: 10.1002/eji.1830090215. [DOI] [PubMed] [Google Scholar]
- Sadahira Y., Ruan F., Hakomori S., Igarashi Y. Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9686–9690. doi: 10.1073/pnas.89.20.9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann G. A simple and novel method for tritium labeling of gangliosides and other sphingolipids. Biochim Biophys Acta. 1978 Apr 28;529(1):106–114. doi: 10.1016/0005-2760(78)90108-x. [DOI] [PubMed] [Google Scholar]
- Shaposhnikova G. I., Prokazova N. V., Buznikov G. A., Zvezdina N. D., Teplitz N. A., Bergelson L. D. Shedding of gangliosides from tumor cells depends on cell density. Eur J Biochem. 1984 May 2;140(3):567–570. doi: 10.1111/j.1432-1033.1984.tb08139.x. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Katopodis N., Prendergast J. S., Stock C. C. Gangliosides in blood serum of normal rats and Morris hepatoma 5123tc-bearing rats. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1122–1127. doi: 10.1016/0006-291x(75)90790-1. [DOI] [PubMed] [Google Scholar]
- Valentino L., Moss T., Olson E., Wang H. J., Elashoff R., Ladisch S. Shed tumor gangliosides and progression of human neuroblastoma. Blood. 1990 Apr 1;75(7):1564–1567. [PubMed] [Google Scholar]
- Whisler R. L., Yates A. J. Regulation of lymphocyte responses by human gangliosides. I. Characteristics of inhibitory effects and the induction of impaired activation. J Immunol. 1980 Nov;125(5):2106–2111. [PubMed] [Google Scholar]
- Young W. W., Jr, Borgman C. A., Wolock D. M. Modes of shedding of glycosphingolipids from mouse lymphoma cells. J Biol Chem. 1986 Feb 15;261(5):2279–2283. [PubMed] [Google Scholar]
- Zhang H., Desai N. N., Olivera A., Seki T., Brooker G., Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991 Jul;114(1):155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]